CHAPTER 13 CHEMICAL KINETICS RATE LAWS FIRST ORDER





![Rate Laws: General Comments • REACTION ORDER – RATE = [A]1 – RATE = Rate Laws: General Comments • REACTION ORDER – RATE = [A]1 – RATE =](https://slidetodoc.com/presentation_image/5d94bb4f48077ed2bcfb9a417210ead7/image-13.jpg)
![Effect of Concentration: Rate Laws First-Order Reactions • rate = k[A]1 • A plot Effect of Concentration: Rate Laws First-Order Reactions • rate = k[A]1 • A plot](https://slidetodoc.com/presentation_image/5d94bb4f48077ed2bcfb9a417210ead7/image-14.jpg)
![Problem • ln[A]t = -kt +ln [A]0 • 4. 5 E-2 s-1 • [A]t Problem • ln[A]t = -kt +ln [A]0 • 4. 5 E-2 s-1 • [A]t](https://slidetodoc.com/presentation_image/5d94bb4f48077ed2bcfb9a417210ead7/image-17.jpg)


![Reaction Rate: Concentration Second-Order Reactions: rate = k [A] [B] • For a second Reaction Rate: Concentration Second-Order Reactions: rate = k [A] [B] • For a second](https://slidetodoc.com/presentation_image/5d94bb4f48077ed2bcfb9a417210ead7/image-22.jpg)
- Slides: 26

CHAPTER 13: CHEMICAL KINETICS • RATE LAWS • FIRST ORDER REACTIONS

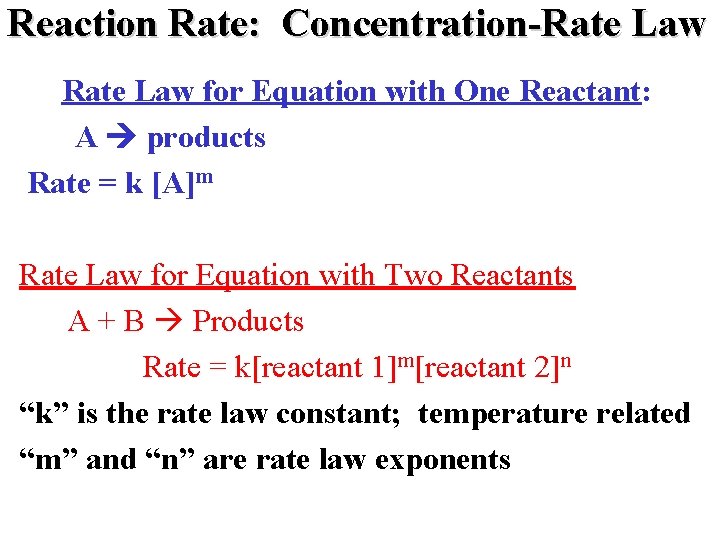
Reaction Rate: Concentration-Rate Law for Equation with One Reactant: A products Rate = k [A]m Rate Law for Equation with Two Reactants A + B Products Rate = k[reactant 1]m[reactant 2]n “k” is the rate law constant; temperature related “m” and “n” are rate law exponents

Reaction Rate: Concentration-Rate Law To Deduce Intuitively. Rate Law Exponents: From data, observe correlation between change in concentration and rate. • rate law exponent is zero (zero order) for a reactant if the change in concentration of that reactant produces no effect. • Rate law exponent is one (first order) if doubling the concentration causes the rate to double.

To Deduce, Intuitively, Rate Law Exponents: • Rate law exponent is two (second order) if doubling the concentration results in a 22 increase in rate. • Note that the rate constant does not depend on concentration.

