Reactions Reaction Types Rxn Reaction Chemical Reaction Process


- Slides: 9

Reactions & Reaction Types Rxn = Reaction

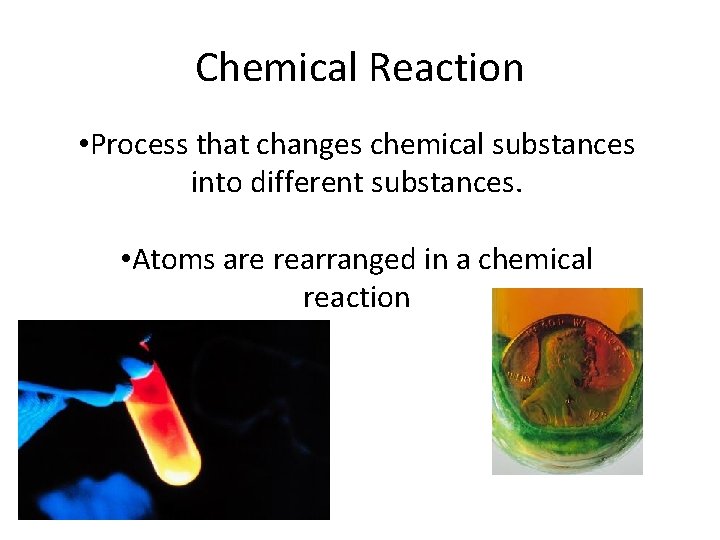
Chemical Reaction • Process that changes chemical substances into different substances. • Atoms are rearranged in a chemical reaction

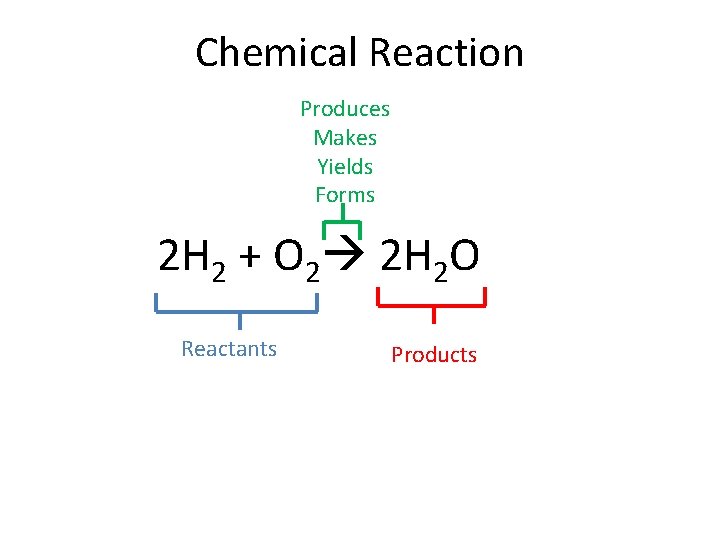
Chemical Reaction Produces Makes Yields Forms 2 H 2 + O 2 2 H 2 O Reactants Products

5 MAIN TYPES OF CHEM RXNS

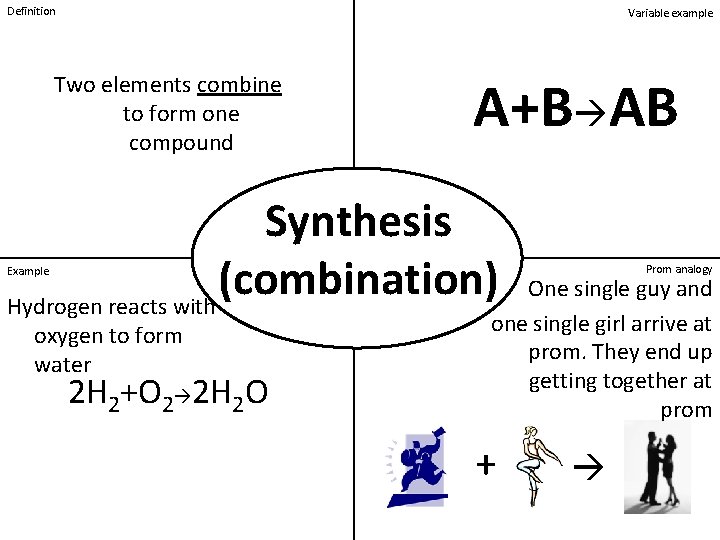
Definition Variable example Two elements combine to form one compound A+B AB Synthesis (combination) Hydrogen reacts with Example oxygen to form water 2 H 2+O 2 2 H 2 O Prom analogy One single guy and one single girl arrive at prom. They end up getting together at prom +

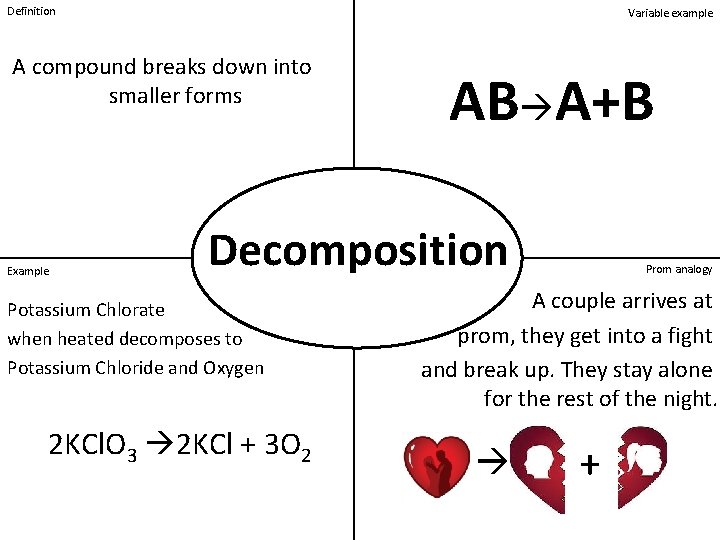
Definition Variable example A compound breaks down into smaller forms Example AB A+B Decomposition Potassium Chlorate when heated decomposes to Potassium Chloride and Oxygen 2 KCl. O 3 2 KCl + 3 O 2 Prom analogy A couple arrives at prom, they get into a fight and break up. They stay alone for the rest of the night. +

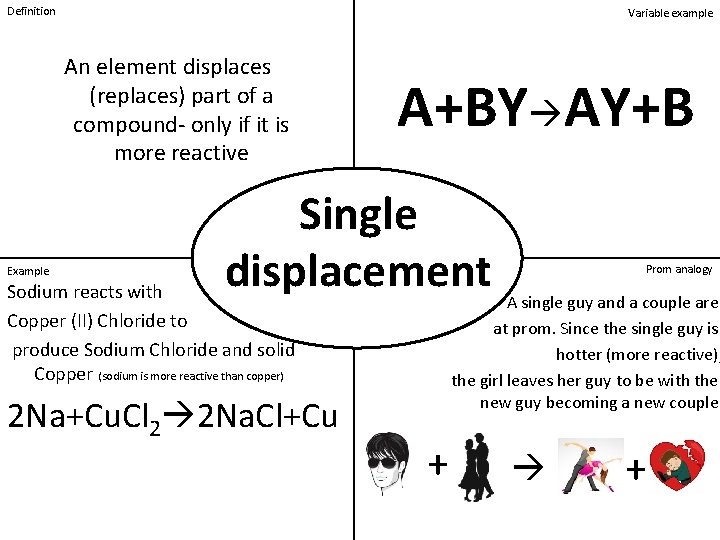
Definition Variable example An element displaces (replaces) part of a compound- only if it is more reactive Example A+BY AY+B Single displacement Sodium reacts with Copper (II) Chloride to produce Sodium Chloride and solid Copper (sodium is more reactive than copper) 2 Na+Cu. Cl 2 2 Na. Cl+Cu Prom analogy A single guy and a couple are at prom. Since the single guy is hotter (more reactive), the girl leaves her guy to be with the new guy becoming a new couple. + +

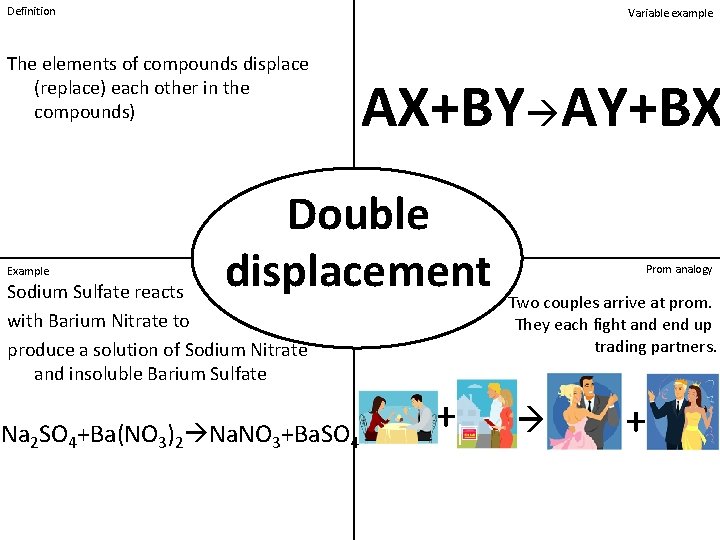
Definition Variable example The elements of compounds displace (replace) each other in the compounds) Example AX+BY AY+BX Double displacement Sodium Sulfate reacts with Barium Nitrate to produce a solution of Sodium Nitrate and insoluble Barium Sulfate Na 2 SO 4+Ba(NO 3)2 Na. NO 3+Ba. SO 4 + Prom analogy Two couples arrive at prom. They each fight and end up trading partners. +

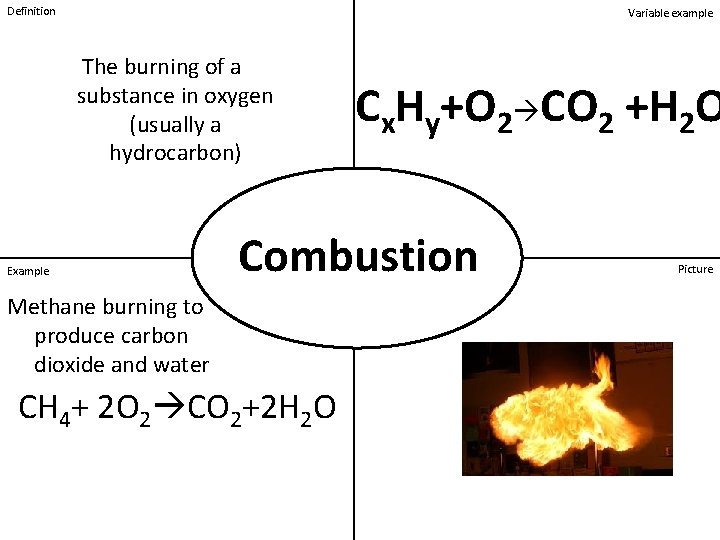
Definition Variable example The burning of a substance in oxygen (usually a hydrocarbon) Example Cx. Hy+O 2 CO 2 +H 2 O Combustion Methane burning to produce carbon dioxide and water CH 4+ 2 O 2 CO 2+2 H 2 O Picture