Chemical Reactions Factors Affecting Rates of Reaction January





- Slides: 25

Chemical Reactions Factors Affecting Rates of Reaction January 9, 2015

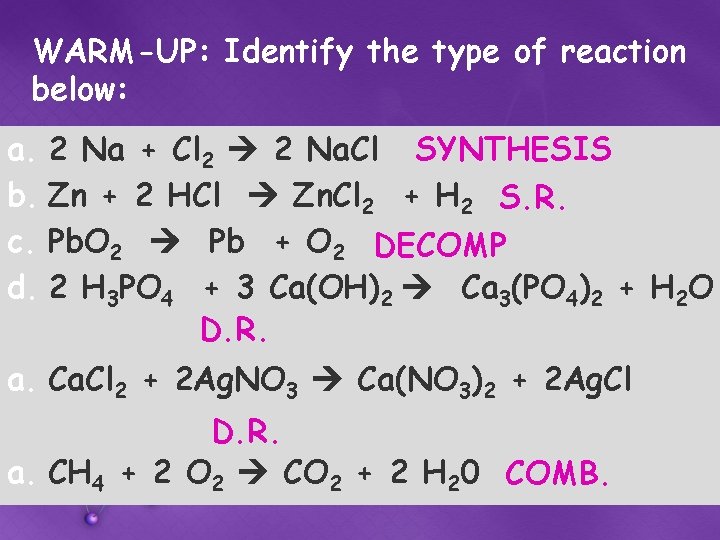
WARM-UP: Identify the type of reaction below: a. 2 Na + Cl 2 2 Na. Cl SYNTHESIS b. Zn + 2 HCl Zn. Cl 2 + H 2 S. R. c. Pb. O 2 Pb + O 2 DECOMP d. 2 H 3 PO 4 + 3 Ca(OH)2 Ca 3(PO 4)2 + H 2 O D. R. a. Cl 2 + 2 Ag. NO 3 Ca(NO 3)2 + 2 Ag. Cl D. R. a. CH 4 + 2 O 2 CO 2 + 2 H 20 COMB.

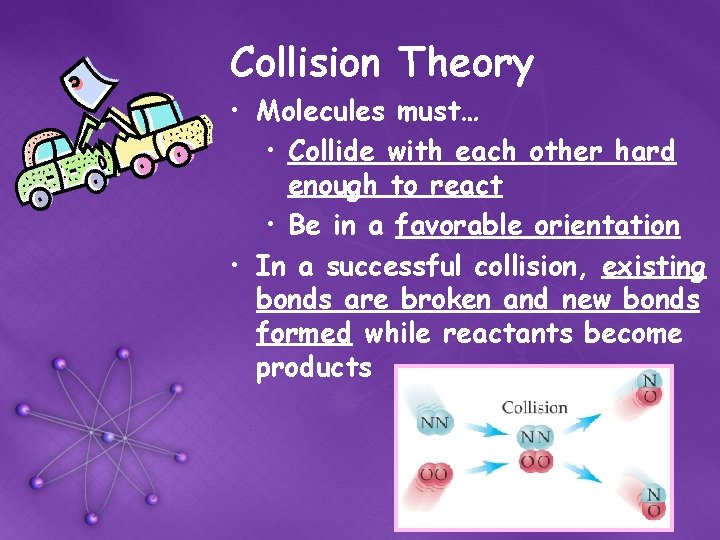
Collision Theory • Molecules must… • Collide with each other hard enough to react • Be in a favorable orientation • In a successful collision, existing bonds are broken and new bonds formed while reactants become products

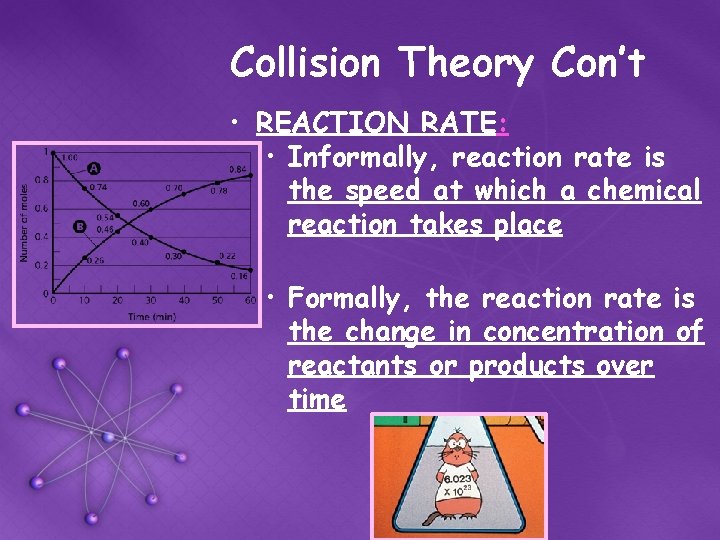
Collision Theory Con’t • REACTION RATE: • Informally, reaction rate is the speed at which a chemical reaction takes place • Formally, the reaction rate is the change in concentration of reactants or products over time

