Chemical Reactions and Change Chemical Reaction Chemical Reaction

















- Slides: 31

Chemical Reactions and Change

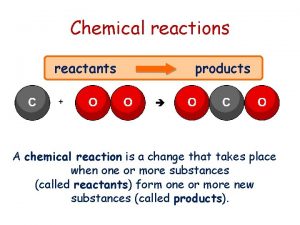
Chemical Reaction • Chemical Reaction – a process in which the physical and chemical properties of the original (beginning) substances change as new substances with different physical and chemical properties are formed. • In every chemical • reaction, A NEW • SUBSTANCE(S) • is formed.

Chemical Reaction In every chemical reaction, there is ALWAYS a change in the properties and a change in the energy of the substances involved. A. Substances present before the reaction called REACTENTS B. Substances produced by the reaction called PRODUCTS

Chemical Reaction EXAMPLES: Energy is absorbed when making homemade caramels Energy is released when anything burns (combustion)

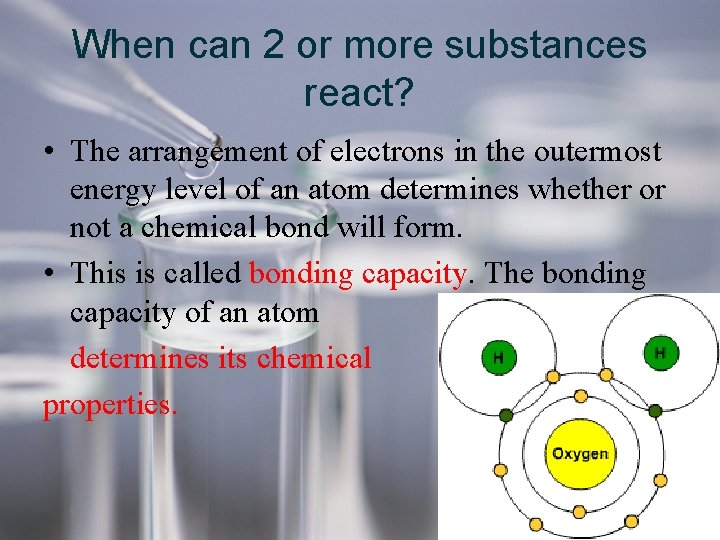
When can 2 or more substances react? • The arrangement of electrons in the outermost energy level of an atom determines whether or not a chemical bond will form. • This is called bonding capacity. The bonding capacity of an atom determines its chemical properties.

During a Chemical Reaction • • 1) Atoms can combine to form molecules 2) Molecules can break apart 3) Molecules can react with other molecules In any case new substances are produced as existing bonds are broken, atoms are rearranged, and new bonds are formed.

Clues that a Chemical Reaction has occurred between 2 substances • • • Bubbling – gas being produced Heat or light given off Substance gets colder Change in color A new odor is produced

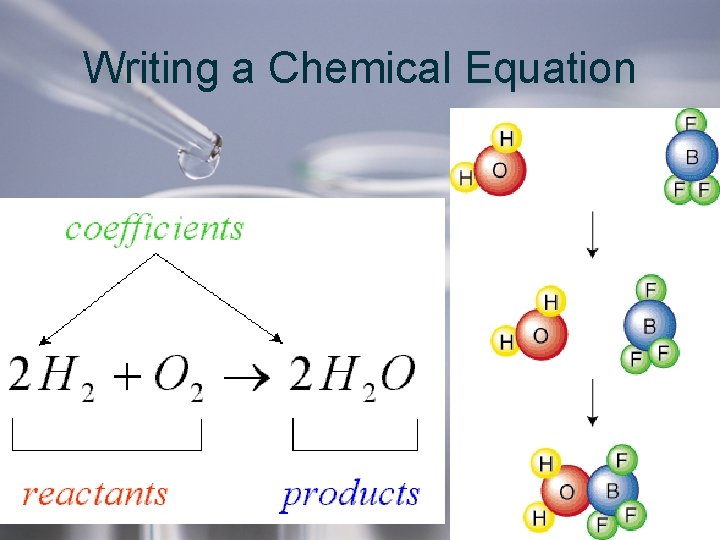
Chemical Equations • A chemical equation is a description of a chemical reaction using symbols to represent elements and formulas.