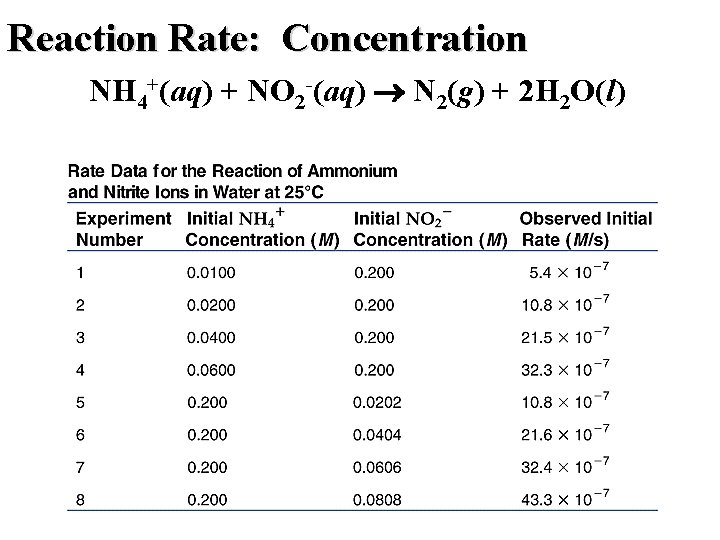
Reaction Rate: Concentration NH 4+(aq) + NO 2 -(aq) N 2(g) + 2 H 2 O(l)

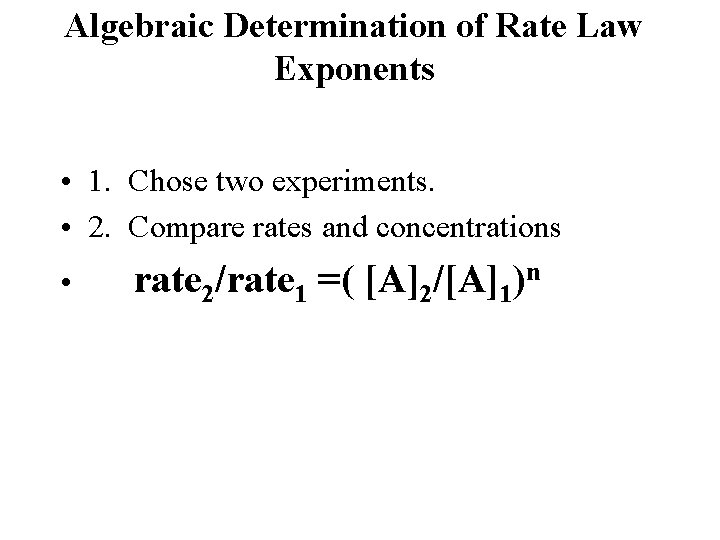
Algebraic Determination of Rate Law Exponents • 1. Chose two experiments. • 2. Compare rates and concentrations • rate 2/rate 1 =( [A]2/[A]1)n

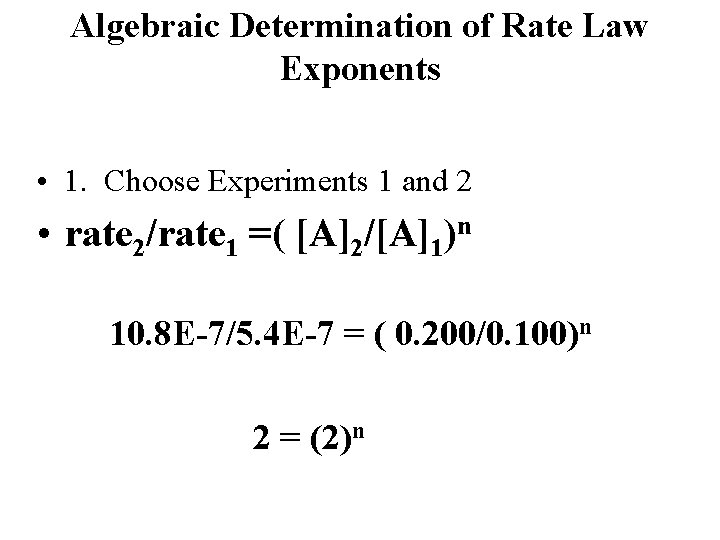
Algebraic Determination of Rate Law Exponents • 1. Choose Experiments 1 and 2 • rate 2/rate 1 =( [A]2/[A]1)n 10. 8 E-7/5. 4 E-7 = ( 0. 200/0. 100)n 2 = (2)n

