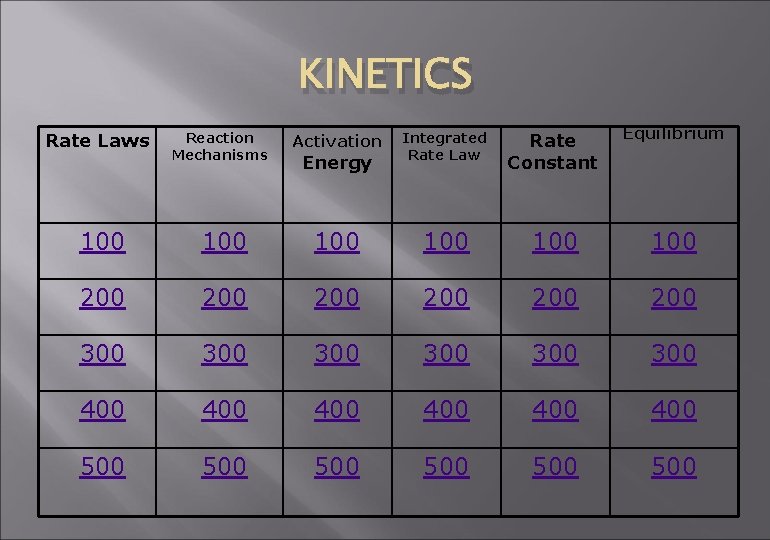
KINETICS JEOPARDY KINETICS Rate Laws Reaction Mechanisms Activation

![Rate Law 100 A reaction has the rate law, Rate = k[A][B]2. What is Rate Law 100 A reaction has the rate law, Rate = k[A][B]2. What is](https://slidetodoc.com/presentation_image_h/0995118842c95eef7f421b4d5602236f/image-3.jpg)
![Rate Law -200 A reaction has the rate law, rate = k[A][B]2. If the Rate Law -200 A reaction has the rate law, rate = k[A][B]2. If the](https://slidetodoc.com/presentation_image_h/0995118842c95eef7f421b4d5602236f/image-4.jpg)














![Integrated Rate Law -500 A graph of [A] vs time gave a straight line. Integrated Rate Law -500 A graph of [A] vs time gave a straight line.](https://slidetodoc.com/presentation_image_h/0995118842c95eef7f421b4d5602236f/image-22.jpg)









- Slides: 32

KINETICS JEOPARDY

KINETICS Rate Laws Reaction Mechanisms Activation Energy Integrated Rate Law Rate Constant Equilibrium 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500
![Rate Law 100 A reaction has the rate law Rate kAB2 What is Rate Law 100 A reaction has the rate law, Rate = k[A][B]2. What is](https://slidetodoc.com/presentation_image_h/0995118842c95eef7f421b4d5602236f/image-3.jpg)
Rate Law 100 A reaction has the rate law, Rate = k[A][B]2. What is the overall order for the reaction? Third Order Overall Return
![Rate Law 200 A reaction has the rate law rate kAB2 If the Rate Law -200 A reaction has the rate law, rate = k[A][B]2. If the](https://slidetodoc.com/presentation_image_h/0995118842c95eef7f421b4d5602236f/image-4.jpg)
Rate Law -200 A reaction has the rate law, rate = k[A][B]2. If the concentration of both A and B are doubled, what happens to the rate? Increases by a factor of 8. Return

Rate law -300 The equation, 2 A + 2 B C + D, describes an elementary reaction which takes place in a single step. What is the rate law? Rate = k[A]2[B]2 Return

Rate Law -400 Based on the following equation, which of the following compounds would you expect to undergo the most change in concentration in a given amount of time? 2 NH 3 N 2 + 3 H 2 Return

Rate Law -500 For the reaction, 2 XO + O 2 2 XO 2, the following data was obtained. What is the rate law for the reaction? Trial [XO] [O 2] rate 1 0. 010 2. 5 2 0. 010 0. 020 5. 0 3 0. 030 0. 020 45. 0 Rate = k [XO]2[O 2] Return

Reaction Mechanism -100 The reaction: A + 3 B D + F was studied and the following mechanism was determined A+B ↔C (fast) C + B D + E (slow) E+B F (very fast) Which step is the rate determining step? The second step (slow step) Return

DAILY DOUBLE 200 * 2 Suppose the reaction: A+B D followed the mechanism A + B ↔ C (fast) C D (slow) What is the rate law for the reaction? Rate = k[A][B] Return

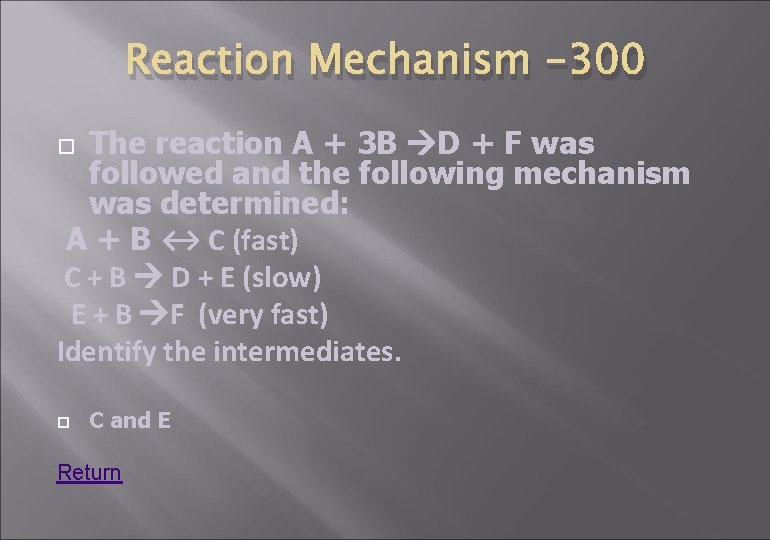
Reaction Mechanism -300 The reaction A + 3 B D + F was followed and the following mechanism was determined: A + B ↔ C (fast) C + B D + E (slow) E + B F (very fast) Identify the intermediates. C and E Return

DAILY DOUBLE -400 * 2 The reaction A +3 B D + F was studied and the mechanism was determined to be: A + B ↔ C (fast) C + B D + E (slow) E + B F (very fast) What is the rate law? Rate = k[A][B]2 Return

Reaction Mechanism 500 The decomposition of H 2 O 2 is determined to occur by the following mechanism: H 2 O 2 + I- H 2 O + IO- (slow) H 2 O 2 + IO- H 2 O + O 2 + I- (fast) What is the overall reaction? 2 H 2 O 2 H 2 O + O 2 Return

Activation Energy-100 How does a catalyst alter the rate of a chemical reaction? Lowers the activation energy (provides an alternative pathway) Return

Activation Energy-200 For a one step reaction, the activation energy for the forward reaction is 40 k. J/mol and the enthalpy of reaction is -20 k. J/mole. Which reaction is slower; the forward or the reverse? The reverse reaction is slower Return

Activation Energy-300 According to the collision theory, what two conditions must be met in order for a reaction to occur? Reactants must possess a minimum amount of energy and the collisions must occur with the correct orientation Return

Activation Energy -400 How can the slope of a plot of ln(k) vs 1/T for a reaction be used to determine the activation energy? By multiplying by –R (-8. 314) Return

Activation Energy -500 For a one step reaction the activation energy is 40 k. J/mol and the enthalpy of reaction is 20 k. J/mol. What is the activation energy of the reverse reaction? 20 k. J/mol Return

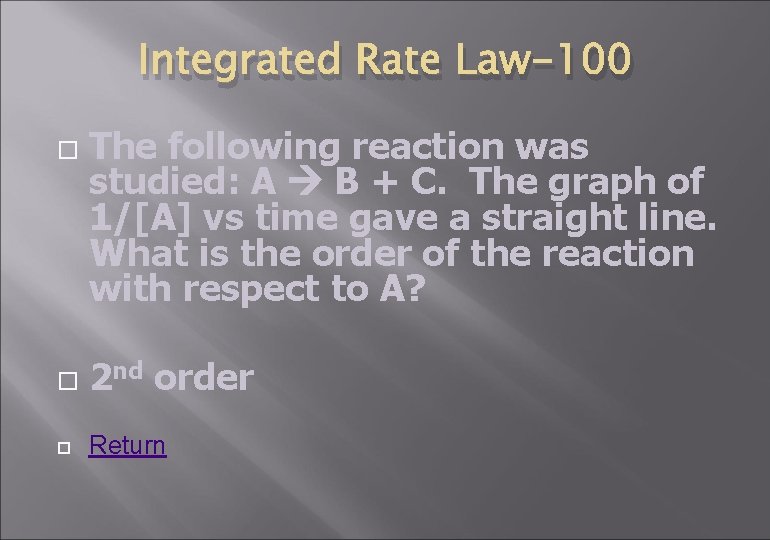
Integrated Rate Law-100 The following reaction was studied: A B + C. The graph of 1/[A] vs time gave a straight line. What is the order of the reaction with respect to A? 2 nd order Return

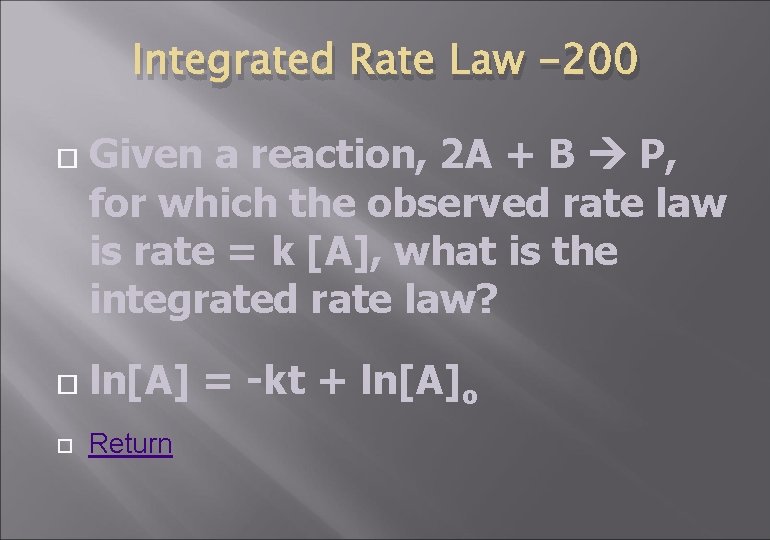
Integrated Rate Law -200 Given a reaction, 2 A + B P, for which the observed rate law is rate = k [A], what is the integrated rate law? ln[A] = -kt + ln[A]o Return

Integrated Rate Law -300 In a first order reaction, what fraction of the material will remain after 4 half lives? 1/16 Return

Integrated Rate Law -400 A first order reaction has a rate constant of 0. 00318 min-1. What is the half life of the reaction? 21. 8 minutes Return
![Integrated Rate Law 500 A graph of A vs time gave a straight line Integrated Rate Law -500 A graph of [A] vs time gave a straight line.](https://slidetodoc.com/presentation_image_h/0995118842c95eef7f421b4d5602236f/image-22.jpg)
Integrated Rate Law -500 A graph of [A] vs time gave a straight line. How does doubling the concentration of A affect the rate of the reaction? It has no affect Return

Rate Constant-100 How does changing the concentration affect the rate constant? It has no affect Return

Rate Constant -200 How does increasing the temperature affect the rate and the rate constant? It increases both the rate constant and the rate Return

Rate Constant - 300 What is the unit for the rate constant in a reaction that is 2 nd order overall? L/mol s Return

Rate Constant -400 In a zero order rate law, what units must the rate concept possess? Mol/L time Return

Rate Constant - 500 A rate constant has the units s-1. Doubling the concentration would have what affect on the overall rate of reaction? It would double the rate Return

Equilibrium -100 What factors affect the value of Keq? Temperature and nature of reactants Return

Equilibrium -200 How does a catalyst affect the value of Keq? It has no effect Return

Equilibrium -300 If K>1, what does that indicate about the equilibrium position? The concentration of products is greater than the concentration of reactants at equilibrium Return

Equilibrium-500 Increasing the temperature increases the value of Keq. Is the reaction endothermic or endothermic? endothermic Return

Equilibrium-400 According to the equation: 3 H 2(g) + N 2(g) 2 NH 3(g), if the volume of the reaction chamber increases, will the forward or reverse reaction be favored? Reverse reaction Return
 Activation tree and activation record
Activation tree and activation record Rate of reaction formula
Rate of reaction formula Factors affecting rate of chemical reaction
Factors affecting rate of chemical reaction Activation energy for reverse reaction
Activation energy for reverse reaction Kinetics reaction
Kinetics reaction Organic chemistry
Organic chemistry Rate mechanisms
Rate mechanisms Charles de secondat
Charles de secondat Exponent rules jeopardy
Exponent rules jeopardy Newton's laws jeopardy
Newton's laws jeopardy E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Equilibrium reaction rate
Equilibrium reaction rate First order rate law
First order rate law Molecularity of reaction
Molecularity of reaction How to calculate rate of reaction
How to calculate rate of reaction Determine
Determine How to calculate instantaneous rate of reaction
How to calculate instantaneous rate of reaction Rate law for first order reaction
Rate law for first order reaction Initial rate of reaction
Initial rate of reaction Factors that affect the rate constant
Factors that affect the rate constant Overall rate law of a reaction
Overall rate law of a reaction What is catalyst and how it affects reaction rate
What is catalyst and how it affects reaction rate How does temperature affect rate of reaction
How does temperature affect rate of reaction Half life for second order reaction
Half life for second order reaction Rate=k a b
Rate=k a b Activation energy and temperature
Activation energy and temperature How to write reaction rate
How to write reaction rate Rates of reaction quiz
Rates of reaction quiz Surface area affecting rate of reaction
Surface area affecting rate of reaction Reaction rate and stoichiometry
Reaction rate and stoichiometry Rate of reaction formula
Rate of reaction formula