When writing a Chemical Equation 1. Write correct symbol and formulas for reactants and products 2. Between the reactants and the products us an which means “yields” or “produces” reactants products 3. Separate the symbols or formulas with a “+” sign. The “+” replaces “and”

Writing a Chemical Equation

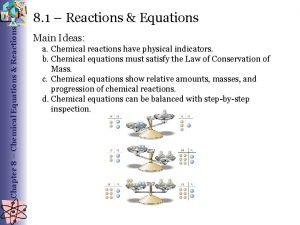
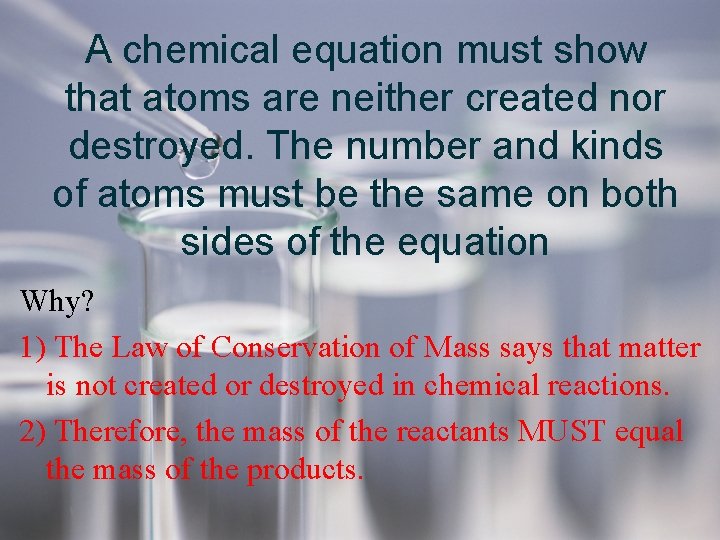
A chemical equation must show that atoms are neither created nor destroyed. The number and kinds of atoms must be the same on both sides of the equation Why? 1) The Law of Conservation of Mass says that matter is not created or destroyed in chemical reactions. 2) Therefore, the mass of the reactants MUST equal the mass of the products.

Writing and Counting Atoms in a Chemical formula • Chemical Symbols – Capitalize the first letter of a chemical symbol. Use lower case for 2 nd or 3 rd Why? Ex: Co = Cobalt, CO = Carbon monoxide • Subscript – a number to the right of a chemical symbol. There is always a subscript for each element in a formula. If you don’t see the subscript, it is an understood 1. Ex: H 2 O

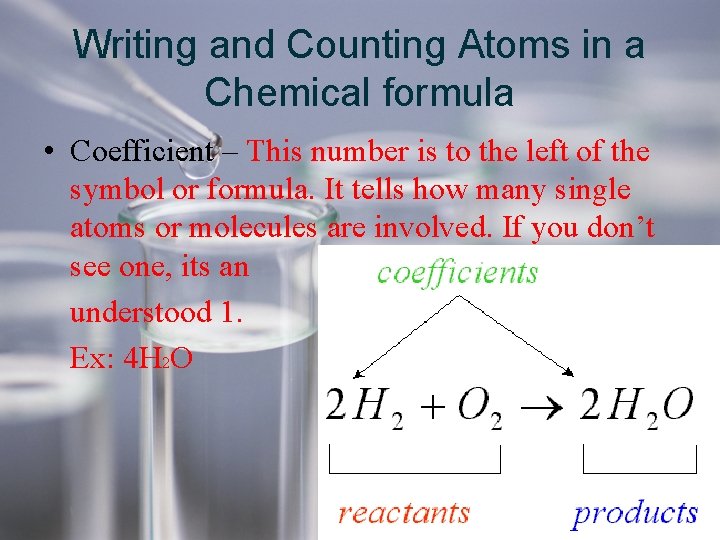
Writing and Counting Atoms in a Chemical formula • Coefficient – This number is to the left of the symbol or formula. It tells how many single atoms or molecules are involved. If you don’t see one, its an understood 1. Ex: 4 H 2 O

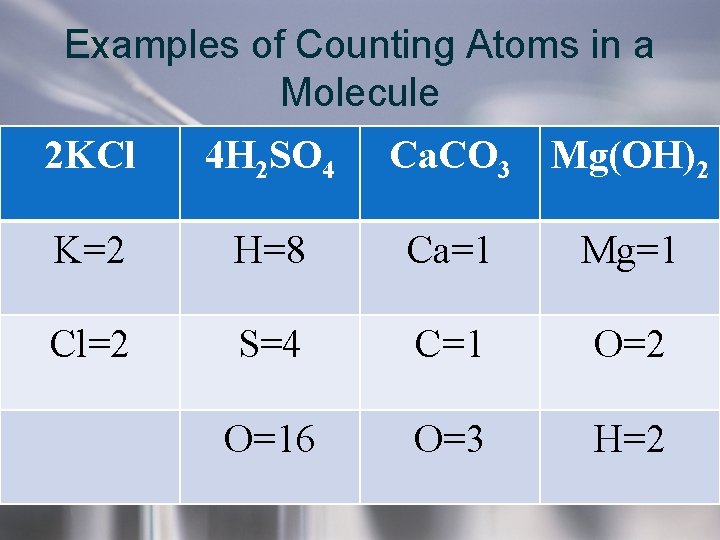
Examples of Counting Atoms in a Molecule • Always - multiply subscript times coefficient to figure out how many of each atom in a chemical formula. If you don’t see a coefficient or subscript, it is an understood 1.

Examples of Counting Atoms in a Molecule 2 KCl 4 H 2 SO 4 Ca. CO 3 Mg(OH)2 K=2 H=8 Ca=1 Mg=1 Cl=2 S=4 C=1 O=2 O=16 O=3 H=2

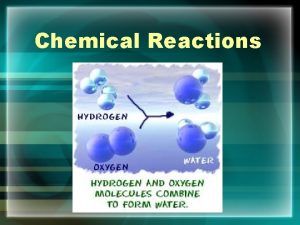
• Balancing Chemical Equations to show that matter is not created or destroyed in a chemical reaction. This must be done when writing chemical equations to show that the Law of Conservation of Mass has been obeyed. Examples: Also the mass of the products must equal the mass of the reactants 2 H 2 + O 2 2 H 2 O H=4, O=2

• Ex. 2 Na N 3 2 Na + 3 Na 500 g 323 g Ex. 2 Na. N 3 2 Na + N 2 500 g 323 g + ? 500 g 177 g -323 g 177 g

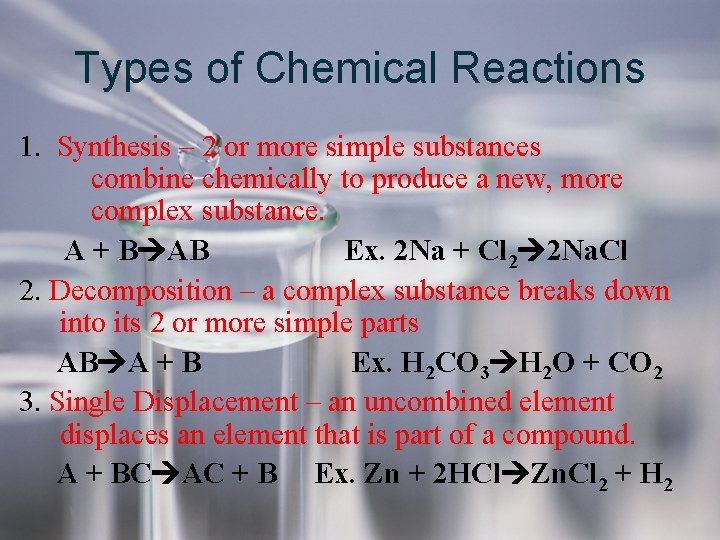
Types of Chemical Reactions 1. Synthesis – 2 or more simple substances combine chemically to produce a new, more complex substance. A + B AB Ex. 2 Na + Cl 2 2 Na. Cl 2. Decomposition – a complex substance breaks down into its 2 or more simple parts AB A + B Ex. H 2 CO 3 H 2 O + CO 2 3. Single Displacement – an uncombined element displaces an element that is part of a compound. A + BC AC + B Ex. Zn + 2 HCl Zn. Cl 2 + H 2

