REACTION RATES FACTORS AFFECTING REACTION RATES I can



























































![�Equation: rate = k[A]x[B]y �[A] and [B] are molar concentrations. �K is a rate �Equation: rate = k[A]x[B]y �[A] and [B] are molar concentrations. �K is a rate](https://slidetodoc.com/presentation_image_h/10c07f8e694b11d4cba4202dd272a26a/image-73.jpg)


![�Example: NO 2(g) + CO(g) NO(g) + CO 2(g) Rate = [NO 2]2 �Example: NO 2(g) + CO(g) NO(g) + CO 2(g) Rate = [NO 2]2](https://slidetodoc.com/presentation_image_h/10c07f8e694b11d4cba4202dd272a26a/image-76.jpg)


- Slides: 78

REACTION RATES

FACTORS AFFECTING REACTION RATES

�I can identify and describe the five factors that affect reaction rates.

�There are five general factors that affect the rate of reaction.

NATURE OF REACTANTS �The rate of reaction depends on the particular reactants and the complexity of the bonds that have to be broken and formed. �Reactions where the rearrangement of atoms is simple occur faster. �More complex reactions are much slower.

NATURE OF REACTANTS �Reactions that involve covalent bonds are usually slower because they are more complex. �The states of a reactant in a chemical reaction can also affect reaction rate.

NATURE OF REACTANTS �Reactions that involve gases will be the fastest, followed by those that involve liquids, and reactions that involve solids are the slowest.

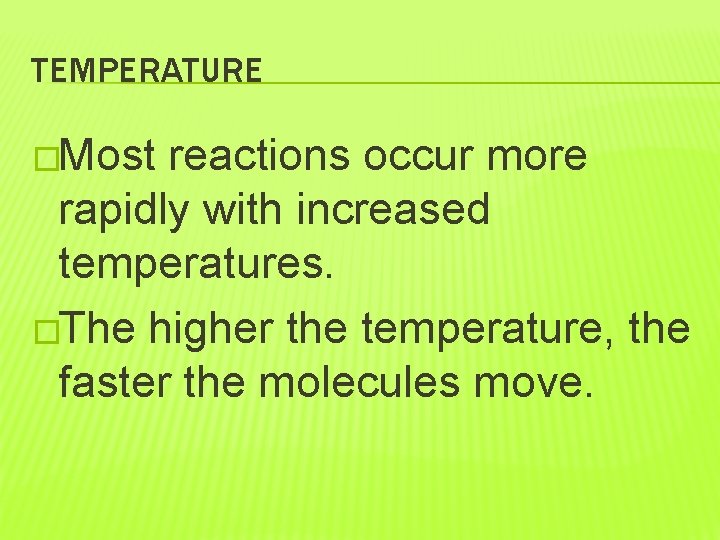
TEMPERATURE �Most reactions occur more rapidly with increased temperatures. �The higher the temperature, the faster the molecules move.

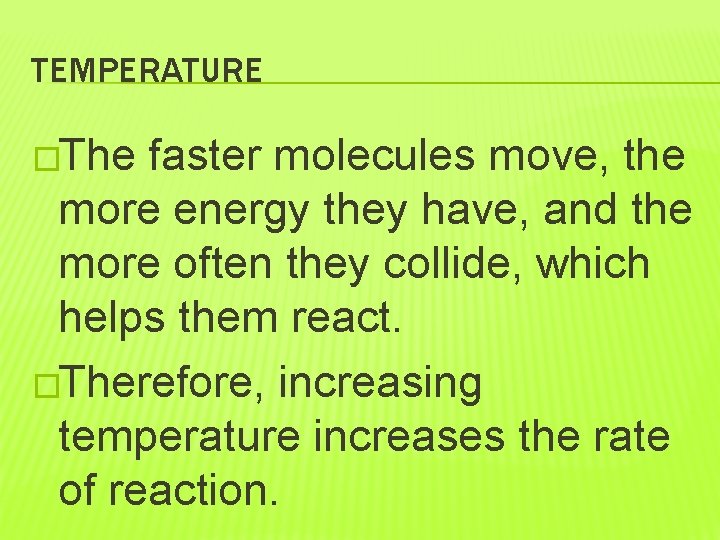
TEMPERATURE �The faster molecules move, the more energy they have, and the more often they collide, which helps them react. �Therefore, increasing temperature increases the rate of reaction.

CONCENTRATION �The rate of reaction depends on concentrations of the reactants. �The higher the concentrations, the faster the reaction rate. �The reason for the increase is due to the fact that higher concentrations result in more collisions.

SURFACE AREA �The more surface area a reactant has, the greater the number of particles that are expose for reaction. �A larger surface area increases the frequency at which particle collide. �To increase the surface area of a solid, scientists use powders.

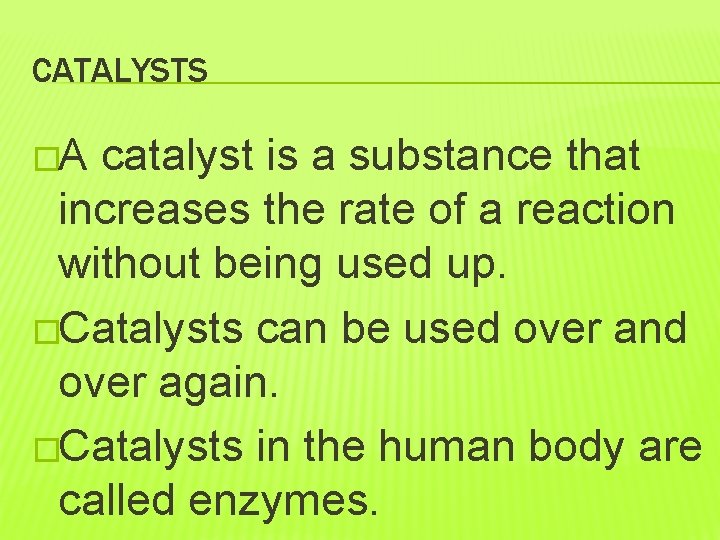
CATALYSTS �A catalyst is a substance that increases the rate of a reaction without being used up. �Catalysts can be used over and over again. �Catalysts in the human body are called enzymes.

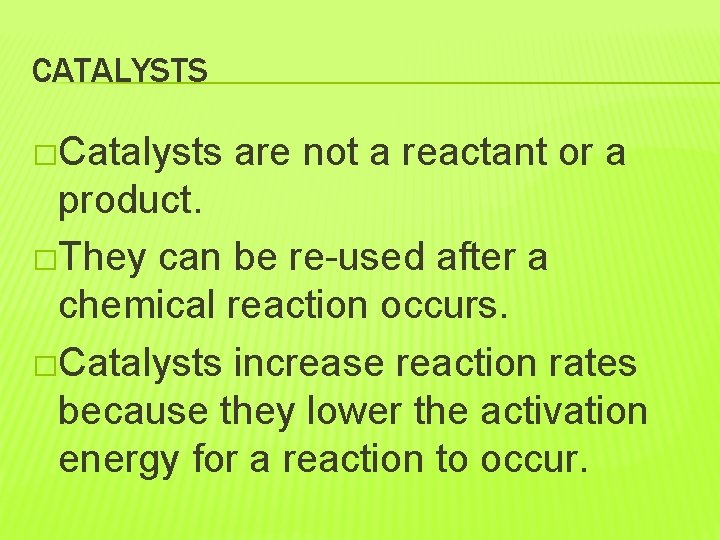
CATALYSTS �Catalysts are not a reactant or a product. �They can be re-used after a chemical reaction occurs. �Catalysts increase reaction rates because they lower the activation energy for a reaction to occur.

CATALYSTS �The lower the activation energy, the faster the reaction rate. �It’s not a good idea to speed up all chemical reactions. �Substances that slow a chemical reaction are inhibitors.

THE COLLISION THEORY

�I can describe the collision theory. � I can define an effective vs. ineffective collision. � I can define both kinetic and potential energy. � I can interpret an energy diagram.

