Reaction Mechanisms Chapter 12 Section 6 Reaction Mechanisms





![Fast Initial Step • Because Ratef = Rater , k 1 [NO] [Br 2] Fast Initial Step • Because Ratef = Rater , k 1 [NO] [Br 2]](https://slidetodoc.com/presentation_image_h/99debac7930052c7bf8a0921d6f6942d/image-13.jpg)
![Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for](https://slidetodoc.com/presentation_image_h/99debac7930052c7bf8a0921d6f6942d/image-14.jpg)
- Slides: 15

Reaction Mechanisms Chapter 12, Section 6

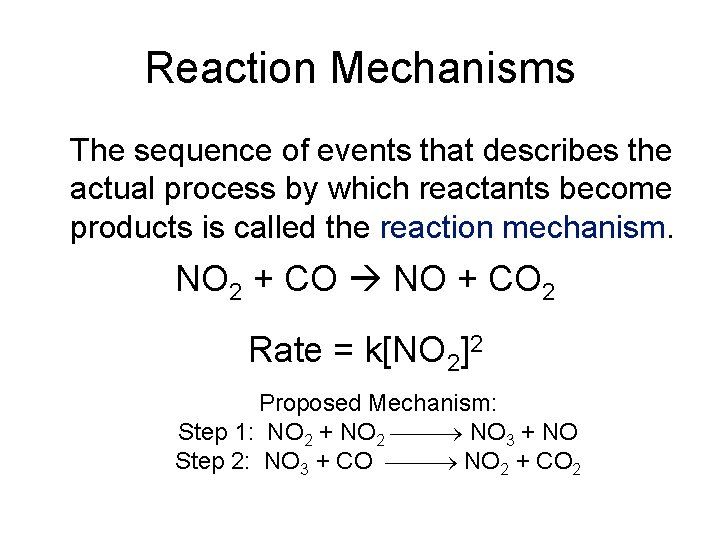
Reaction Mechanisms The sequence of events that describes the actual process by which reactants become products is called the reaction mechanism. NO 2 + CO NO + CO 2 Rate = k[NO 2]2 Proposed Mechanism: Step 1: NO 2 + NO 2 NO 3 + NO Step 2: NO 3 + CO NO 2 + CO 2

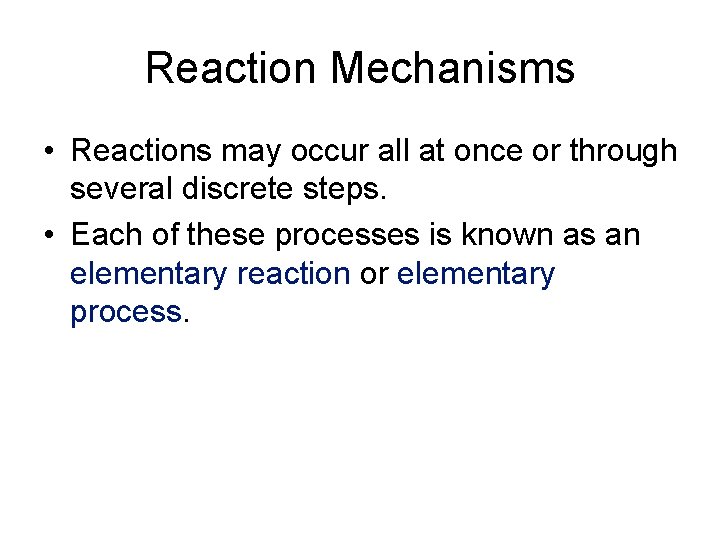
Reaction Mechanisms • Reactions may occur all at once or through several discrete steps. • Each of these processes is known as an elementary reaction or elementary process.

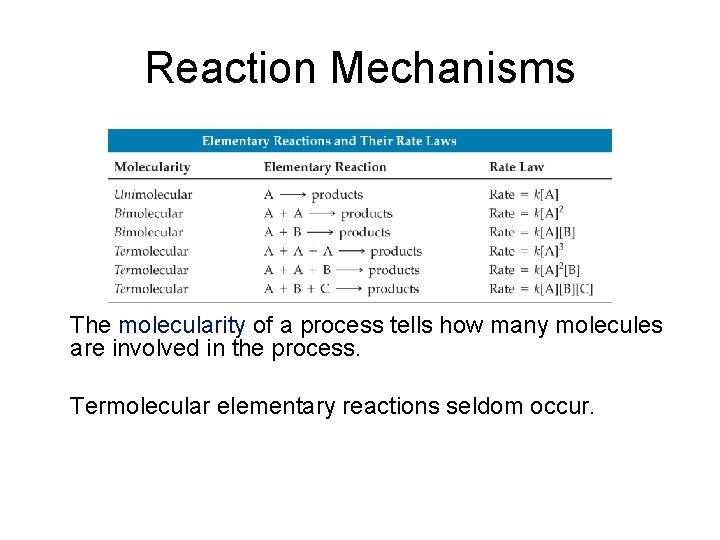
Reaction Mechanisms The molecularity of a process tells how many molecules are involved in the process. Termolecular elementary reactions seldom occur.

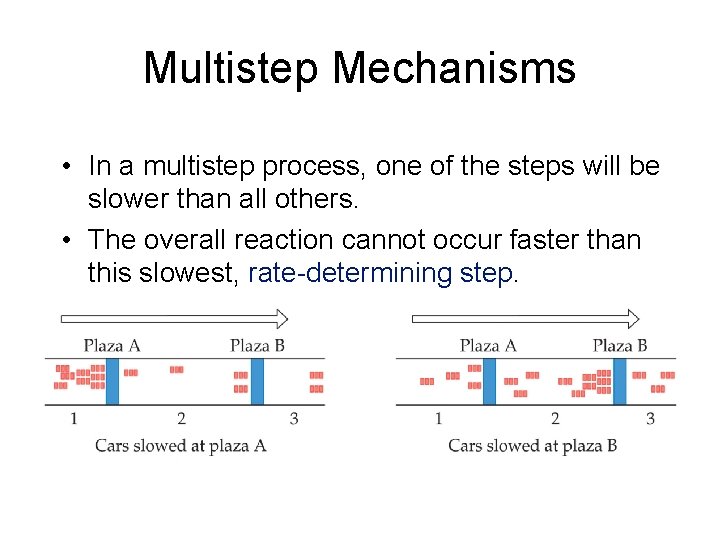
Multistep Mechanisms • In a multistep process, one of the steps will be slower than all others. • The overall reaction cannot occur faster than this slowest, rate-determining step.

Reaction Mechanisms must satisfy 2 requirements: 1. The sum of the elementary steps must give the overall balanced equation. 2. The mechanism must agree with the experimentally determined rate law.

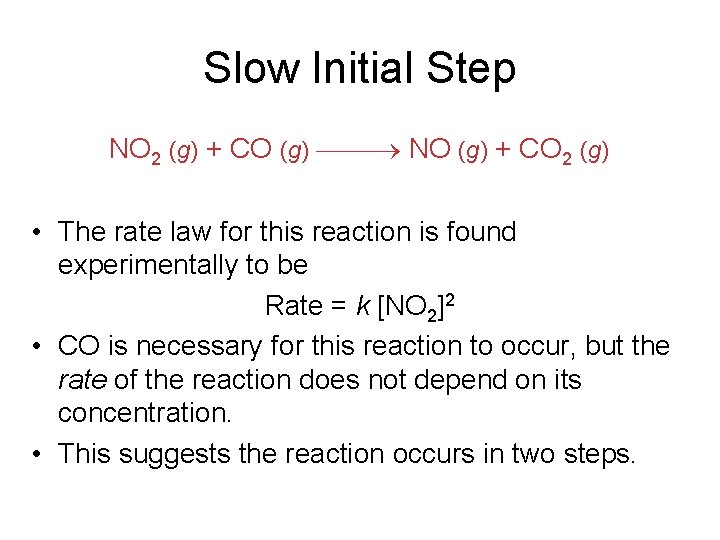
Slow Initial Step NO 2 (g) + CO (g) NO (g) + CO 2 (g) • The rate law for this reaction is found experimentally to be Rate = k [NO 2]2 • CO is necessary for this reaction to occur, but the rate of the reaction does not depend on its concentration. • This suggests the reaction occurs in two steps.

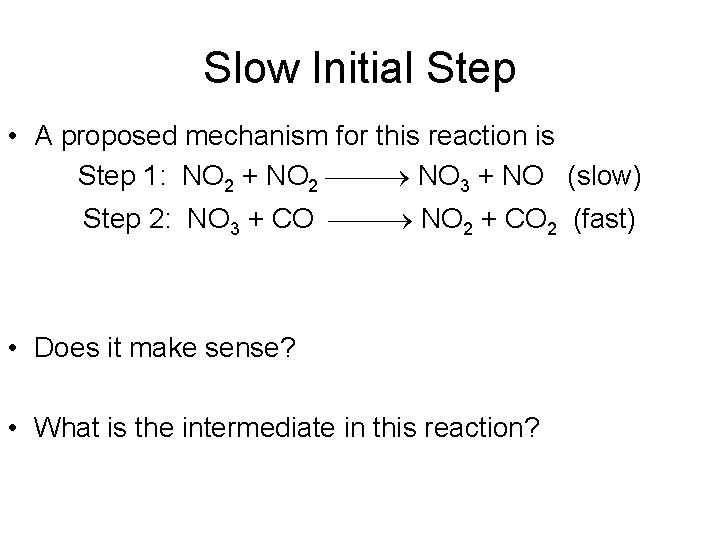
Slow Initial Step • A proposed mechanism for this reaction is Step 1: NO 2 + NO 2 NO 3 + NO (slow) Step 2: NO 3 + CO NO 2 + CO 2 (fast) • Does it make sense? • What is the intermediate in this reaction?

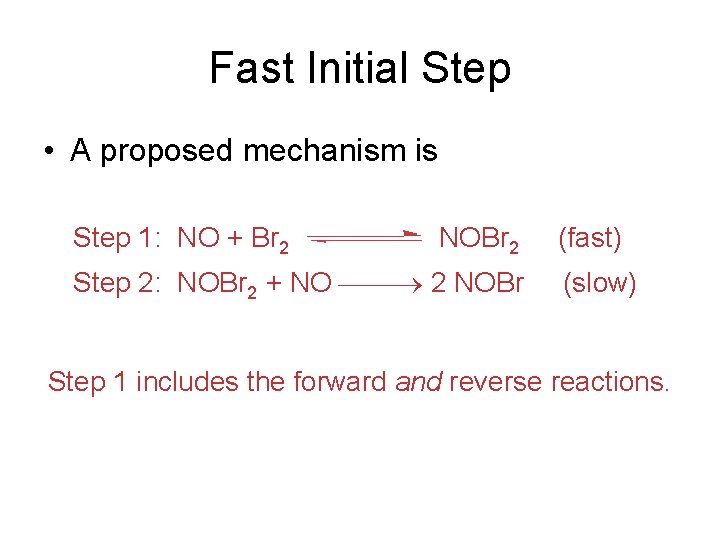
Fast Initial Step 2 NO (g) + Br 2 (g) 2 NOBr (g) • The rate law for this reaction is found to be Rate = k [NO]2 [Br 2] • Because termolecular processes are rare, this rate law suggests a two-step mechanism.

Fast Initial Step • A proposed mechanism is Step 1: NO + Br 2 NOBr 2 Step 2: NOBr 2 + NO 2 NOBr (fast) (slow) Step 1 includes the forward and reverse reactions.

Fast Initial Step • The rate of the overall reaction depends upon the rate of the slow step. • The rate law for that step would be Rate = k 2 [NOBr 2] [NO] • But how can we find [NOBr 2]?

Fast Initial Step • NOBr 2 can react two ways: – With NO to form NOBr – By decomposition to reform NO and Br 2 • The reactants and products of the first step are in equilibrium with each other. • Therefore, Ratef = Rater
![Fast Initial Step Because Ratef Rater k 1 NO Br 2 Fast Initial Step • Because Ratef = Rater , k 1 [NO] [Br 2]](https://slidetodoc.com/presentation_image_h/99debac7930052c7bf8a0921d6f6942d/image-13.jpg)
Fast Initial Step • Because Ratef = Rater , k 1 [NO] [Br 2] = k− 1 [NOBr 2] • Solving for [NOBr 2] gives us k 1 [NO] [Br ] = [NOBr ] 2 2 k− 1
![Fast Initial Step Substituting this expression for NOBr 2 in the rate law for Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for](https://slidetodoc.com/presentation_image_h/99debac7930052c7bf8a0921d6f6942d/image-14.jpg)
Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for the rate-determining step gives Rate = k 2 k 1 k− 1 [NO] [Br 2] [NO] = k [NO]2 [Br 2]

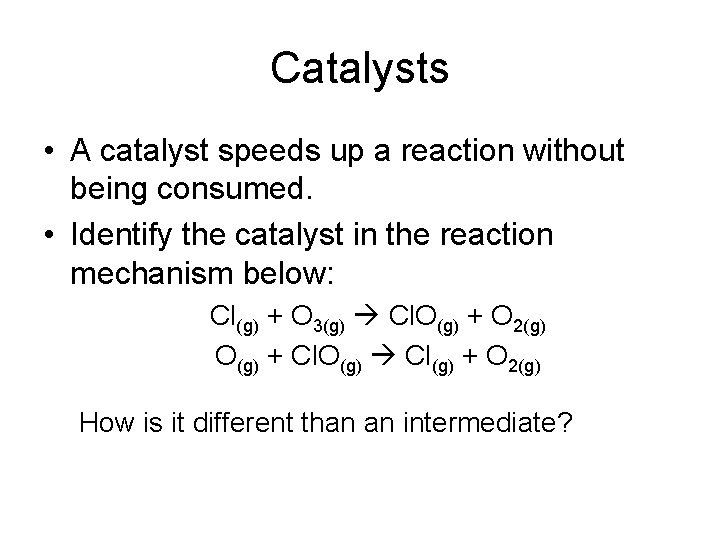
Catalysts • A catalyst speeds up a reaction without being consumed. • Identify the catalyst in the reaction mechanism below: Cl(g) + O 3(g) Cl. O(g) + O 2(g) O(g) + Cl. O(g) Cl(g) + O 2(g) How is it different than an intermediate?