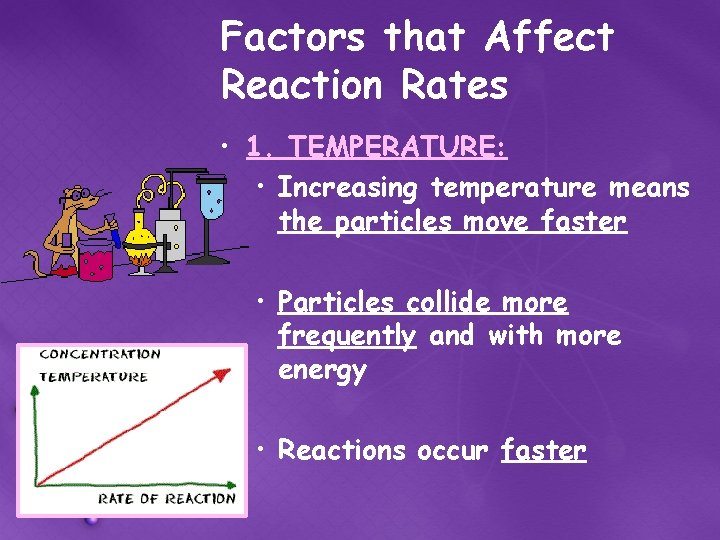
Factors that Affect Reaction Rates • 1. TEMPERATURE: • Increasing temperature means the particles move faster • Particles collide more frequently and with more energy • Reactions occur faster

Factors that Affect Reaction Rates • 2. CONCENTRATION: • Increasing concentration means the molecules are closer together • Particles collide more frequently • Reactions occur faster

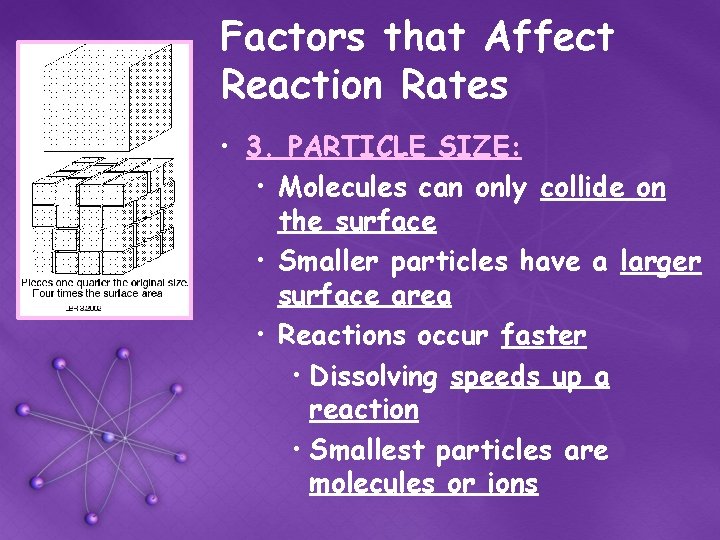
Factors that Affect Reaction Rates • 3. PARTICLE SIZE: • Molecules can only collide on the surface • Smaller particles have a larger surface area • Reactions occur faster • Dissolving speeds up a reaction • Smallest particles are molecules or ions