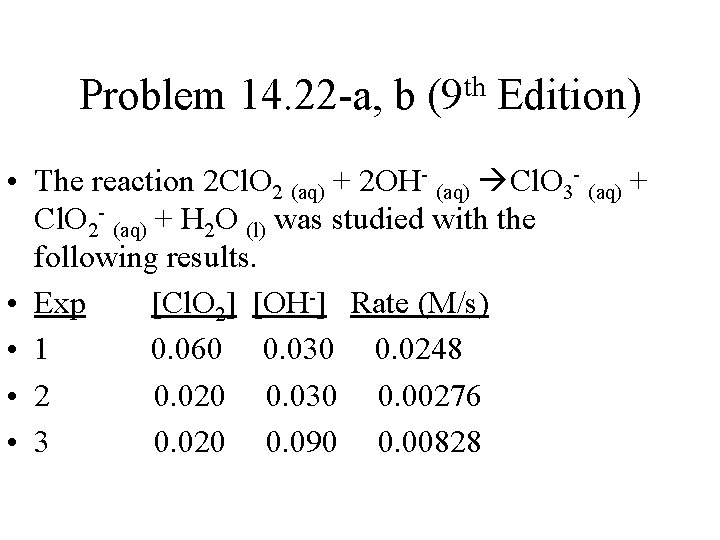
Problem 14. 22 -a, b th (9 Edition) • The reaction 2 Cl. O 2 (aq) + 2 OH- (aq) Cl. O 3 - (aq) + Cl. O 2 - (aq) + H 2 O (l) was studied with the following results. • Exp [Cl. O 2] [OH-] Rate (M/s) • 1 0. 060 0. 030 0. 0248 • 2 0. 020 0. 030 0. 00276 • 3 0. 020 0. 090 0. 00828

Problem 14. 22 -a, b (9 th Edition) • (a) Determine the rate law • (b) Calculate the value of the rate law constant

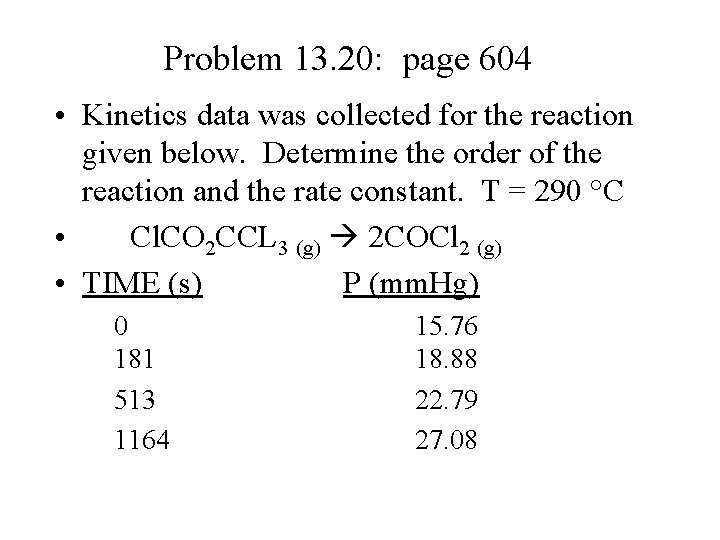
Problem 13. 20: page 604 • Kinetics data was collected for the reaction given below. Determine the order of the reaction and the rate constant. T = 290 °C • Cl. CO 2 CCL 3 (g) 2 COCl 2 (g) • TIME (s) P (mm. Hg) 0 181 513 1164 15. 76 18. 88 22. 79 27. 08