�In order for a chemical reaction to occur, particles must come together. �Combining two substances means forcing their particles to hit or collide. �The collision theory states that molecules must collide to react.

� In order for a molecule, atom, or ion to react, it must collide with another molecule, atom, or ion or with the wall of a container. � If there were no collisions among reactants, there would be no reactions. � Not all collisions are effective in leading to the formation of products.

�Effective collisions lead to the formation of products. �Ineffective collisions do not lead to the formation of products. �If every collision lead to a reaction, the rates of reaction would be much faster.

� For a reaction to occur, particles must be in the correct position that allows the bonds to break and atoms to rearrange. � If the orientation is not right, the molecules bounce off each other. � Colliding particles must also have enough energy so that bonds can be broken and new bonds formed.

ENERGY IN REACTIONS �Energy is required to break bonds that hold reactants together. �There are two types of energy: Kinetic and potential. �Potential energy is stored and kinetic energy is the energy of motion.

�Remember, energy can not be created or destroyed, only transformed. �Particles of matter have both potential and kinetic energy. �The energy to break bonds comes form the energy of the reacting particles.

�During a collision, kinetic energy is converted to potential energy as particles are formed, bonds are broken, and atoms rearranged. �Kinetic energy depends on the mass and velocity of a particle. �Particles must have a minimum amount of energy to react.

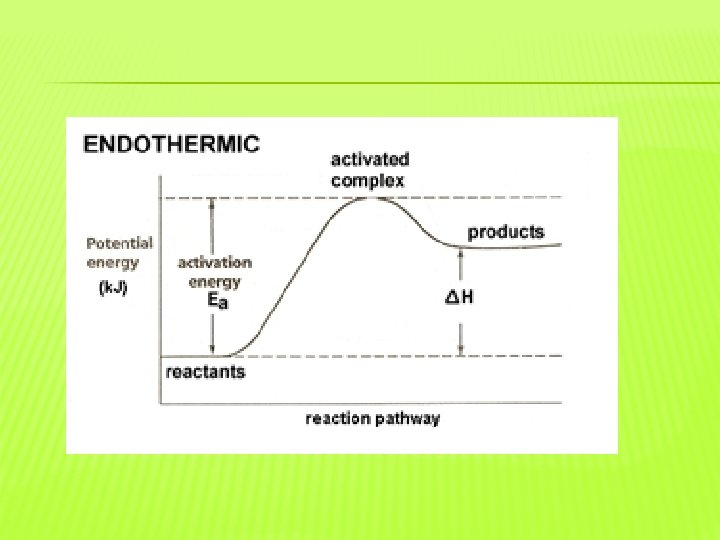
�Activation energy is the energy needed to start a reaction. �Energy diagrams show the changes in energy that occur during a chemical reaction. �The difference between the peak and reactants is the activation energy.



�An exothermic reaction releases heat. �Therefore, the products have less energy than the reactants. �An endothermic reaction absorbs heat. �Therefore the products have more energy than the reactants.

�The activated complex is a structure that forms in a reaction that is not a reactant or a product. �The activated complex exists where the energy is greatest. �Reactions occur at different rates because of energy.

� Particles must collide at the correct orientation to react. � Particles must also have enough energy to overcome the activation energy to react. � Reaction rates are directly related to activation energy because every reaction has a different activation energy.

FREE ENERGY

�I can define free energy. � I can describe a spontaneous and nonspontaneous reaction. � I can define both entropy and enthalpy. � I can describe the conditions under which a spontaneous and nonspontaneous reaction occur.

�Many chemical and physical processes release energy that can be used to bring about other changes. �Free energy is the energy that is available to do work.

�Even though free energy is available, that does not mean it can be used efficiently. �Car engines are only 30% efficient. �That means only 30% of the free energy released from burning gasoline is used to move the car.

�The other 70% is lost to friction and heat. �Efforts are constantly underway to increase efficiency. �However, no mechanical process can be made 100% efficient.

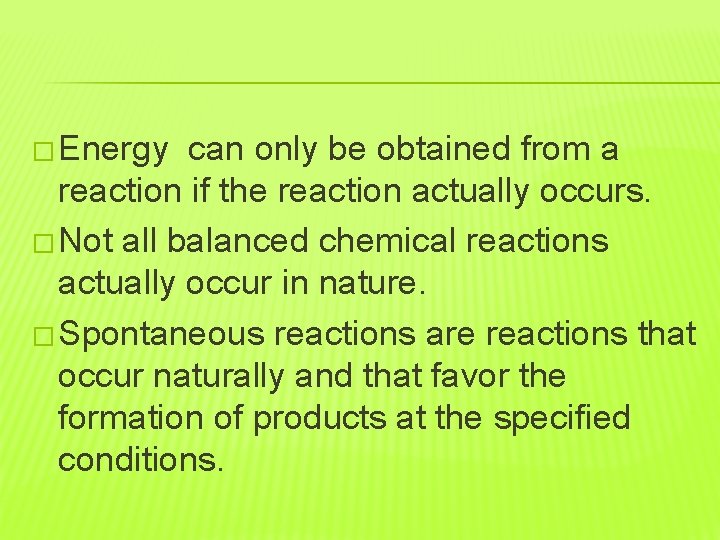
� Energy can only be obtained from a reaction if the reaction actually occurs. � Not all balanced chemical reactions actually occur in nature. � Spontaneous reactions are reactions that occur naturally and that favor the formation of products at the specified conditions.

� All spontaneous reactions release free energy. � Nonspontaneous reactions are reactions that do not favor the formation of products at the specified conditions. � In most reversible reactions, one reaction is spontaneous and one is nonspontaneous.

�The terms spontaneous and nonspontaneous do not refer to how fast reactants go to products. �Some spontaneous reactions are extremely slow, and they look like they are nonspontaneous.

�Some reactions that are nonspontaneous at one set of conditions may be spontaneous at other conditions. �Changing the temperature or pressure can change a reaction to be spontaneous.

�Heat change accompanies most chemical and physical processes. �Both exothermic and endothermic reactions can be spontaneous. �Heat is also called enthalpy. �Enthalpy change is symbolized as H.

�To determine whether or not a reaction is spontaneous, we must consider heat and disorder. �The disorder of a system is called entropy. �The more disorder, the more entropy.

�The law of disorder states that processes move in the direction of maximum disorder or randomness. �The entropy of gasses is higher than the entropy of liquids. �The entropy of liquids is higher than the entropy of solids.

�Entropy increases when solid reactants form liquids. �Entropy also increases when liquid reactants form gases. �Entropy increases when a substance is divided into parts.

�Therefore, entropy increases when a solid is dissolved in water because the solute particles are more separated than the solid. �Entropy tends to increase in chemical reactions when the total number of product molecules is greater than the total number of

�Entropy tends to increase when temperature increases. �As the temperature increases, the molecules move faster and faster, which increases disorder.

�The size and direction of heat changes and entropy changes determine whether a reaction is spontaneous. �In other words, whether it favors products and releases free energy.

� Releasing heat is a favorable condition for a reaction to be spontaneous. � Absorbing heat is an unfavorable condition. � Increasing entropy is a favorable condition for a reaction to be spontaneous. � Decreasing entropy is an unfavorable condition.

�Spontaneous reactions occur under the following conditions: �Heat is released and Entropy increases �The entropy increase is larger than the heat absorbed. �The heat released is larger than the decrease in entropy.

� Reactions will be nonspontaneous under the following conditions: � The heat absorbed is greater than the increase in entropy. � The heat released is less than the decrease in entropy. � Heat is absorbed and entropy decreases.

ENTROPY CALCULATIONS

�Entropy is the measure of disorder of a system. �The symbol for entropy is S. �The units for S is J/K �The standard entropy of a liquid or solid substance at 25 C is designated So.

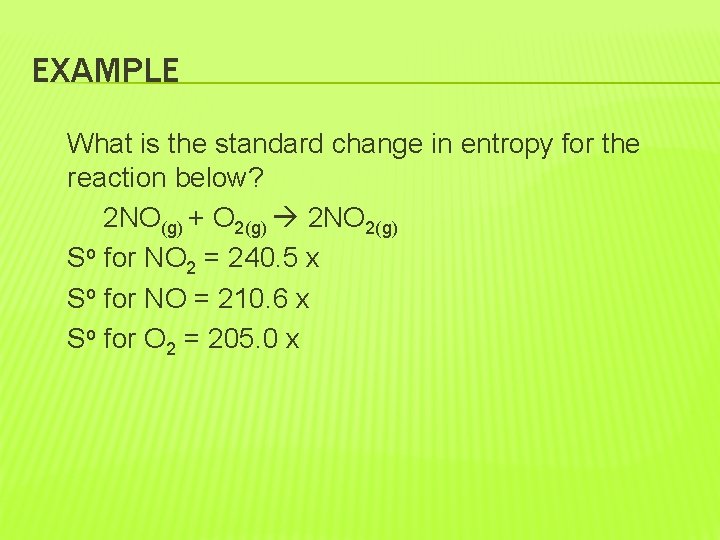
�The pressure at So for gases is 101. 3 k. Pa. �Standard entropy changes can be calculated using the following equation: So = So(products) – So (reactants)

EXAMPLE What is the standard change in entropy for the reaction below? 2 NO(g) + O 2(g) 2 NO 2(g) So for NO 2 = 240. 5 x So for NO = 210. 6 x So for O 2 = 205. 0 x

GIBBS FREE ENERGY

�In every spontaneous process, some energy becomes available to do work. �This energy is called the Gibbs-free energy change ( G) �It is the maximum amount of energy that can be coupled to another process to do useful work.

�The change in Gibbs free energy is related to the change in entropy and the change in enthalpy. �The change in enthalpy is the change in heat.

�The equation for Gibbs Free Energy: G= H–T S H= Change in enthalpy S = Change in entropy T = Temperature in Kelvin

�All spontaneous reactions release free energy. � G for all spontaneous processes is negative. �All nonspontaneous reactions require work to make them happen. �Therefore, the G for nonspontaneous reactions is

�You can calculate G if you know H and S. �However, if H and S are not known, you can still calculate G.

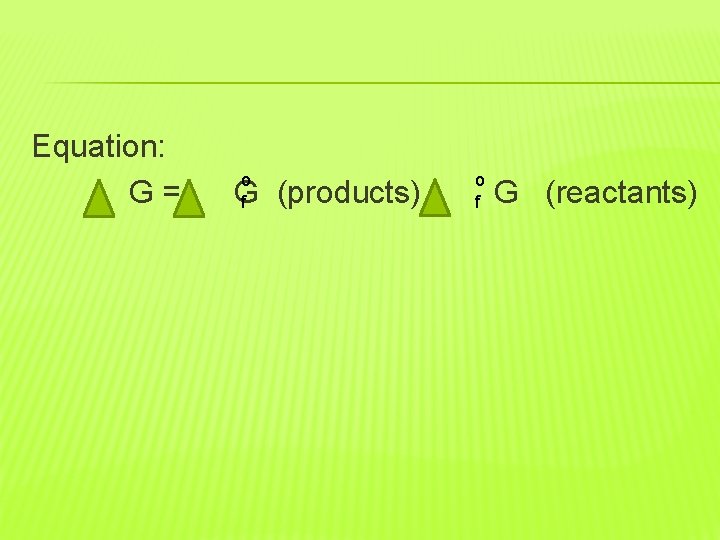
Equation: G= o f G (products) - o f G (reactants)

�Using G to calculate whether a reaction is spontaneous or not only works if the reactants and products are in their standard states. �A reaction that is nonspontaneous under one set of conditions may be spontaneous under another set.

CHEMICAL KINETICS

�I can explain the term rate. � I can define reaction rates. � I can explain a reaction mechanism. � I can use a rate law to determine what would happen to the reaction rate when concentrations are changed.

�The speed at which chemical reactions occur depends on external conditions. �It’s important to know how fast chemical reactions occur and to understand factors that control speed.

�Knowing the speed at which food spoils, metal rusts, and dental fillings set is important. �Chemical kinetics is the chemistry concerned with the speed of reactions. �Rates measure change over time.

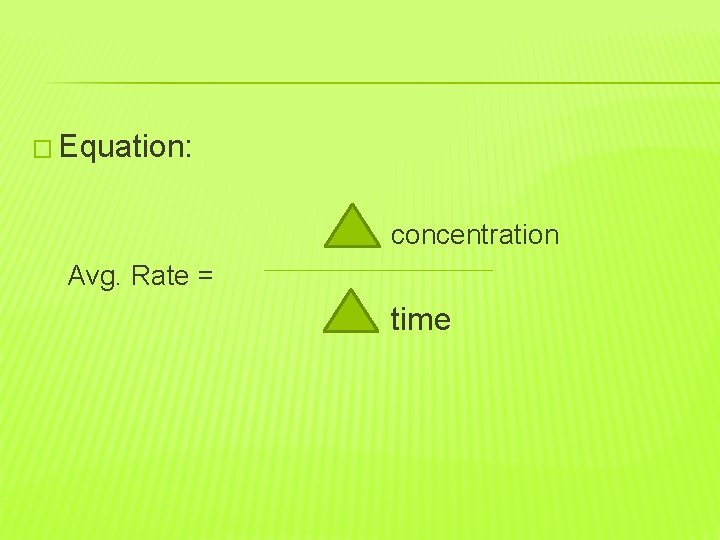
�Chemical changes in a chemical reaction do not occur all at once. �The reaction rate is the rate at which reactants disappear and products appear. �It is the change in concentration of reactants and products in a certain amount of time.

� Equation: concentration Avg. Rate = time

�The Greek letter delta means change in. �Reaction rates change throughout a chemical reaction so it is important to get an average.

REACTION MECHANISMS �During chemical reactions, the rearrangement of atoms is often complicated. �Chemical reactions do not necessarily occur in one step. �Usually chemical reactions occur in several steps made of simple reactions.

�A reaction mechanism is a series of steps that lead from reactants to products. �Detailed reaction mechanisms describe the order in which bonds break and atoms rearrange during a reaction.

�The simple steps in a reaction mechanism are called elementary steps. �The product of the first step becomes the reactant of the second step.

�An intermediate product is a substance that is produces in one step, but used in a later step. �Determining reaction mechanisms is difficult and time consuming and they are determined by experimentation.

RATE LAWS �A rate law is an equation that can be used to calculate the reaction rate for any given concentration of reactants.
![Equation rate kAxBy A and B are molar concentrations K is a rate �Equation: rate = k[A]x[B]y �[A] and [B] are molar concentrations. �K is a rate](https://slidetodoc.com/presentation_image_h/10c07f8e694b11d4cba4202dd272a26a/image-73.jpg)
�Equation: rate = k[A]x[B]y �[A] and [B] are molar concentrations. �K is a rate constant.

�K has a fixed value for a reaction at a particular temperature and is determined by experiment. �X and y are also determined by experiment. �Not all reactants appear in the

�If changing the concentration of a particular reactant does not change the rate, the reactant does not appear in the law.
![Example NO 2g COg NOg CO 2g Rate NO 22 �Example: NO 2(g) + CO(g) NO(g) + CO 2(g) Rate = [NO 2]2](https://slidetodoc.com/presentation_image_h/10c07f8e694b11d4cba4202dd272a26a/image-76.jpg)
�Example: NO 2(g) + CO(g) NO(g) + CO 2(g) Rate = [NO 2]2

�CO is not included in the rate law because it does not change the rate. �What would happen to the reaction rate if the concentration increased 5 times?

�What would happen if the concentration went from 4. 0 M to 3. 0 M? Hint: Take the final concentration and divide by the starting concentration, and then put that number in the rate law.
 What factors influence the rate of a chemical reaction
What factors influence the rate of a chemical reaction Elimination reaction
Elimination reaction Rate of reaction quiz
Rate of reaction quiz Unit rate
Unit rate Equivalent ratios guided notes
Equivalent ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Ocean currents
Ocean currents Factors affecting microbial growth in food
Factors affecting microbial growth in food Factors affecting volcanic eruption
Factors affecting volcanic eruption Factor affecting volcanic eruption
Factor affecting volcanic eruption Simple carburetor
Simple carburetor Factors affecting tga curve pdf
Factors affecting tga curve pdf Factors that affect housing choices
Factors that affect housing choices Factors of bilingualism
Factors of bilingualism Human movement impact factor
Human movement impact factor Ejection fraction vs stroke volume
Ejection fraction vs stroke volume Factors affecting movement in physical education
Factors affecting movement in physical education Baricity definition
Baricity definition Factors affecting soil formation
Factors affecting soil formation Factors affecting health
Factors affecting health Section 17.2 factors affecting chemical equilibrium
Section 17.2 factors affecting chemical equilibrium Factors affecting sample size
Factors affecting sample size Factors affecting the merchandising function
Factors affecting the merchandising function Factors affecting planning
Factors affecting planning Factors affecting evaporation
Factors affecting evaporation Psychological factors affecting sports performance
Psychological factors affecting sports performance Psychological factors affecting sports performance
Psychological factors affecting sports performance What are the different routes of drug administration
What are the different routes of drug administration Cardiac output
Cardiac output Factors affecting transportation decisions
Factors affecting transportation decisions Factors affecting span of control
Factors affecting span of control Incisal guidance in complete denture
Incisal guidance in complete denture Factors affecting bacterial growth ppt
Factors affecting bacterial growth ppt Contingency factors affecting structural choice
Contingency factors affecting structural choice Us geological survey floating pan
Us geological survey floating pan Factors affecting milling process
Factors affecting milling process Lush
Lush Factors affecting enzyme activity bbc bitesize
Factors affecting enzyme activity bbc bitesize Explain the factors affecting gfr
Explain the factors affecting gfr Optimum weight gcse pe
Optimum weight gcse pe Factors affecting fermentation
Factors affecting fermentation Factors affecting fermentation
Factors affecting fermentation Powder flowing angle
Powder flowing angle Factors affecting interior design
Factors affecting interior design Factors affecting interior design
Factors affecting interior design Factors affecting communication skills
Factors affecting communication skills Local factors of wound healing
Local factors of wound healing A factor that affects the flight of a projectile?
A factor that affects the flight of a projectile? Factors affecting population explosion
Factors affecting population explosion Factors affecting organizational design
Factors affecting organizational design Factors affecting option prices
Factors affecting option prices What factors affect motion
What factors affect motion Intrinsic factors affecting the growth of microorganisms
Intrinsic factors affecting the growth of microorganisms Factors affecting interpersonal skills
Factors affecting interpersonal skills Social factors affecting human resource management
Social factors affecting human resource management Factors that affect equilibrium
Factors that affect equilibrium What are the factors affecting bulk density
What are the factors affecting bulk density Factors affecting absorption of drug
Factors affecting absorption of drug Objective of plant layout
Objective of plant layout Factors affecting esp course design
Factors affecting esp course design Factors affecting entrepreneurship growth
Factors affecting entrepreneurship growth Different social factors
Different social factors Dr burs
Dr burs Crystallinity
Crystallinity Factor affecting consensus
Factor affecting consensus Function of management
Function of management Factors affecting strategic choice
Factors affecting strategic choice Hit another home run strategy
Hit another home run strategy Factors affecting oxygen administration
Factors affecting oxygen administration Factors affecting fermentation
Factors affecting fermentation Factors affecting inflation
Factors affecting inflation First step of decision making
First step of decision making What are the three factors that affect solvation
What are the three factors that affect solvation Factors affecting cardiac output
Factors affecting cardiac output Intrinsic conduction system
Intrinsic conduction system Site selection for bridge
Site selection for bridge Factors affecting absorption of drug
Factors affecting absorption of drug Factors affecting sleep
Factors affecting sleep