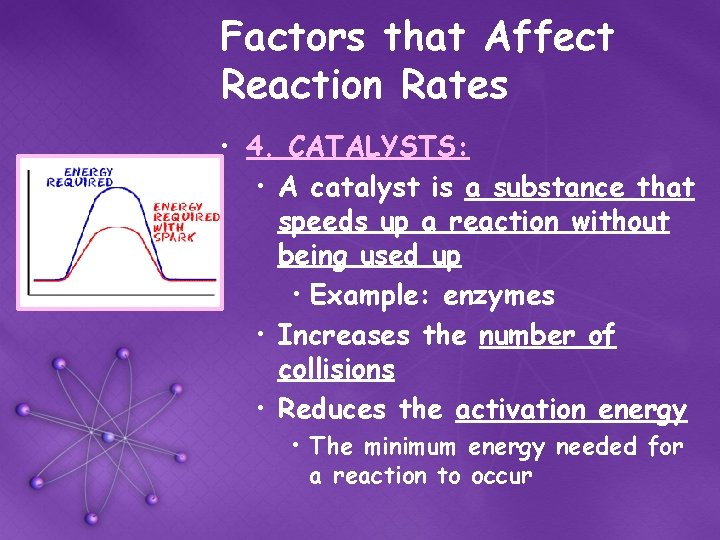
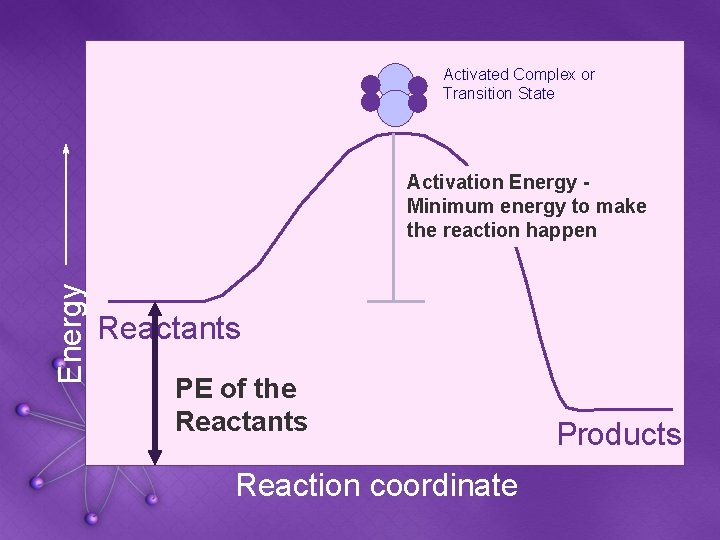
Factors that Affect Reaction Rates • 4. CATALYSTS: • A catalyst is a substance that speeds up a reaction without being used up • Example: enzymes • Increases the number of collisions • Reduces the activation energy • The minimum energy needed for a reaction to occur

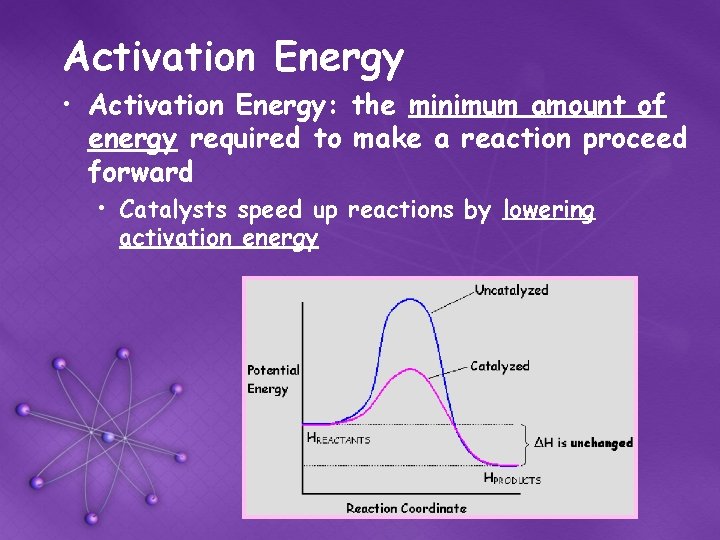
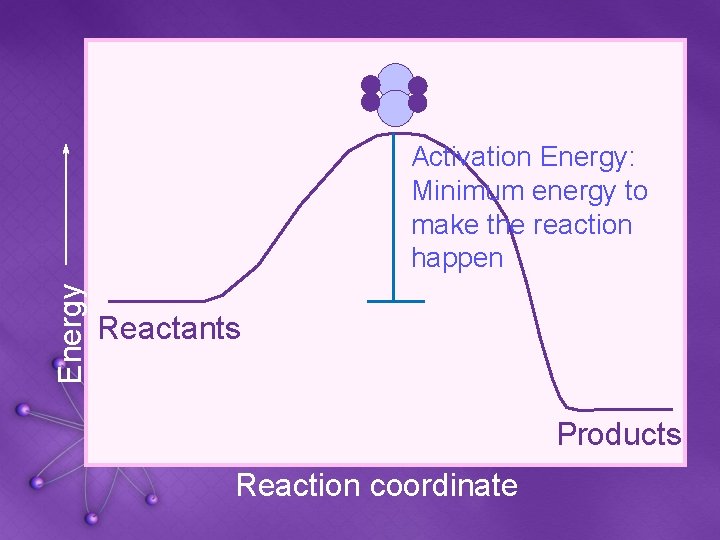
Activation Energy • Activation Energy: the minimum amount of energy required to make a reaction proceed forward • Catalysts speed up reactions by lowering activation energy