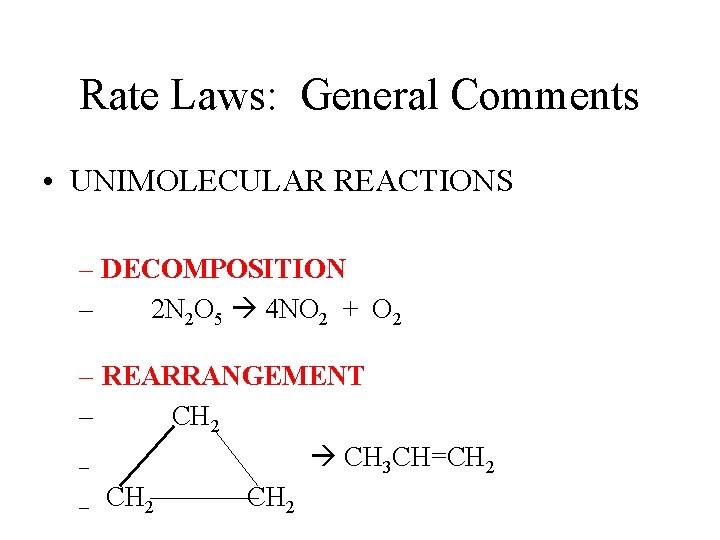
Rate Laws: General Comments • Write rate laws for ELEMENTARY REACTIONS – Unimolecular – A B – Bimolecular – A + B Products

Rate Laws: General Comments • UNIMOLECULAR REACTIONS – DECOMPOSITION – 2 N 2 O 5 4 NO 2 + O 2 – REARRANGEMENT – CH 2 CH 3 CH=CH 2 – CH 2
![Rate Laws General Comments REACTION ORDER RATE A1 RATE Rate Laws: General Comments • REACTION ORDER – RATE = [A]1 – RATE =](https://slidetodoc.com/presentation_image/5d94bb4f48077ed2bcfb9a417210ead7/image-13.jpg)
Rate Laws: General Comments • REACTION ORDER – RATE = [A]1 – RATE = [A]2 – RATE = [A] [B] – RATE =[A]0
![Effect of Concentration Rate Laws FirstOrder Reactions rate kA1 A plot Effect of Concentration: Rate Laws First-Order Reactions • rate = k[A]1 • A plot](https://slidetodoc.com/presentation_image/5d94bb4f48077ed2bcfb9a417210ead7/image-14.jpg)
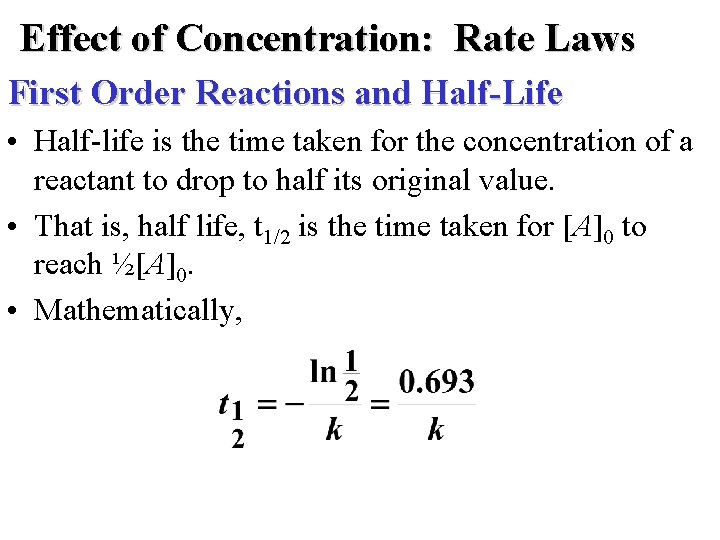
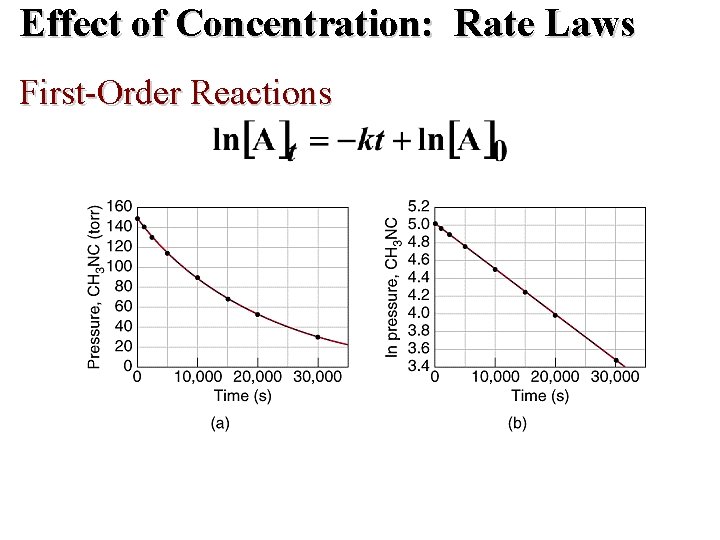
Effect of Concentration: Rate Laws First-Order Reactions • rate = k[A]1 • A plot of ln[A]t versus t is a straight line with slope -k and intercept ln[A]0.