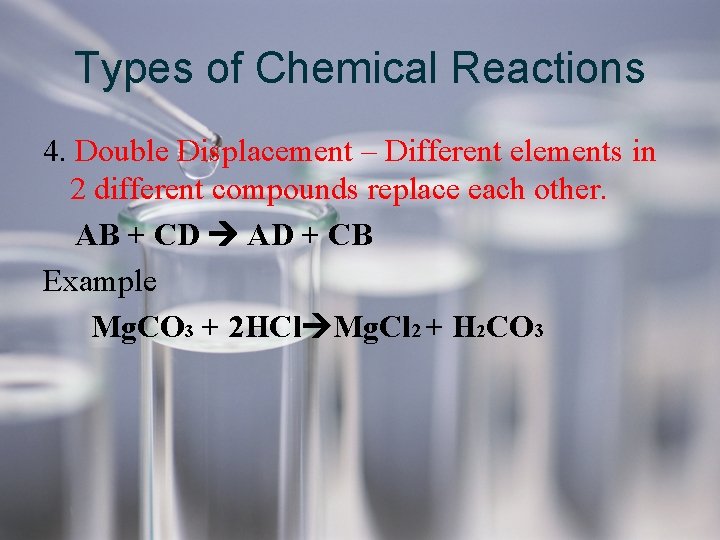
Types of Chemical Reactions 4. Double Displacement – Different elements in 2 different compounds replace each other. AB + CD AD + CB Example Mg. CO 3 + 2 HCl Mg. Cl 2 + H 2 CO 3

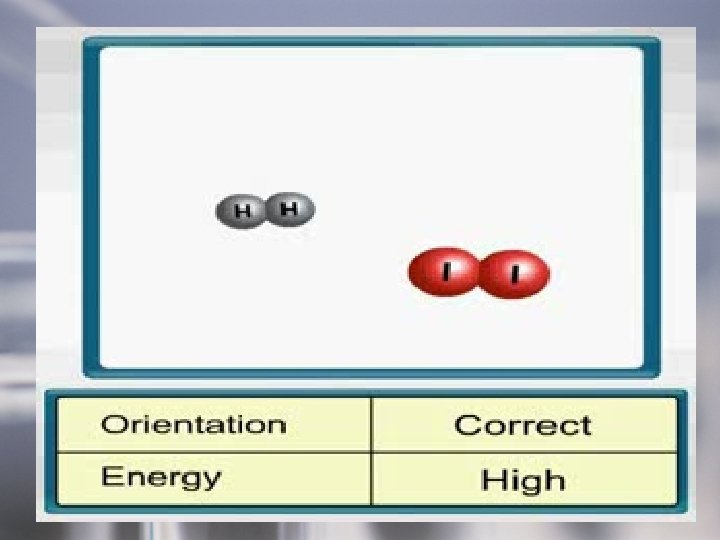
• Rates of Chemical Reactions: Kinetics is the study of reaction rates. • Collision theory – theory that relates molecular collisions to reaction rate. Reactants must come in physical contact with each other for a chemical reaction to occur.



Factors Which Affect Reaction Rate • Factors which affect reaction rate – ways to speed up or slow down reactions 1. Temperature – Often, raising the temperature of the reactants speeds up a chemical reaction. Why? Molecules or atoms move faster so they bump into each other more often. 2. Concentration – The higher the concentration of reactants, the more often they will bump into each other, which speeds up the reaction.