Check for Understanding 1. According to “Collision Theory”, what two conditions must be fulfilled for a reaction to occur? 2. How does increasing temp cause the reaction to occur faster? 3. Which has a larger surface area, a single stick of butter or butter cubed into small pieces? Which would cause a reaction proceed faster? 4. What is the activation energy?

Check for Understanding ANSWERS 1. The molecules must collide with enough strength to react and be in a favorable orientation 2. It speeds up the molecules and they collide more frequently 3. Cubed butter because it is smaller in size; cubed butter will react faster 4. Minimum amount of energy required for a reaction to occur

Reaction Rate Reaction rate can be stated as: 1. The decrease in the concentration of reactants with time or 2. The increase in the concentration of products with time

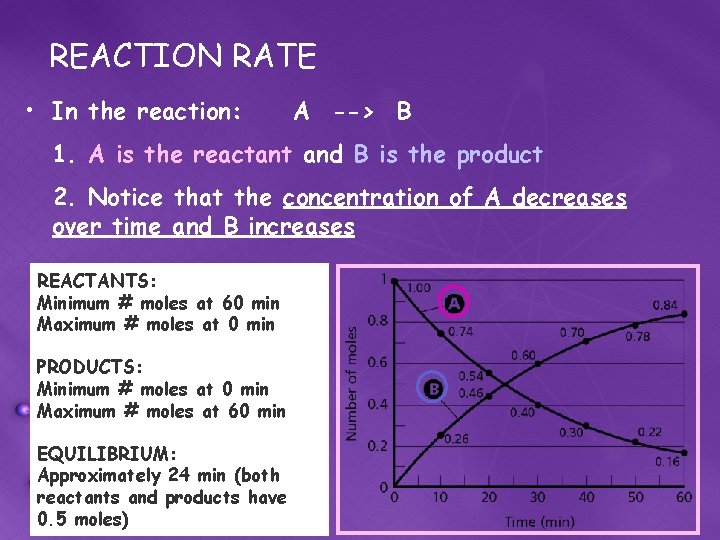
REACTION RATE • In the reaction: A --> B 1. A is the reactant and B is the product 2. Notice that the concentration of A decreases over time and B increases REACTANTS: Minimum # moles at 60 min Maximum # moles at 0 min PRODUCTS: Minimum # moles at 0 min Maximum # moles at 60 min EQUILIBRIUM: Approximately 24 min (both reactants and products have 0. 5 moles)

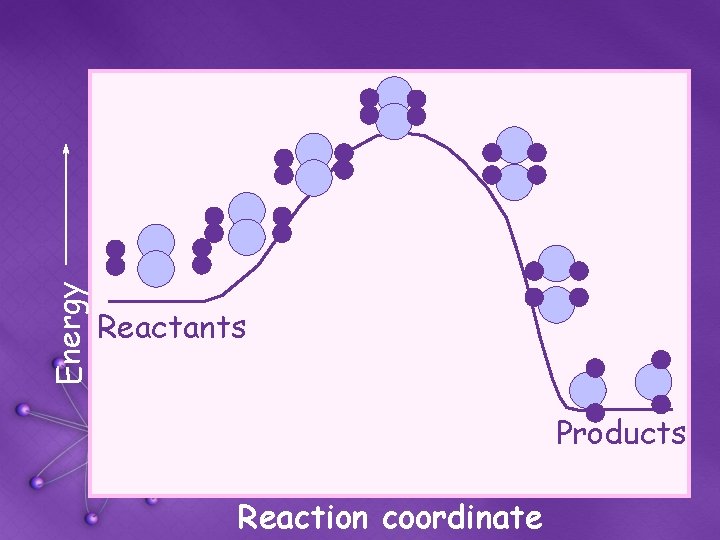
Energy Reactants Products Reaction coordinate