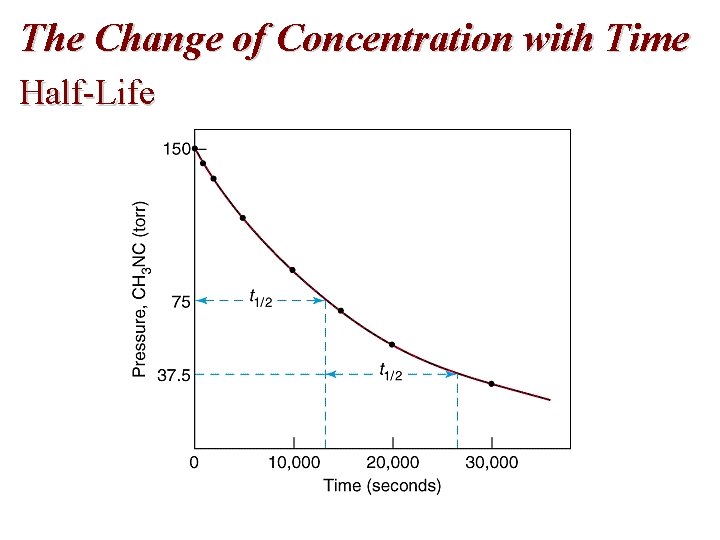
The Change of Concentration with Time Half-Life

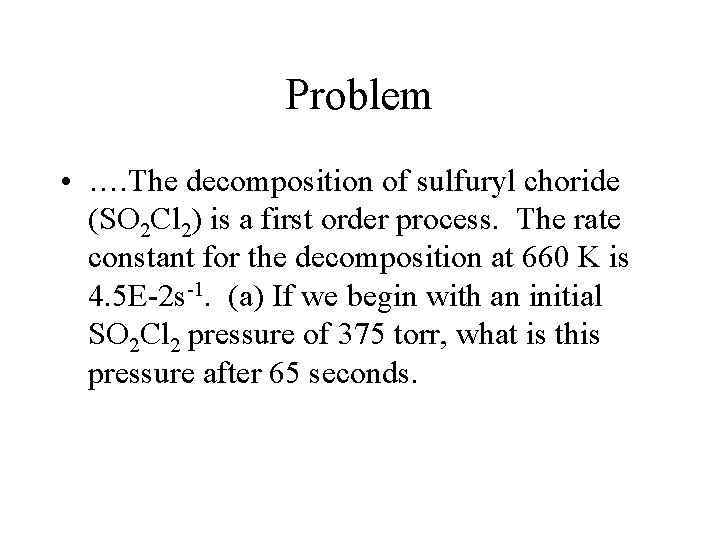
Problem • …. The decomposition of sulfuryl choride (SO 2 Cl 2) is a first order process. The rate constant for the decomposition at 660 K is 4. 5 E-2 s-1. (a) If we begin with an initial SO 2 Cl 2 pressure of 375 torr, what is this pressure after 65 seconds.
![Problem lnAt kt ln A0 4 5 E2 s1 At Problem • ln[A]t = -kt +ln [A]0 • 4. 5 E-2 s-1 • [A]t](https://slidetodoc.com/presentation_image/5d94bb4f48077ed2bcfb9a417210ead7/image-17.jpg)
Problem • ln[A]t = -kt +ln [A]0 • 4. 5 E-2 s-1 • [A]t = ? • [A]0 = 375 torr • ln (x) = -(4. 5 E-2 s-1)(65 seconds) + (ln 375) • ln (x) = -2. 925 + 5. 927 = 3. 0019 • inverse/antilog/ex = 20. 1 torr