Factors Which Affect Reaction Rate 3. Surface Area – The greater the surface area the more molecules are available to react with each other. Turning a chunk into a powder will up reaction rate. 4. Stirring – stirring physically gets the reactants together quicker which increases reaction rate. 5. Catalyst and Inhibitors – A catalyst speeds up a chemical reaction without being changed itself during the reaction. Inhibitors slow down chemical reactions Ex. BHA & BHT prevent spoilage in foods.

Energy of Chemical Reactions • Energy is changed (but not created or destroyed) in a chemical reaction. Chemical Reactions are classified as exothermic or endothermic

Energy of Chemical Reactions • 1. Exothermic – Energy is released in a chemical reaction as heat and/or light ANY KIND OF BURNING IS EXOTHERMIC. The energy of the reactants energy of the products.

Energy of Chemical Reactions 2. Endothermic – Chemical reaction where energy is absorbed The energy of the products > energy of the reactants Remember – No Matter what kind of chemical reaction occurs, Energy is not created or destroyed only transferred or changed.

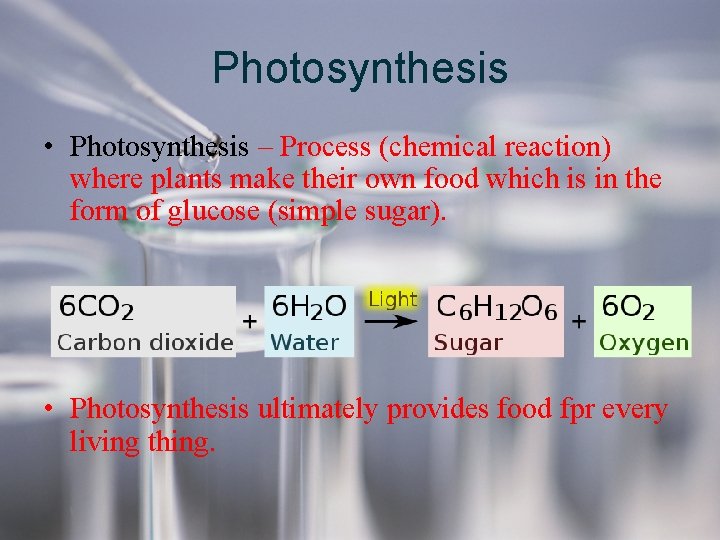
Photosynthesis • Photosynthesis – Process (chemical reaction) where plants make their own food which is in the form of glucose (simple sugar). • Photosynthesis ultimately provides food fpr every living thing.

Food Chain

Cellular Respiration, often called respiration • The process where living things get energy from food. • It is the exact opposite of photosynthesis. 6 O 2 + C 6 H 12 O 6 6 CO 2 + 6 H 2 O + Energy

Rusting • 4 Fe+ 3 O 2 2 Fe 2 O 3
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Balancing redox reactions
Balancing redox reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Example of physical and chemical change
Example of physical and chemical change Differences between chemical and physical changes
Differences between chemical and physical changes What is an example of chemical and physical change
What is an example of chemical and physical change Whats the difference between chemical and physical change
Whats the difference between chemical and physical change Half time equation
Half time equation What is a physical change
What is a physical change Spare change physical versus chemical change
Spare change physical versus chemical change How does a physical change differ from a chemical change
How does a physical change differ from a chemical change Physical or chemical change
Physical or chemical change Chopping wood is a physical or chemical change
Chopping wood is a physical or chemical change How to balance chemical equations step by step
How to balance chemical equations step by step Is the halloween clock reaction a chemical change
Is the halloween clock reaction a chemical change Odor change chemical reaction example
Odor change chemical reaction example Chemical reactions reactants and products
Chemical reactions reactants and products Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical reaction of copper and percent yield
Chemical reaction of copper and percent yield Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes