Reaction rates Reaction rates Some reactions go faster





- Slides: 16
Reaction rates

Reaction rates Some reactions go faster than others. We measure reactant rates by looking at how quickly reactants are forming or how quickly products are being used up. Reaction rates can be understood when you understand collision theory

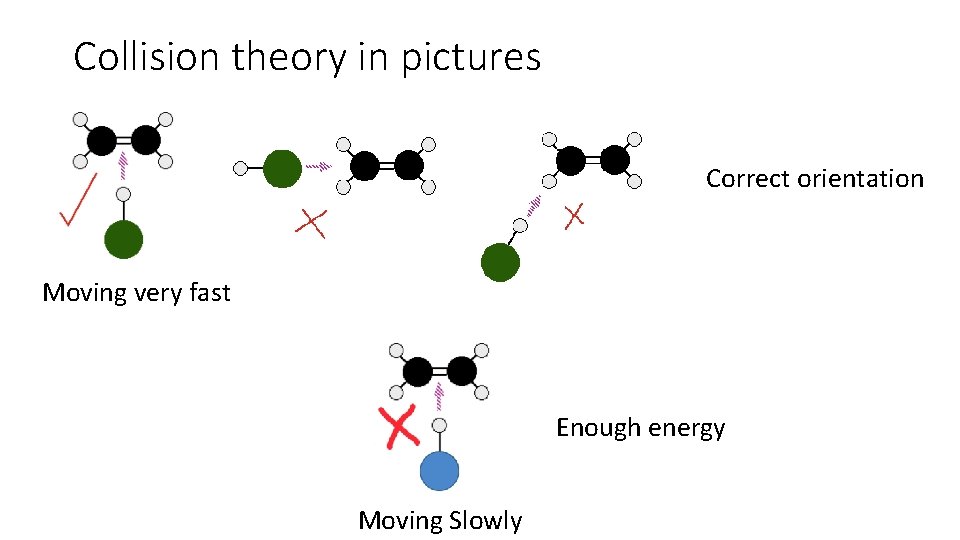
Collision theory and reaction rates 1. For a reaction to occur particles must collide 2. When the particles collide they must have sufficient energy to break bonds and form new ones. 3. The particles must collide with the correct orientation for bond breaking and making to occur.

Collision theory in pictures Correct orientation Moving very fast Enough energy Moving Slowly

Booklet questions • Now read answer the questions page 67 – 68 of booklet • If you finish this read energy profile diagrams on page 71.

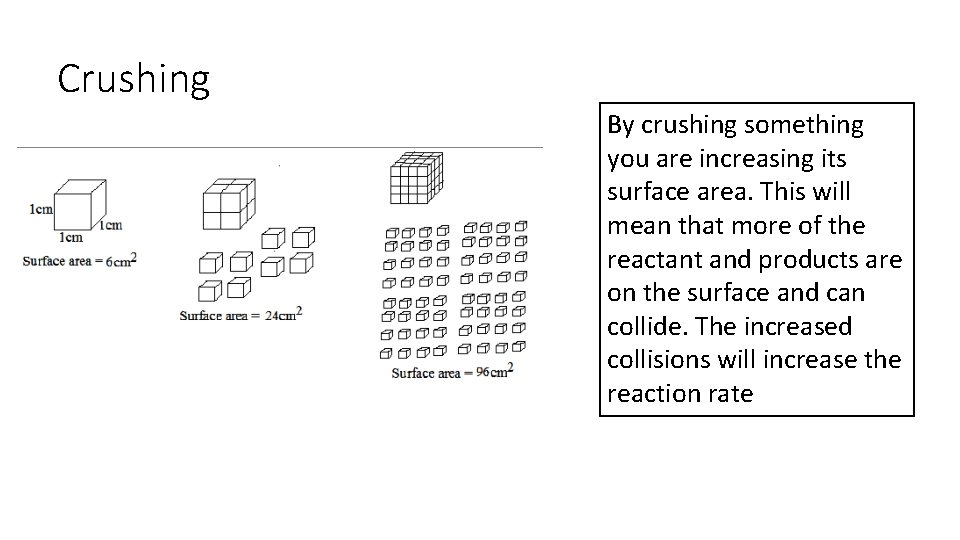
Crushing By crushing something you are increasing its surface area. This will mean that more of the reactant and products are on the surface and can collide. The increased collisions will increase the reaction rate

Stirring By stirring reactants you are increasing their kinetic energy and therefore increasing the chances that a collision will have enough energy to cause a reaction. You are also increasing the number of collisions as you are making the particles move more quickly.

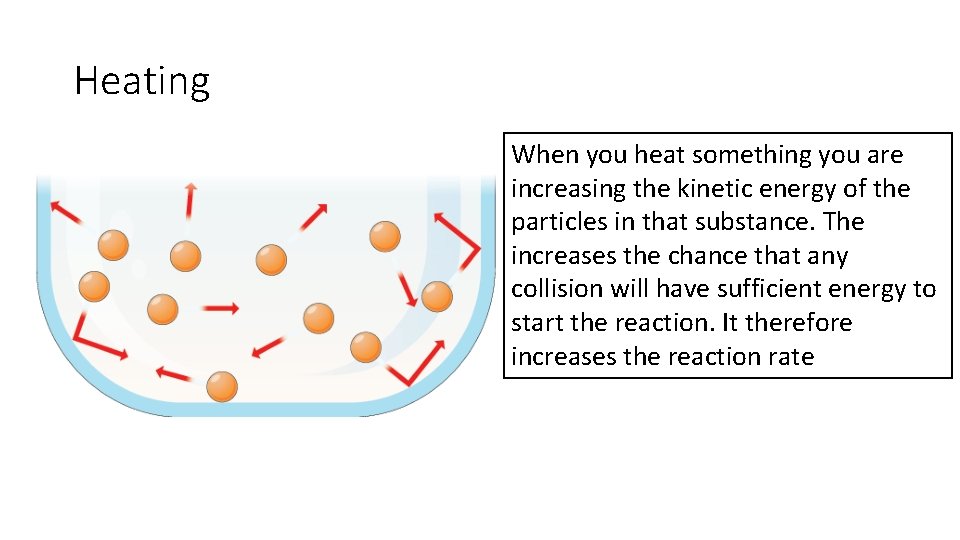
Heating When you heat something you are increasing the kinetic energy of the particles in that substance. The increases the chance that any collision will have sufficient energy to start the reaction. It therefore increases the reaction rate

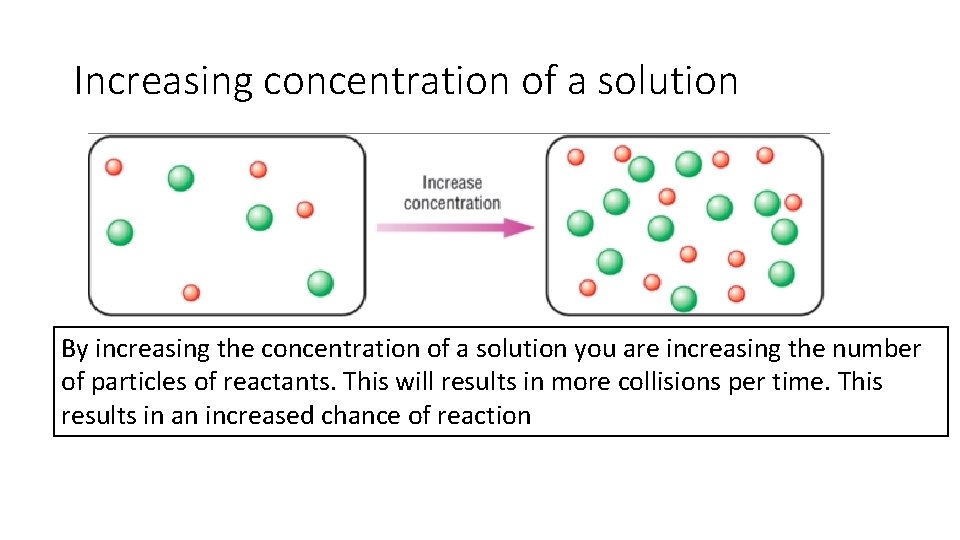
Increasing concentration of a solution By increasing the concentration of a solution you are increasing the number of particles of reactants. This will results in more collisions per time. This results in an increased chance of reaction

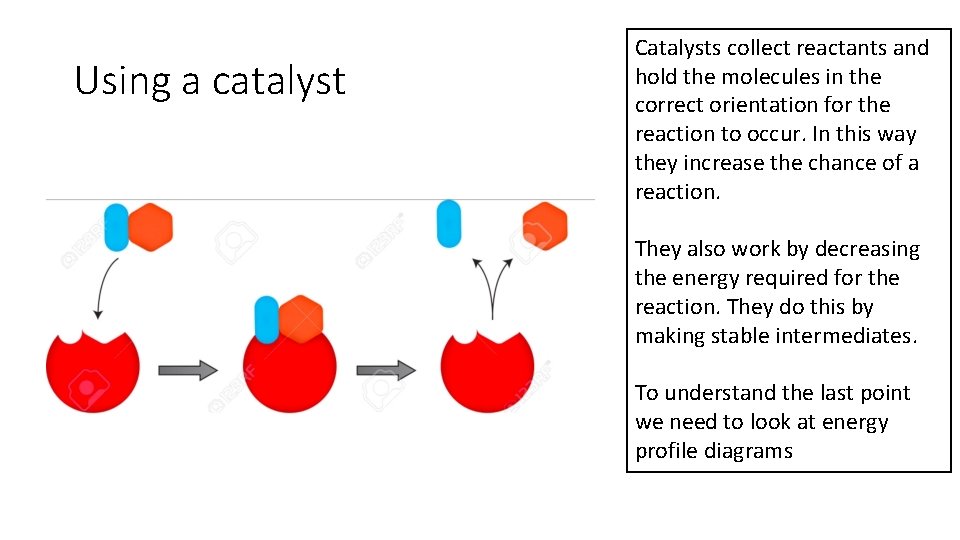
Using a catalyst Catalysts collect reactants and hold the molecules in the correct orientation for the reaction to occur. In this way they increase the chance of a reaction. They also work by decreasing the energy required for the reaction. They do this by making stable intermediates. To understand the last point we need to look at energy profile diagrams

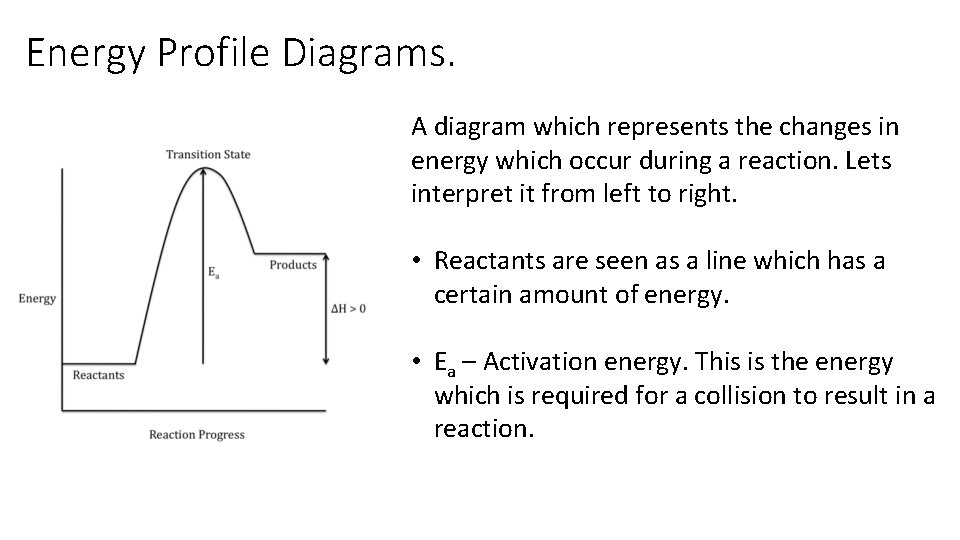
Energy Profile Diagrams. A diagram which represents the changes in energy which occur during a reaction. Lets interpret it from left to right. • Reactants are seen as a line which has a certain amount of energy. • Ea – Activation energy. This is the energy which is required for a collision to result in a reaction.

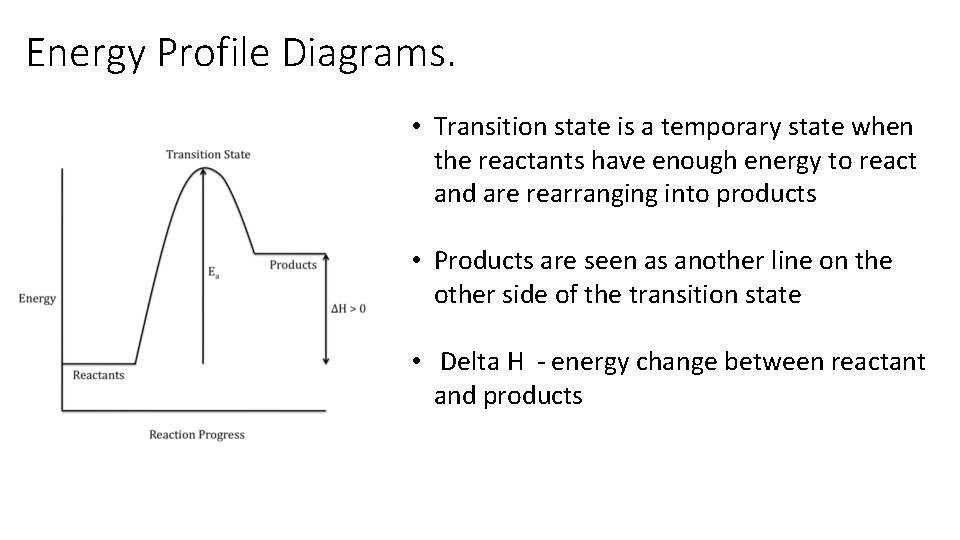
Energy Profile Diagrams. • Transition state is a temporary state when the reactants have enough energy to react and are rearranging into products • Products are seen as another line on the other side of the transition state • Delta H - energy change between reactant and products

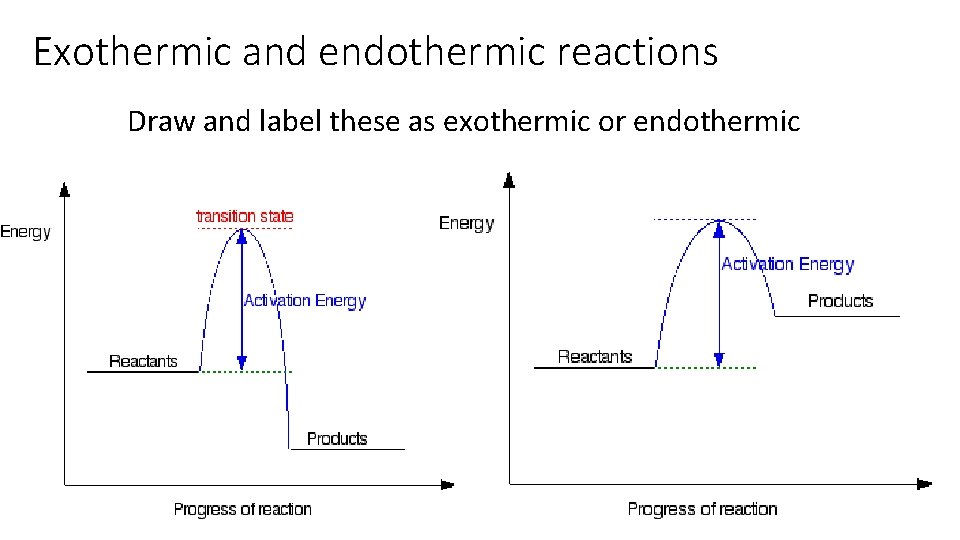
Exothermic and endothermic reactions An endothermic reaction is one which absorbs energy An exothermic reaction is one which releases energy If products have more energy than reactants the reaction would be? If reactants have more energy than products the reaction would be?

Exothermic and endothermic reactions Draw and label these as exothermic or endothermic

Energy Profile Diagrams and reaction rate Which of these reactions do you think would occur faster? Look at diagram on the board answer this question in note books

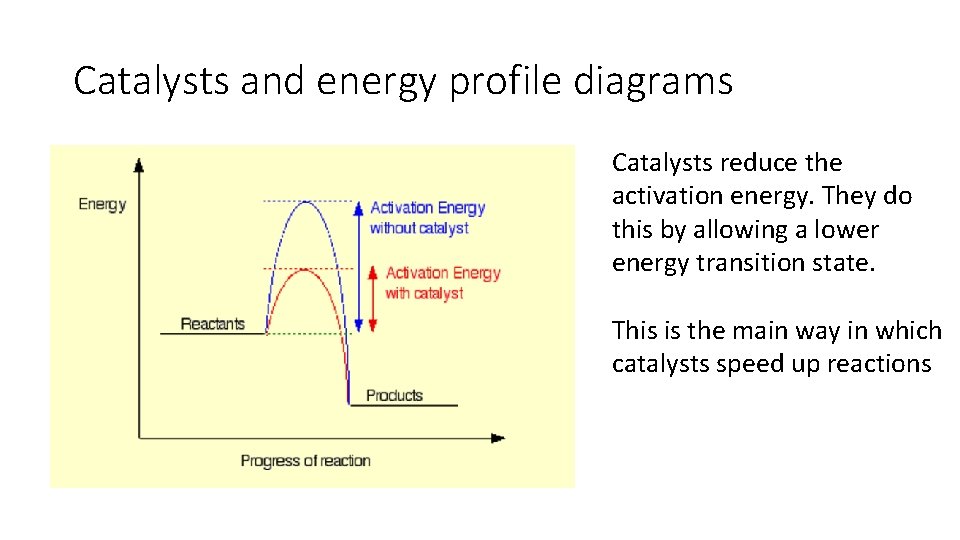
Catalysts and energy profile diagrams Catalysts reduce the activation energy. They do this by allowing a lower energy transition state. This is the main way in which catalysts speed up reactions
 Rate of reaction graph
Rate of reaction graph Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Unit 5 chemical reactions answers
Unit 5 chemical reactions answers How to write a balanced redox reaction
How to write a balanced redox reaction Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates A rate is a ratio that compares
A rate is a ratio that compares Equivalent ratios guided notes
Equivalent ratios guided notes Is ice cream countable noun
Is ice cream countable noun Contact and non contact forces
Contact and non contact forces They say it only takes a little faith
They say it only takes a little faith Some say the world will end in fire some say in ice
Some say the world will end in fire some say in ice Fire and ice diamante poem
Fire and ice diamante poem They say sometimes you win some
They say sometimes you win some Some trust in horses
Some trust in horses