Chapter 17 REACTION RATES DETERMINING RATES OF REACTIONS




- Slides: 8

Chapter 17 REACTION RATES

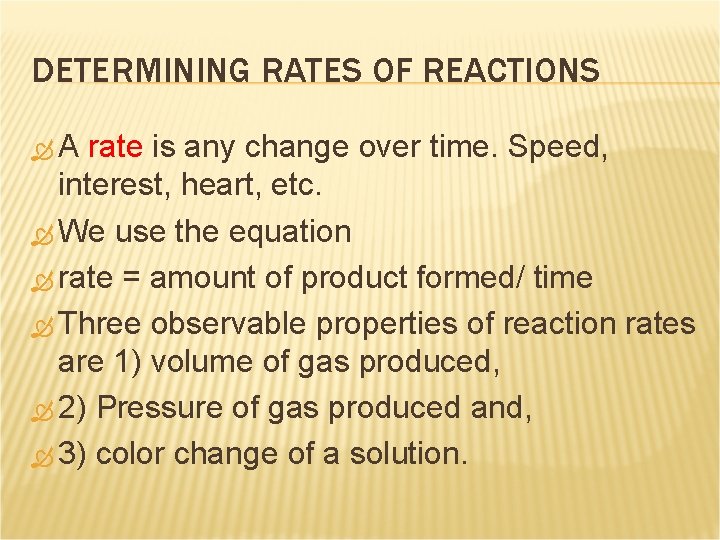
DETERMINING RATES OF REACTIONS A rate is any change over time. Speed, interest, heart, etc. We use the equation rate = amount of product formed/ time Three observable properties of reaction rates are 1) volume of gas produced, 2) Pressure of gas produced and, 3) color change of a solution.

FACTORS DETERMINING RATES OF REACTIONS Collision Theory is the root. “The rates of chemical reactions depend upon the collisions between reacting particles. ” Surface area. Sugar cube vs granulated Concentration. Higher concentrations = more collisions Nature of reactants. Some things just react more readily. Alkali metals and halogens.

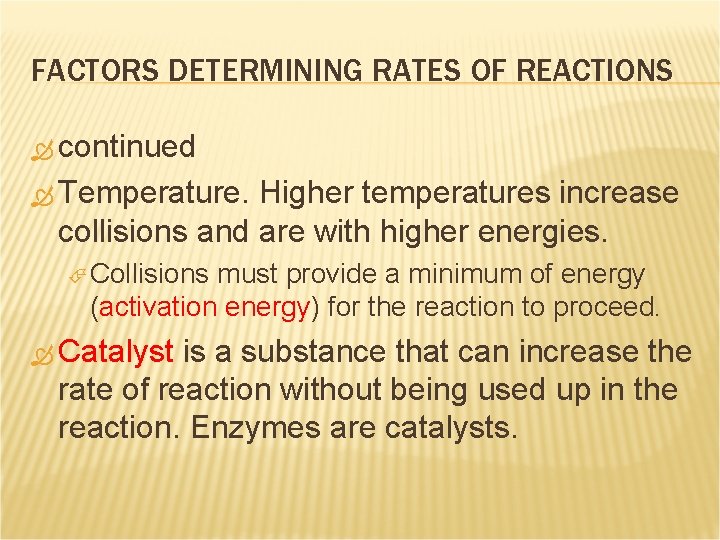
FACTORS DETERMINING RATES OF REACTIONS continued Temperature. Higher temperatures increase collisions and are with higher energies. Collisions must provide a minimum of energy (activation energy) for the reaction to proceed. Catalyst is a substance that can increase the rate of reaction without being used up in the reaction. Enzymes are catalysts.

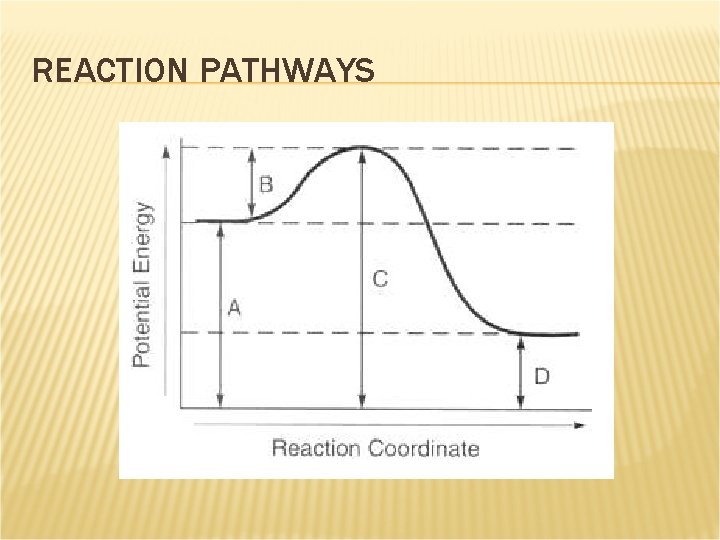
REACTION PATHWAYS Activation energy is the minimum amount of energy needed to cause a reaction to proceed. When molecules collide with each other, their kinetic energy is changed to potential energy. This higher energy state is referred to as an activated complex.

REACTION PATHWAYS

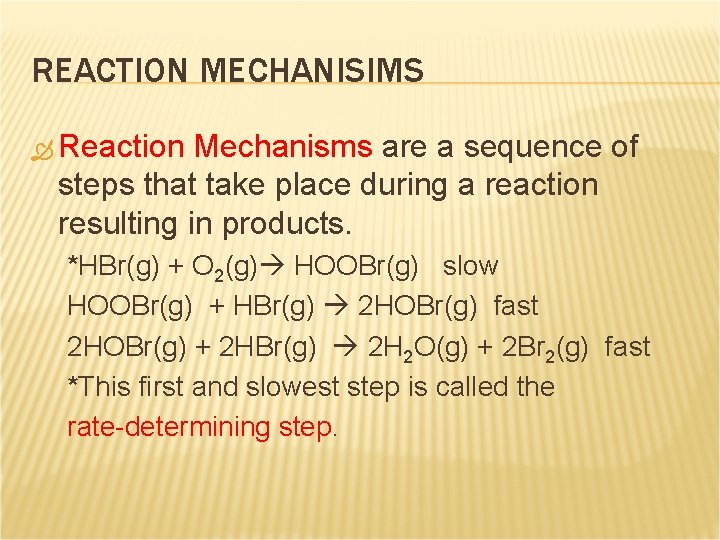
REACTION MECHANISIMS Reaction Mechanisms are a sequence of steps that take place during a reaction resulting in products. *HBr(g) + O 2(g) HOOBr(g) slow HOOBr(g) + HBr(g) 2 HOBr(g) fast 2 HOBr(g) + 2 HBr(g) 2 H 2 O(g) + 2 Br 2(g) fast *This first and slowest step is called the rate-determining step.

PRACTICE ? Page 575 #’s 3, 4 Page 582 #’s 11 -13 Page 583 #’s 15, 17 Page 587 #’s 18, 20, 21, 22
 Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Balance redox half reactions
Balance redox half reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Unit rate
Unit rate