Energy Activation Energy: Minimum energy to make the reaction happen Reactants Products Reaction coordinate

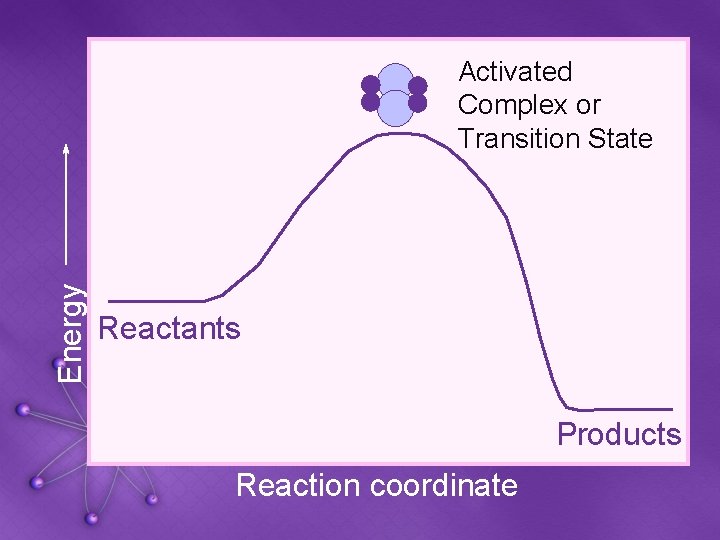
Energy Activated Complex or Transition State Reactants Products Reaction coordinate

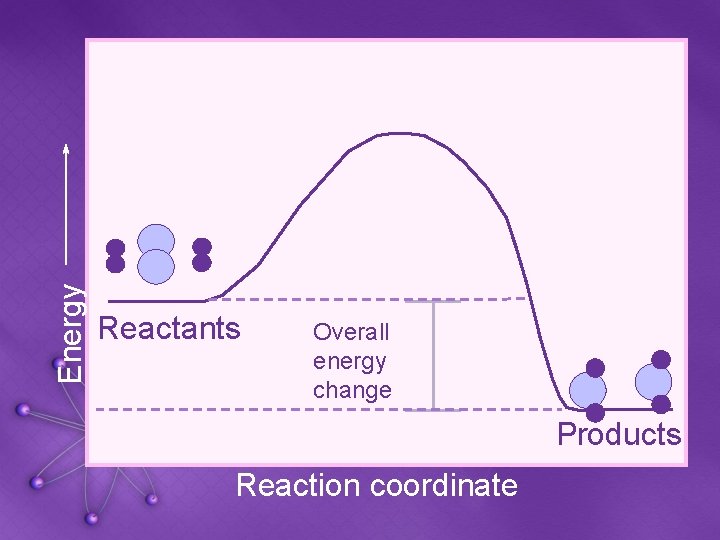
Energy Reactants Overall energy change Products Reaction coordinate

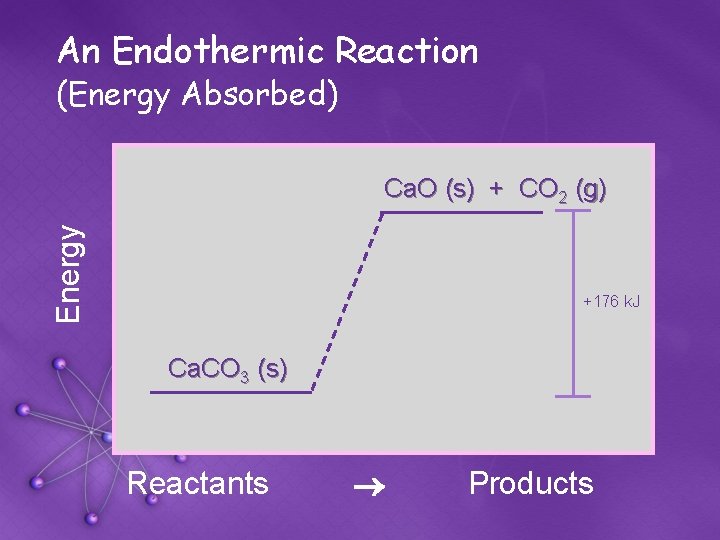
An Endothermic Reaction (Energy Absorbed) Energy Ca. O (s) + CO 2 (g) +176 k. J Ca. CO 3 (s) Reactants ® Products