Reaction Rate & Concentration: First Order Reactions • The first-order rate constant for the decomposition of an antibiotic is 6. 82 E-3 hour – 1. The initial concentration of the antibiotic is 125 mg. How long will it take for the antibiotic to decompose to 25. 0 mg?

Effect of Concentration: Rate Laws First Order Reactions and Half-Life • Half-life is the time taken for the concentration of a reactant to drop to half its original value. • That is, half life, t 1/2 is the time taken for [A]0 to reach ½[A]0. • Mathematically,

First Order Reactions: Half-Life • The first-order rate constant for the decomposition of an antibiotic is 6. 82 E-3 hour– 1. Calculate the half-life for this antibiotic.

Effect of Concentration: Rate Laws First-Order Reactions
![Reaction Rate Concentration SecondOrder Reactions rate k A B For a second Reaction Rate: Concentration Second-Order Reactions: rate = k [A] [B] • For a second](https://slidetodoc.com/presentation_image/5d94bb4f48077ed2bcfb9a417210ead7/image-22.jpg)
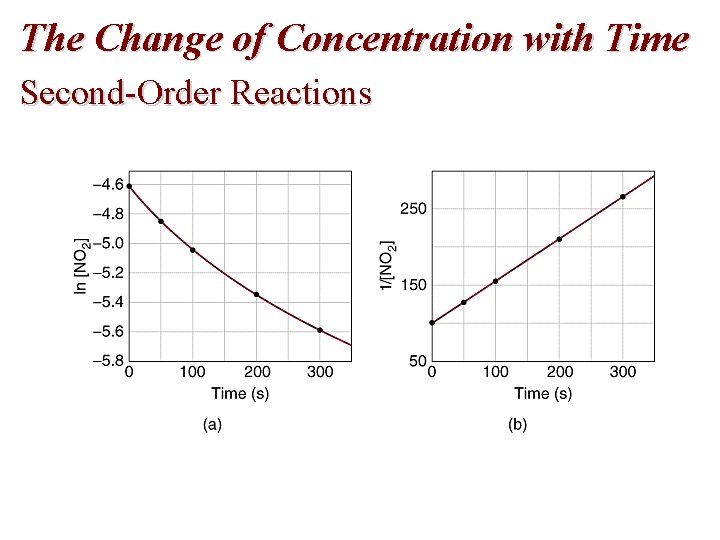
Reaction Rate: Concentration Second-Order Reactions: rate = k [A] [B] • For a second order reaction with just one reactant • A plot of 1/[A]t versus t is a straight line with slope k and intercept 1/[A]0 • For a second order reaction, a plot of ln[A]t vs. t is not linear.

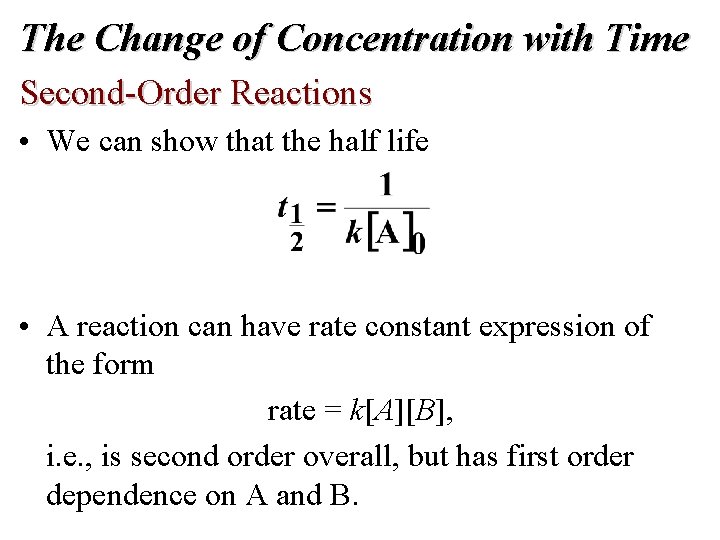
The Change of Concentration with Time Second-Order Reactions

The Change of Concentration with Time Second-Order Reactions • We can show that the half life • A reaction can have rate constant expression of the form rate = k[A][B], i. e. , is second order overall, but has first order dependence on A and B.

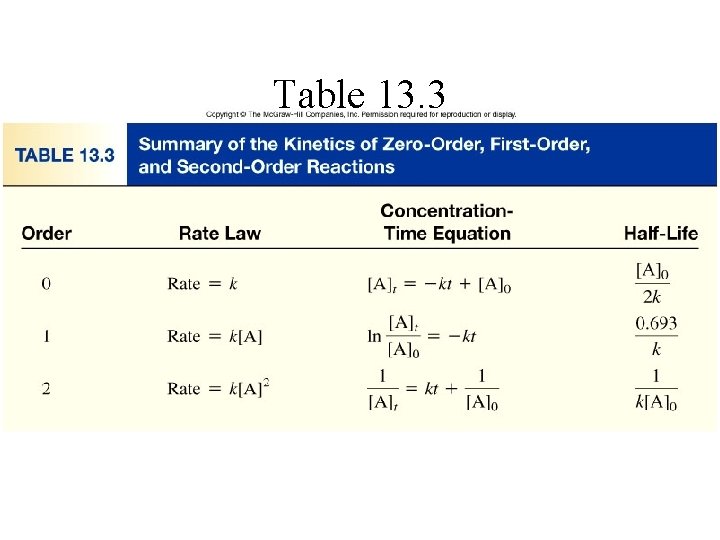
Table 13. 3

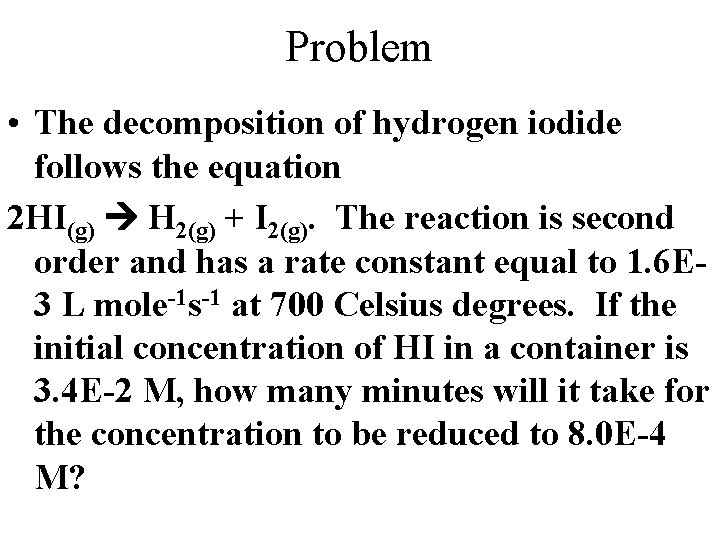
Problem • The decomposition of hydrogen iodide follows the equation 2 HI(g) H 2(g) + I 2(g). The reaction is second order and has a rate constant equal to 1. 6 E 3 L mole-1 s-1 at 700 Celsius degrees. If the initial concentration of HI in a container is 3. 4 E-2 M, how many minutes will it take for the concentration to be reduced to 8. 0 E-4 M?