NMR SPECTROSCOPY Dr Sarish S Assistant Professor PG


- Slides: 26

NMR SPECTROSCOPY Dr. Sarish S Assistant Professor PG Dept. Chemistry N. S. S. College Pandalam N. S. S. COLLEGE–PANDALAM 2017 -2018 Part I

INTRODUCTION Spectroscopy is concerned with interactions between electromagnetic radiations and matter. NMR spectroscopy use NMR phenomenon to study physical, chemical and biological properties of matter NMR phenomenon was discovered in 1945 Research groups at Stanford (Bloch, Hansen and Packard )and Harvard University(Purcell, Torrey and Pound) , in the USA. 2

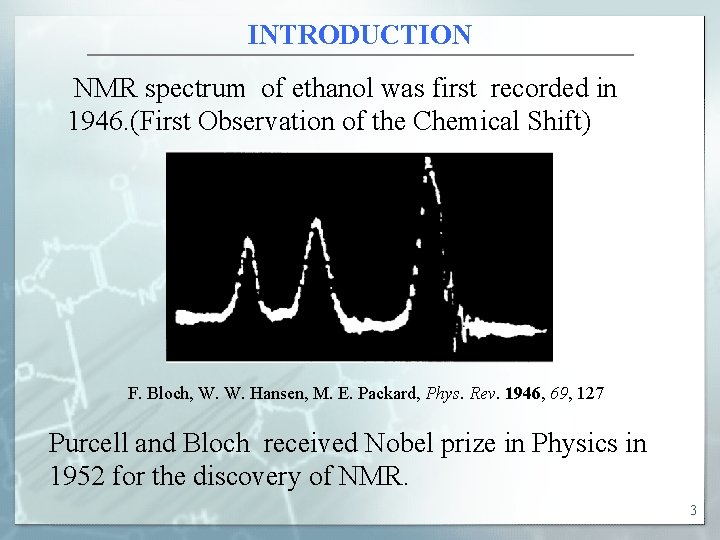
INTRODUCTION NMR spectrum of ethanol was first recorded in 1946. (First Observation of the Chemical Shift) F. Bloch, W. W. Hansen, M. E. Packard, Phys. Rev. 1946, 69, 127 Purcell and Bloch received Nobel prize in Physics in 1952 for the discovery of NMR. 3

INTRODUCTION The utility of NMR spectroscopy for structural elucidation arises because different atoms in a molecule experience slightly different magnetic fields and therefore transitions at different resonance frequencies. In addition , splitting of spectral lines arises due to interactions between different nuclei provides valuable information about the proximity of different atoms in a molecule. 4

INTRODUCTION NMR spectroscopy is considered as one of most powerful for the structural elucidation of organic compounds NMR spectroscopy uses radiofrequency radiations to induce transitions between different nuclear spin states of a molecule, which are created by keeping the sample in a magnetic filed 5

THEORY OF NMR The NMR phenomenon is based on the fact that nuclei of atoms have magnetic properties that can be utilized to yield chemical information. Atomic nucleus has mass and it spins on its own axis. Due to the spin, it possesses angular momentum (P) Nuclear spin is the total nuclear angular momentum quantum number. This is characterized by a quantum number I, which may be integral, half-integral or 0. Only nuclei with spin number I 0 can absorb/emit electromagnetic radiation. The magnetic quantum number m. I has values of –I, -I+1, …. . +I. ( e. g. for I=3/2, m. I=-3/2, -1/2, 3/2 ) 6

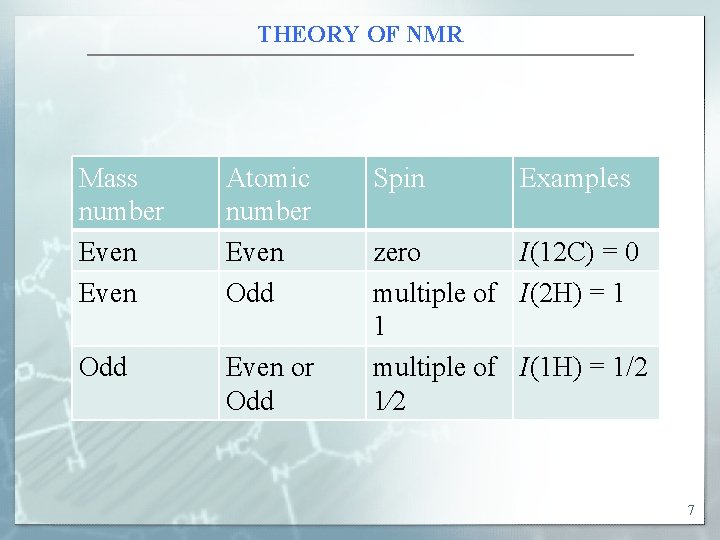
THEORY OF NMR Mass number Even Atomic number Even Odd Even or Odd Spin Examples zero I(12 C) = 0 multiple of I(2 H) = 1 1 multiple of I(1 H) = 1/2 1⁄2 7

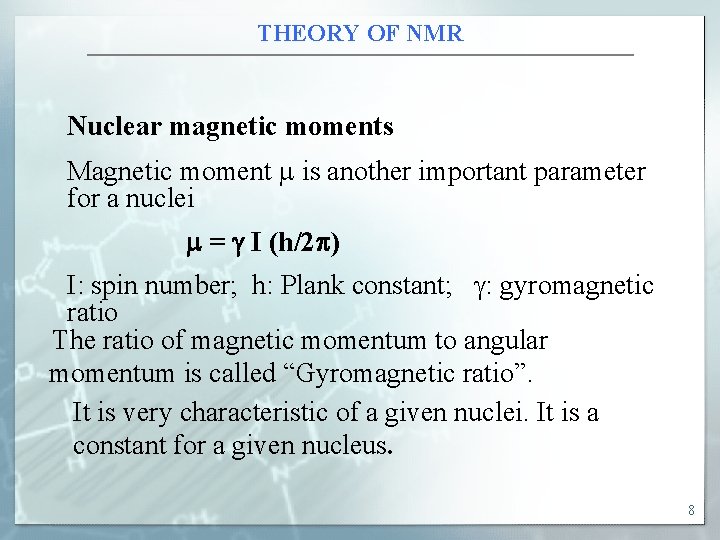
THEORY OF NMR Nuclear magnetic moments Magnetic moment is another important parameter for a nuclei = I (h/2 ) I: spin number; h: Plank constant; : gyromagnetic ratio The ratio of magnetic momentum to angular momentum is called “Gyromagnetic ratio”. It is very characteristic of a given nuclei. It is a constant for a given nucleus. 8

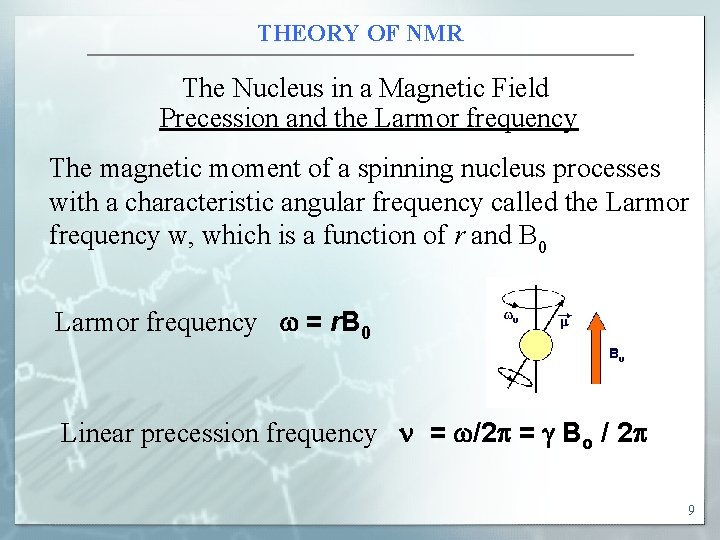
THEORY OF NMR The Nucleus in a Magnetic Field Precession and the Larmor frequency The magnetic moment of a spinning nucleus processes with a characteristic angular frequency called the Larmor frequency w, which is a function of r and B 0 Larmor frequency w = r. B 0 Linear precession frequency n = w/2 = Bo / 2 9