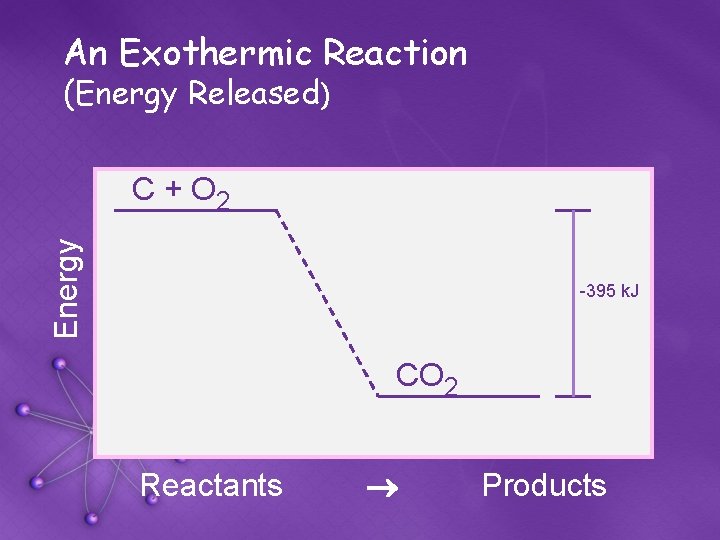
An Exothermic Reaction (Energy Released) Energy C + O 2 -395 k. J CO 2 Reactants ® Products

Energy Activation Energy Minimum energy to make the reaction happen Reactants Products Reaction coordinate

Activated Complex or Transition State Energy Activation Energy Minimum energy to make the reaction happen Reactants PE of the Reactants Reaction coordinate Products

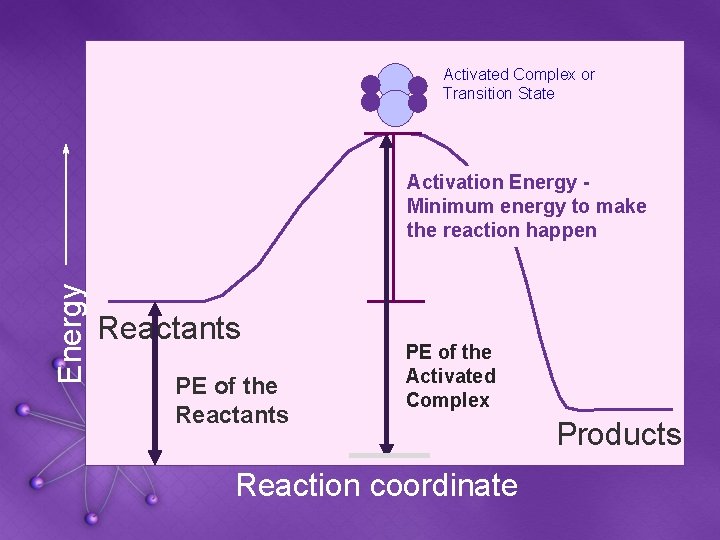
Activated Complex or Transition State Energy Activation Energy Minimum energy to make the reaction happen Reactants PE of the Activated Complex Reaction coordinate Products

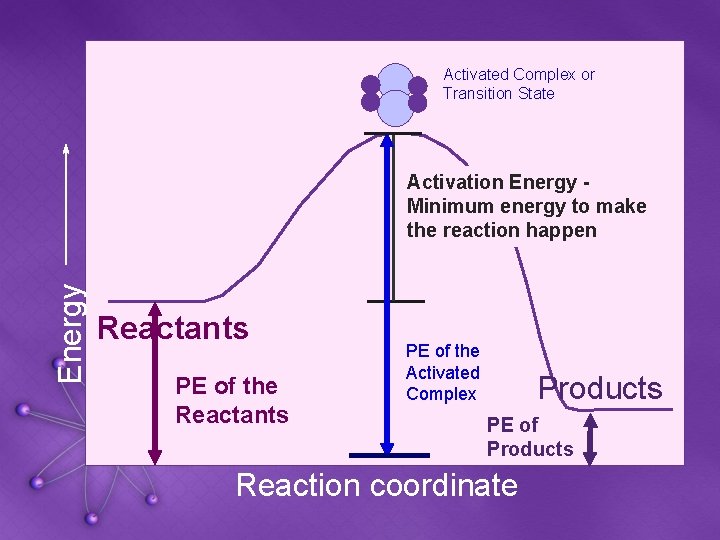
Activated Complex or Transition State Energy Activation Energy Minimum energy to make the reaction happen Reactants PE of the Activated Complex Products PE of Products Reaction coordinate

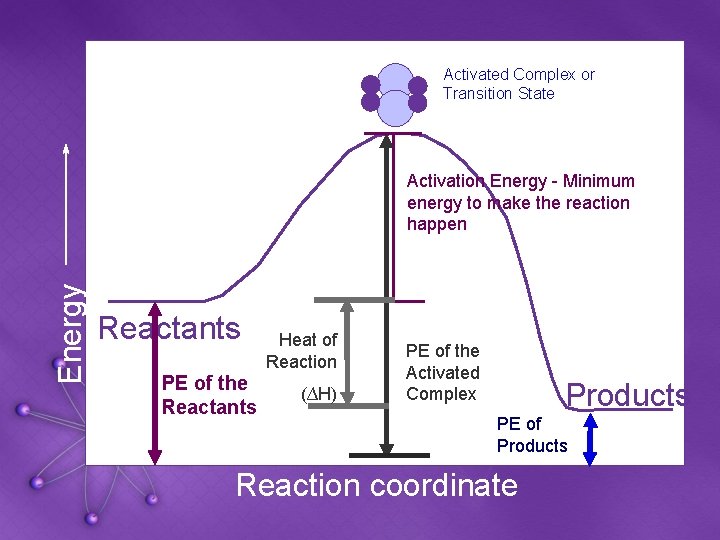
Activated Complex or Transition State Energy Activation Energy - Minimum energy to make the reaction happen Reactants PE of the Reactants Heat of Reaction (∆H) PE of the Activated Complex Products PE of Products Reaction coordinate

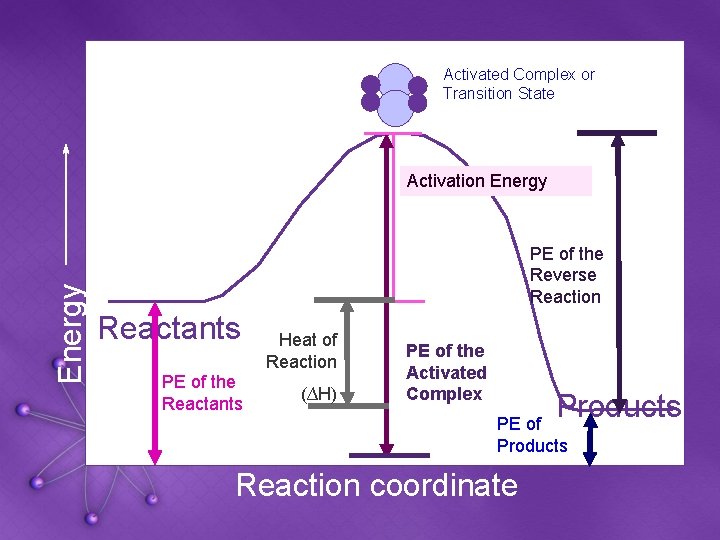
Activated Complex or Transition State Energy Activation Energy PE of the Reverse Reaction Reactants PE of the Reactants Heat of Reaction (∆H) PE of the Activated Complex Products PE of Products Reaction coordinate
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Factors affecting the rate of chemical reaction temperature
Factors affecting the rate of chemical reaction temperature Types of reactions
Types of reactions Elimination reaction conditions
Elimination reaction conditions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Section 17.2 factors affecting chemical equilibrium
Section 17.2 factors affecting chemical equilibrium Spin of hydrogen nucleus is
Spin of hydrogen nucleus is Factors affecting chemical shift in nmr spectroscopy
Factors affecting chemical shift in nmr spectroscopy Factors affecting chemical shift
Factors affecting chemical shift Proton capture equation
Proton capture equation Unit rate
Unit rate Equivalent ratios definition
Equivalent ratios definition Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Redox reactions examples
Redox reactions examples Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Reaction rate
Reaction rate