Chemical Reactions Chemical Reactions A Chemical reaction a





- Slides: 11

Chemical Reactions

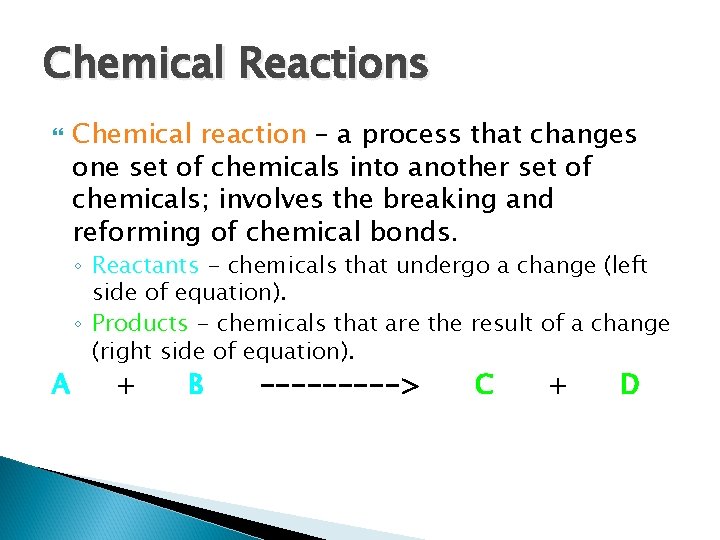
Chemical Reactions A Chemical reaction – a process that changes one set of chemicals into another set of chemicals; involves the breaking and reforming of chemical bonds. ◦ Reactants - chemicals that undergo a change (left side of equation). ◦ Products - chemicals that are the result of a change (right side of equation). + B -----> C + D

Energy in Chemical Reactions Energy is stored within chemical bonds. ◦ When bonds are broken, energy is released. ◦ All living organisms must have a source of energy to carry out chemical reactions! Two types or reactions deal with the energy stored in chemical bonds: Endergonic reactions Exergonic reactions

Endergonic Reactions Endergonic reactions – reactions that absorb energy. ◦ Need a source of energy (usu. electricity) to trigger the reaction (don’t occur spontaneously). ◦ Reactions tend to feel cold. ◦ Ex: electroplating, aluminum metal obtained from its ore ◦ When the energy absorbed is thermal we call it an endothermic reaction.

Exergonic Reactions Exergonic reactions – reactions that release energy. ◦ Energy is released as heat, light, or gas. ◦ Can occur spontaneously. ◦ Often feel warm. ◦ Ex: glowsticks, heat packs ◦ If energy given off is thermal- we call it an exothermic reaction

5 Types of Reactions 1. Combustion ◦ A substance reacts with oxygen to produce energy in the form of heat and light. ◦ Burning ◦ Ex: C + O 2 CO 2

2. Synthesis Reaction ◦ Two or more substances combine to form another substance ◦ Follows this form: A + B AB ◦ Ex: H 2 + O 2 H 2 O Hydrogen burns in oxygen to form water

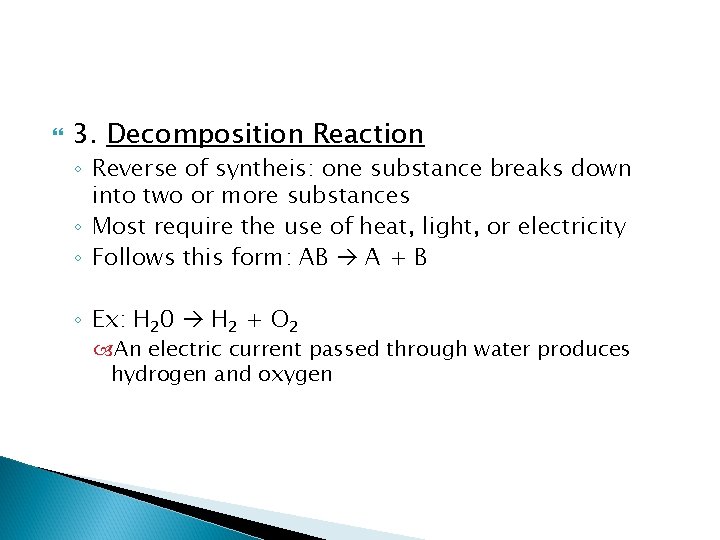
3. Decomposition Reaction ◦ Reverse of syntheis: one substance breaks down into two or more substances ◦ Most require the use of heat, light, or electricity ◦ Follows this form: AB A + B ◦ Ex: H 20 H 2 + O 2 An electric current passed through water produces hydrogen and oxygen