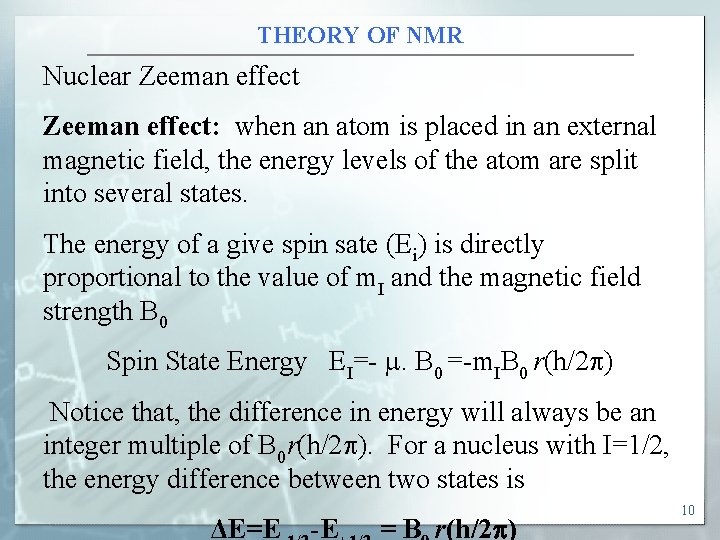
THEORY OF NMR Nuclear Zeeman effect: when an atom is placed in an external magnetic field, the energy levels of the atom are split into several states. The energy of a give spin sate (Ei) is directly proportional to the value of m. I and the magnetic field strength B 0 Spin State Energy EI=- . B 0 =-m. IB 0 r(h/2 p) Notice that, the difference in energy will always be an integer multiple of B 0 r(h/2 p). For a nucleus with I=1/2, the energy difference between two states is ΔE=E -E = B r(h/2 ) 10

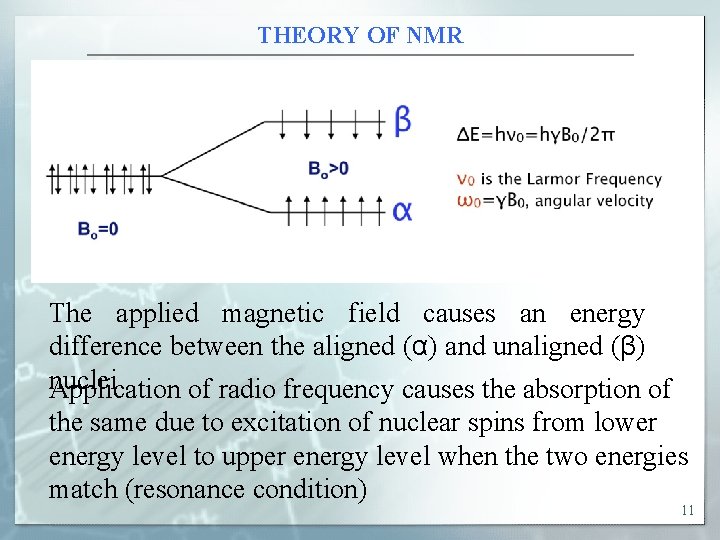
THEORY OF NMR The applied magnetic field causes an energy difference between the aligned (α) and unaligned (β) nuclei Application of radio frequency causes the absorption of the same due to excitation of nuclear spins from lower energy level to upper energy level when the two energies match (resonance condition) 11

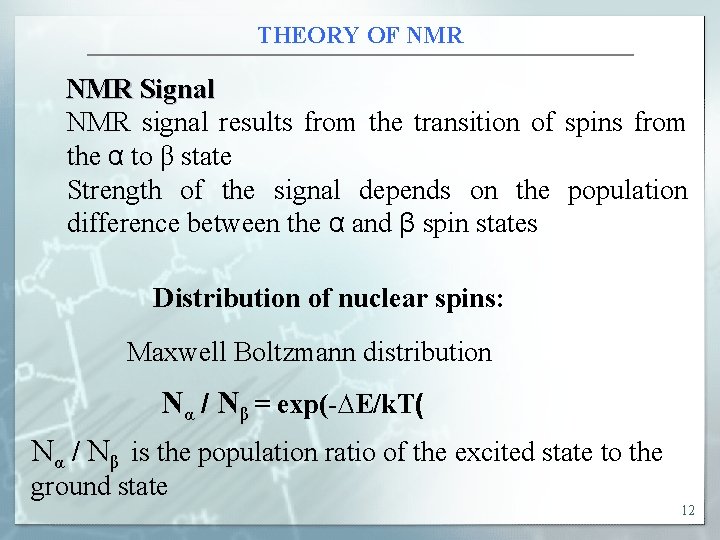
THEORY OF NMR Signal NMR signal results from the transition of spins from the α to β state Strength of the signal depends on the population difference between the α and β spin states Distribution of nuclear spins: Maxwell Boltzmann distribution Nα / Nβ = exp(-∆E/k. T( Nα / Nβ is the population ratio of the excited state to the ground state 12

RELAXATION PROCESS Nuclear spins relax to maintain the population difference. The spins in the excited state return back to ground state by (a) spin lattice relaxation Transfer of energy from the nuclear spin in high energy state to molecular lattice. It is also called longitudinal relaxation Helps to maintain excess population in the ground state And is characterized in terms of time constant T 1 13

RELAXATION PROCESS (b) spin-spin relaxation Mutual exchange of spins by two precessing nucleus I the close proximity of each It is also called transverse relaxation Helps to decrease the half life of the excited state And is characterized in terms of time constant T 2 14

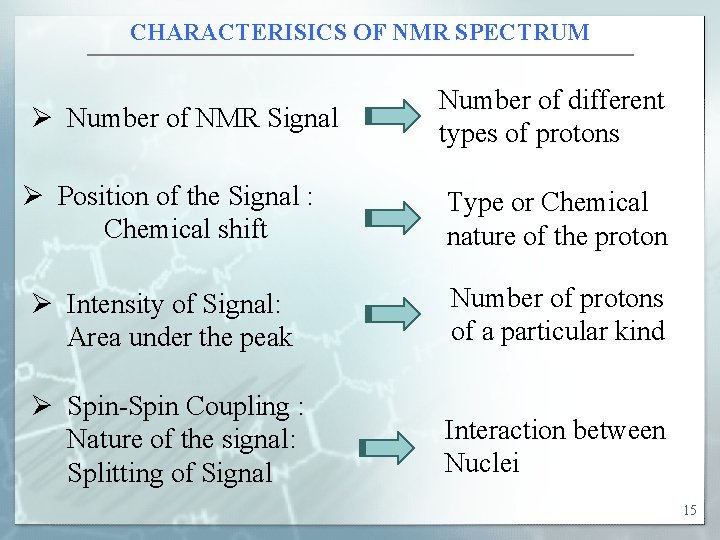
CHARACTERISICS OF NMR SPECTRUM Ø Number of NMR Signal Ø Position of the Signal : Chemical shift Number of different types of protons Type or Chemical nature of the proton Ø Intensity of Signal: Area under the peak Number of protons of a particular kind Ø Spin-Spin Coupling : Nature of the signal: Splitting of Signal Interaction between Nuclei 15

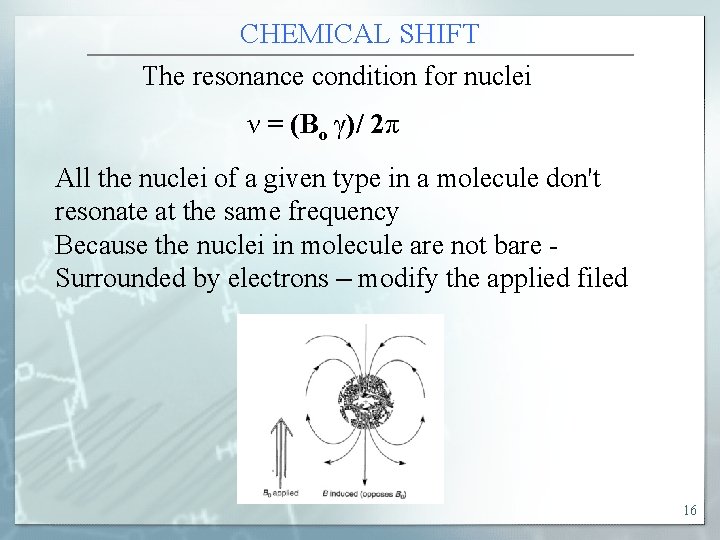
CHEMICAL SHIFT The resonance condition for nuclei ν = (Bo γ)/ 2π All the nuclei of a given type in a molecule don't resonate at the same frequency Because the nuclei in molecule are not bare Surrounded by electrons – modify the applied filed 16

CHEMICAL SHIFT Thus the local magnetic field experienced by nucleus depends on the electronic structure the nucleus of interest. The nuclei are either shielded or deshielded from the applied field. The shift in resonance frequency due to chemical enviroment of the nuclei is chemical shift. Nuclear magnetic field interact with the local magnetic filed 17

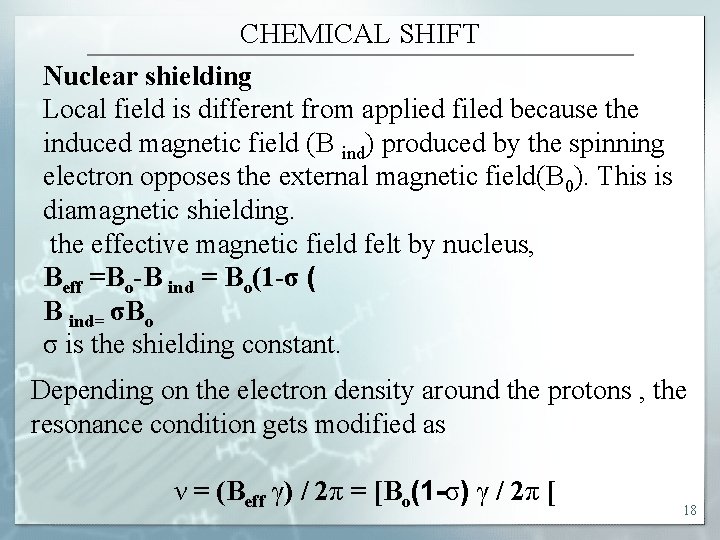
CHEMICAL SHIFT Nuclear shielding Local field is different from applied filed because the induced magnetic field (B ind) produced by the spinning electron opposes the external magnetic field(B 0). This is diamagnetic shielding. the effective magnetic field felt by nucleus, Beff =Bo-B ind = Bo(1 -σ ( B ind= σBo σ is the shielding constant. Depending on the electron density around the protons , the resonance condition gets modified as ν = (Beff γ) / 2π = [Bo(1 -σ) γ / 2π [ 18

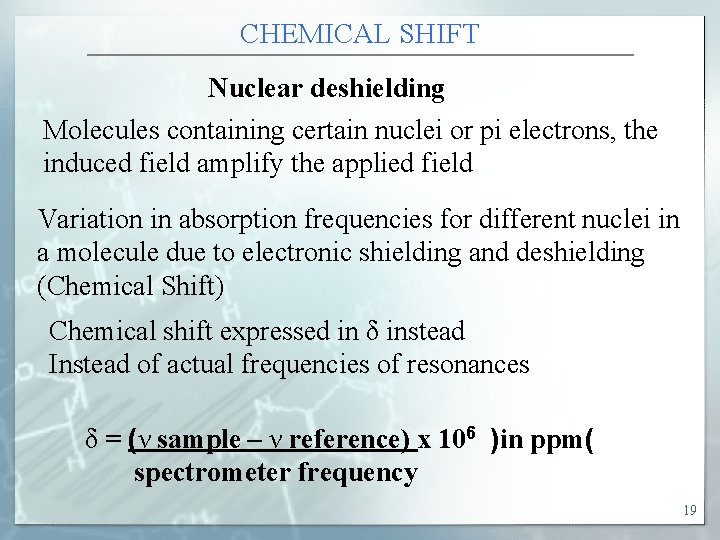
CHEMICAL SHIFT Nuclear deshielding Molecules containing certain nuclei or pi electrons, the induced field amplify the applied field Variation in absorption frequencies for different nuclei in a molecule due to electronic shielding and deshielding (Chemical Shift) Chemical shift expressed in δ instead Instead of actual frequencies of resonances δ = (ν sample – ν reference) x 106 )in ppm( spectrometer frequency 19

REFERENCE COMPOUND FOR 1 H-NMR SPECTROSCOPY Tetramethylsilane (TMS) is used as a reference TMS is a liquid and is soluble in most solvents It is chemically inert All the 12 protons in TMS are equivalent and hence very sharp only one signal Due to large diamagnetic shielding, the chemical shift of TMS is lower than most protons in organic molecules. It is also volatile and hence easy to remove from the sample 20

FACTORS AFFECTING CHEMICAL SHIFT Any factor affecting the electronic environment around the active nuclei would alter its chemical shift Factors ² Electronegativity, inductive and resonance effects ² Hybridization ² Vander Waals deshielding ² Bond anisotropy ² Hydrogen Bonding 1. Electronegativity, inductive and resonance effects CH 4 = 0. 23 CH 3 I - 2. 10 , CH 3 Br - 2. 65, CH 3 Cl -3. 10, CH 3 F 4. 26 With increasing electronegativity, the protons get deshielded 21

FACTORS AFFECTING CHEMICAL SHIFT As the number of EN atoms increases, the protons get deshielded. CH 4 - 0. 23, CH 3 Cl - 3. 10 , CH 2 Cl 2 -5. 33, CHCl 3 -7. 24 2. Hybridization Chemical shifts of protons attached to a carbon atom depends on the hybridization The electronegativity of different hydridised carbon atoms is different SP 3< SP 22

FACTORS AFFECTING CHEMICAL SHIFT 3. Vander Waals deshielding Sterically hindered molecules, The electron cloud of the hindering group will tend to repel the electron cloud of the neighboring protons The proton will be deshielded 4. Bond anisotropy Non-spherical electron density – induced magnetic field will be non-uniform in space – anisotropic effects These effects are paramagnetic in certain directions and diamagnetic in other around the π electron cloud of aromatic ring and unsaturated compounds of the type, C=C and C=O type 23

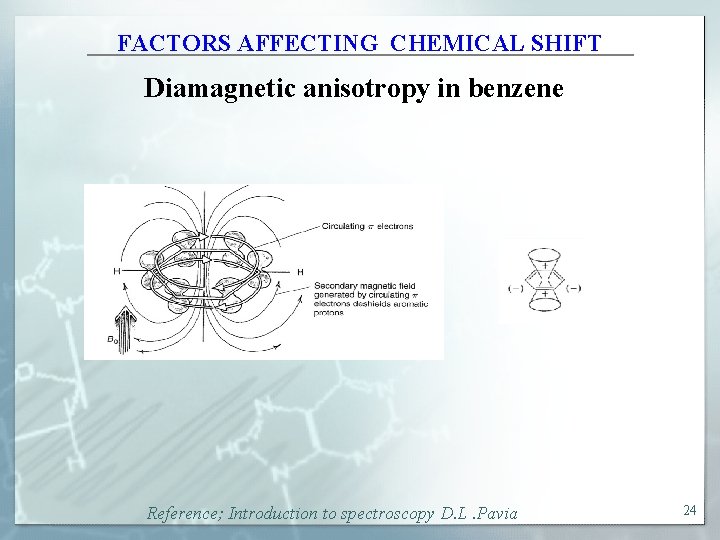
FACTORS AFFECTING CHEMICAL SHIFT Diamagnetic anisotropy in benzene Reference; Introduction to spectroscopy D. L. Pavia 24

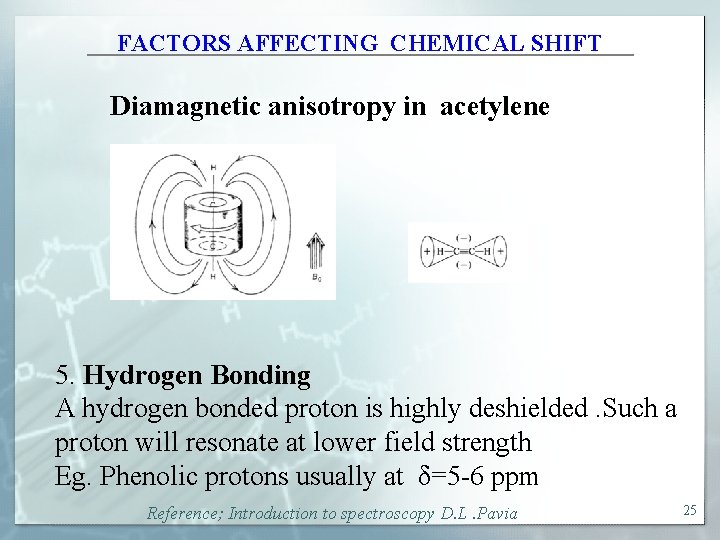
FACTORS AFFECTING CHEMICAL SHIFT Diamagnetic anisotropy in acetylene 5. Hydrogen Bonding A hydrogen bonded proton is highly deshielded. Such a proton will resonate at lower field strength Eg. Phenolic protons usually at δ=5 -6 ppm Reference; Introduction to spectroscopy D. L. Pavia 25

THANK YOU 26