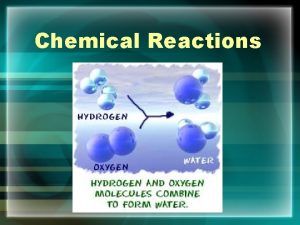
CHEMICAL REACTIONS CHEMICAL REACTIONS Chemical Reaction A process

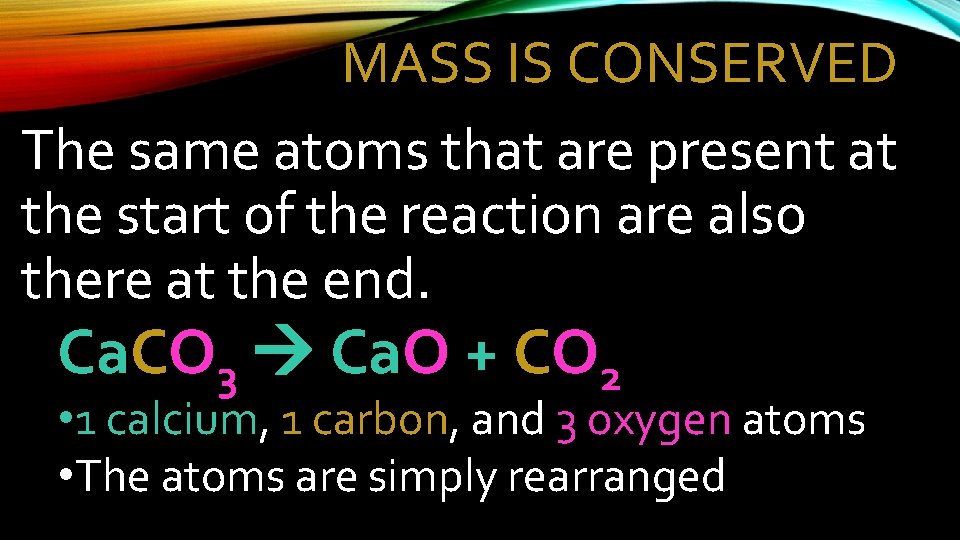




- Slides: 14

CHEMICAL REACTIONS

CHEMICAL REACTIONS Chemical Reaction: A process that transforms one set of chemical substances into another • When atoms break existing chemical bonds and form new ones • Evidence of a chemical reaction: • Change in properties (gas or precipitate, color) • Change in energy (temperature or light emission)

CHEMICAL REACTIONS Reactants are the chemicals present BEFORE the reaction Products are the chemicals present AFTER the reaction

CHEMICAL EQUATIONS Chemical Equations symbolize what happens during a reaction • like a math equation…with instead of = • Reactants Products • Reactants and products are shown as chemical formulas • Ex: Ca. CO 3 Ca. O + CO 2 • When limestone is heated, it changes into calcium oxide and carbon dioxide • Notice that the elements are the same before and after the reaction.
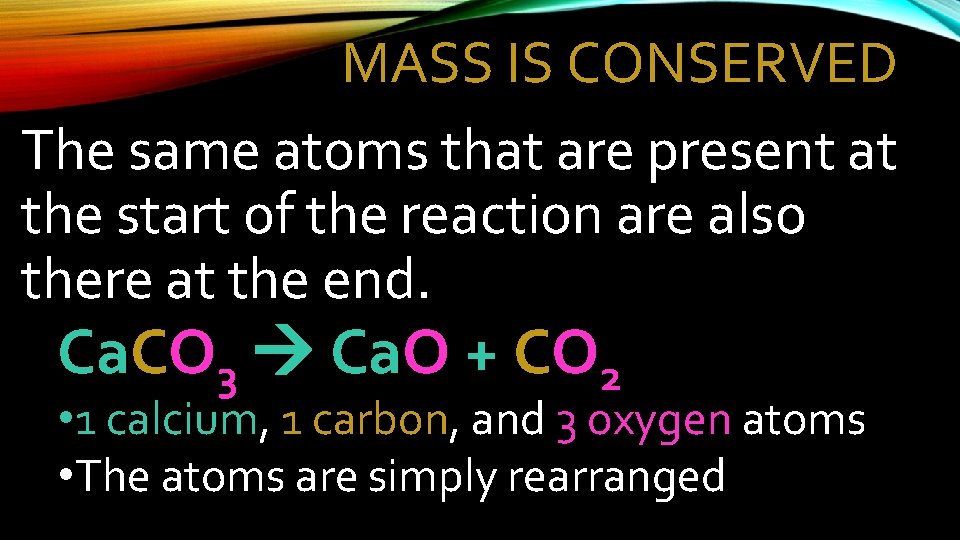
MASS IS CONSERVED The same atoms that are present at the start of the reaction are also there at the end. Ca. CO 3 Ca. O + CO 2 • 1 calcium, 1 carbon, and 3 oxygen atoms • The atoms are simply rearranged

MASS IS CONSERVED Law of Conservation of Mass: The mass of the products will equal the mass of the reactants • Matter is neither created nor destroyed. • The atoms present in the reactants are the same atoms that form the products…they are just rearranged.

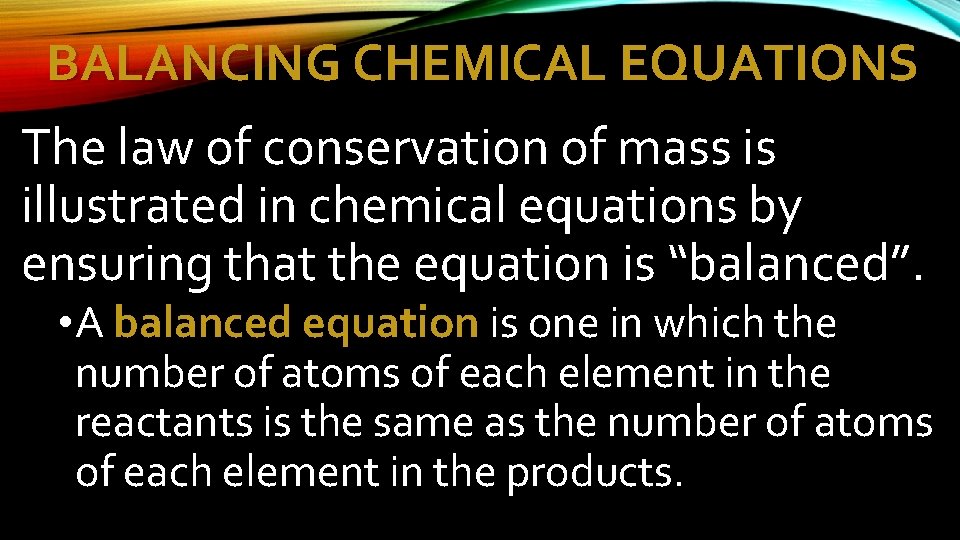
BALANCING CHEMICAL EQUATIONS The law of conservation of mass is illustrated in chemical equations by ensuring that the equation is “balanced”. • A balanced equation is one in which the number of atoms of each element in the reactants is the same as the number of atoms of each element in the products.

BALANCED EQUATIONS Ex) N 2 O 5 + H 2 O 2 HNO 3 • Subscripts belong only to the element preceding them. • N 2 O 5 = 2 N + 5 O • Coefficients are distributed to each element in the compound. (multiply by subscripts) • 2 HNO 3 = 2(HNO 3) = 2 H + 2 N + 6 O

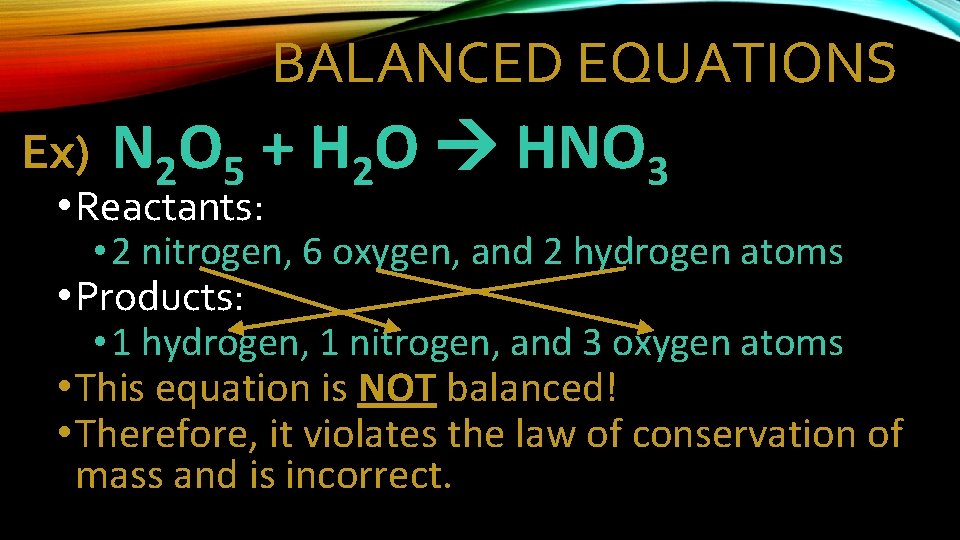
BALANCED EQUATIONS Ex) N 2 O 5 + H 2 O HNO 3 • Reactants: • 2 nitrogen, 6 oxygen, and 2 hydrogen atoms • Products: • 1 hydrogen, 1 nitrogen, and 3 oxygen atoms • This equation is NOT balanced! • Therefore, it violates the law of conservation of mass and is incorrect.

BALANCED EQUATIONS Ex) N 2 O 5 + H 2 O 2 HNO 3 • Reactants: • 2 nitrogen, 6 oxygen, and 2 hydrogen atoms • Products: • 2 hydrogen, 2 nitrogen, and 6 oxygen atoms • This equation is balanced! • It shows that matter is neither created nor destroyed.

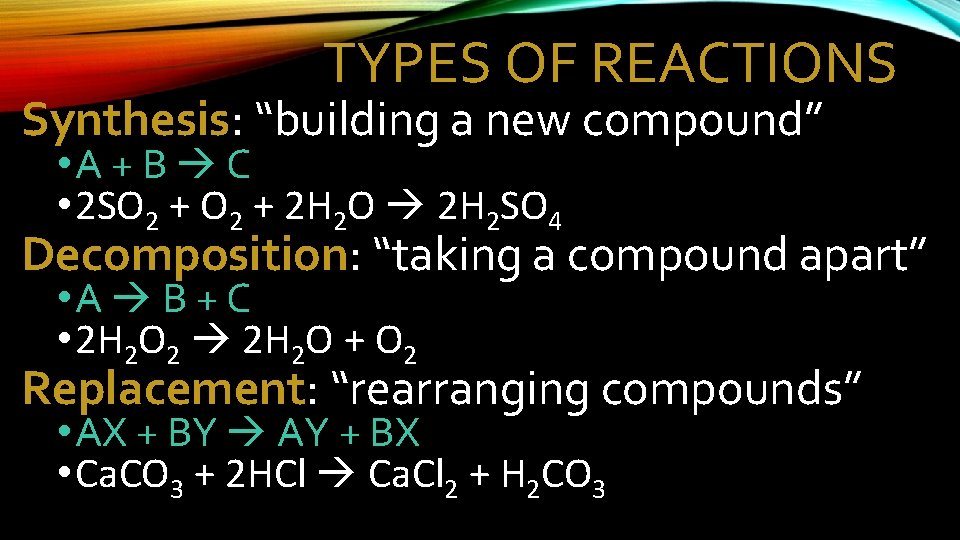
TYPES OF REACTIONS Synthesis: “building a new compound” • A + B C • 2 SO 2 + 2 H 2 O 2 H 2 SO 4 Decomposition: “taking a compound apart” • A B + C • 2 H 2 O 2 2 H 2 O + O 2 Replacement: “rearranging compounds” • AX + BY AY + BX • Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 CO 3

ENERGY Activation Energy: the energy required to begin a reaction • ex) some reactions require heat to begin Exothermic Reactions: give off energy (they feel hot) • ex) calcium chloride and water give off heat Endothermic Reactions: absorb energy (they feel cold) • ex) water and ammonium nitrate absorb heat

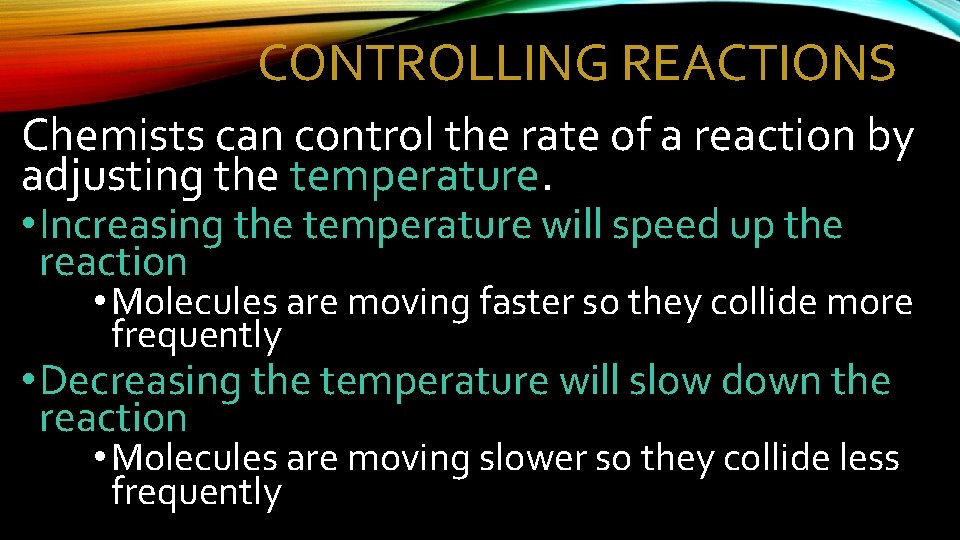
CONTROLLING REACTIONS Chemists can control the rate of a reaction by adjusting the temperature. • Increasing the temperature will speed up the reaction • Molecules are moving faster so they collide more frequently • Decreasing the temperature will slow down the reaction • Molecules are moving slower so they collide less frequently

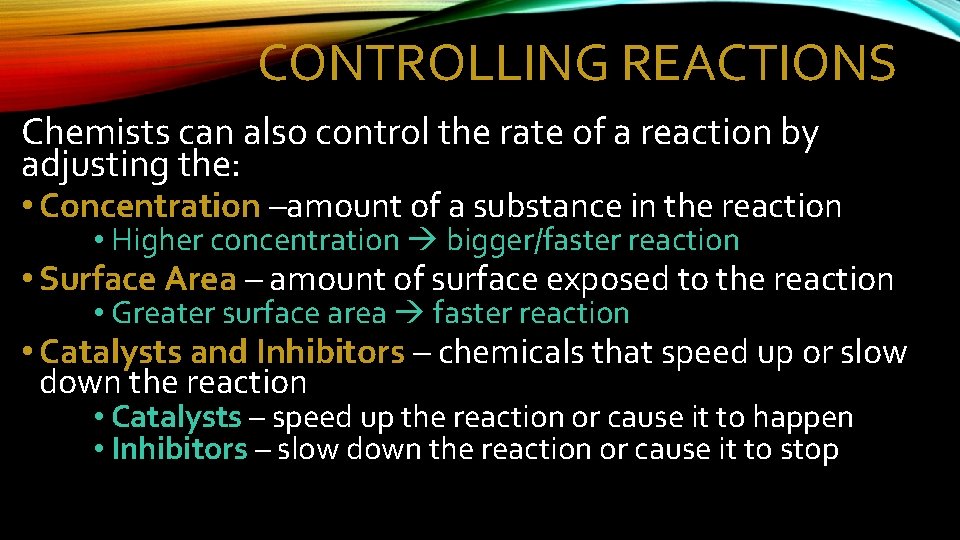
CONTROLLING REACTIONS Chemists can also control the rate of a reaction by adjusting the: • Concentration –amount of a substance in the reaction • Higher concentration bigger/faster reaction • Surface Area – amount of surface exposed to the reaction • Greater surface area faster reaction • Catalysts and Inhibitors – chemicals that speed up or slow down the reaction • Catalysts – speed up the reaction or cause it to happen • Inhibitors – slow down the reaction or cause it to stop
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Half-life formula
Half-life formula Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Examples of redox reaction
Examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Order of reaction
Order of reaction Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Proportional relationships in chemical reactions
Proportional relationships in chemical reactions I intro
I intro Types of redox reactions
Types of redox reactions Types of reactions
Types of reactions