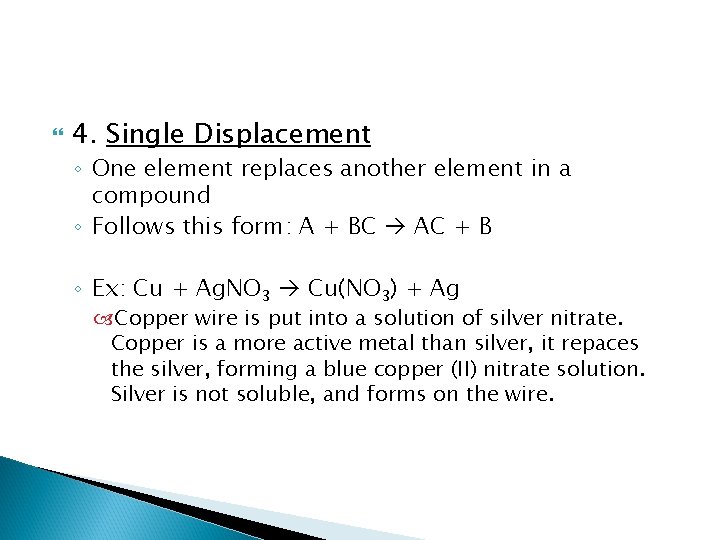
4. Single Displacement ◦ One element replaces another element in a compound ◦ Follows this form: A + BC AC + B ◦ Ex: Cu + Ag. NO 3 Cu(NO 3) + Ag Copper wire is put into a solution of silver nitrate. Copper is a more active metal than silver, it repaces the silver, forming a blue copper (II) nitrate solution. Silver is not soluble, and forms on the wire.

5. Double Displacement ◦ The positive ion of one compound replaces the positive ion of the other to form two new compounds. ◦ Takes place if a precipitate, water, or a gas forms when two ionic compounds in solution are combined. ◦ Precipitate- an insoluble compound that comes out of solution ◦ Follows this form: AB + CD AD + CB ◦ Ex: Ba(NO 3) + K 2 SO 4 Ba. SO 4 + KNO 3 Reaction of barium nitrate with potassium sulfate – a precipitate (barium sulfate) forms.

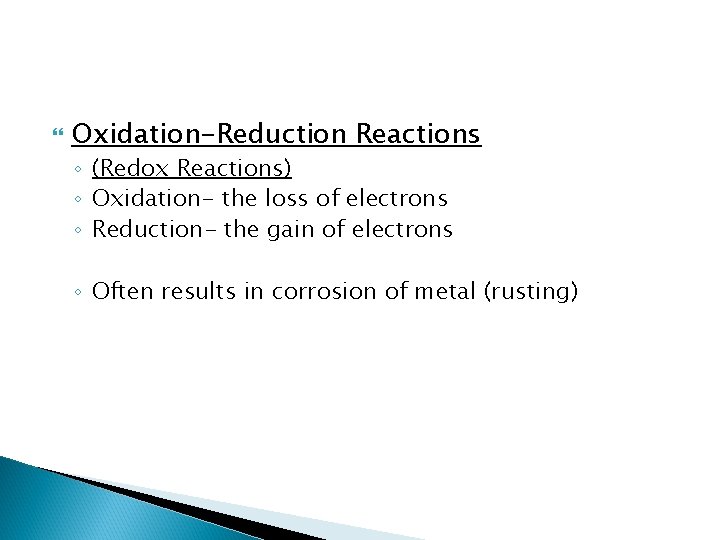
Oxidation-Reduction Reactions ◦ (Redox Reactions) ◦ Oxidation- the loss of electrons ◦ Reduction- the gain of electrons ◦ Often results in corrosion of metal (rusting)
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Proton capture equation
Proton capture equation Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Redox reactions half reactions
Redox reactions half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Equation for rate of reaction
Equation for rate of reaction Ictahedron
Ictahedron Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry