Reaction Rate Activation Energy Activation energy is the
- Slides: 4

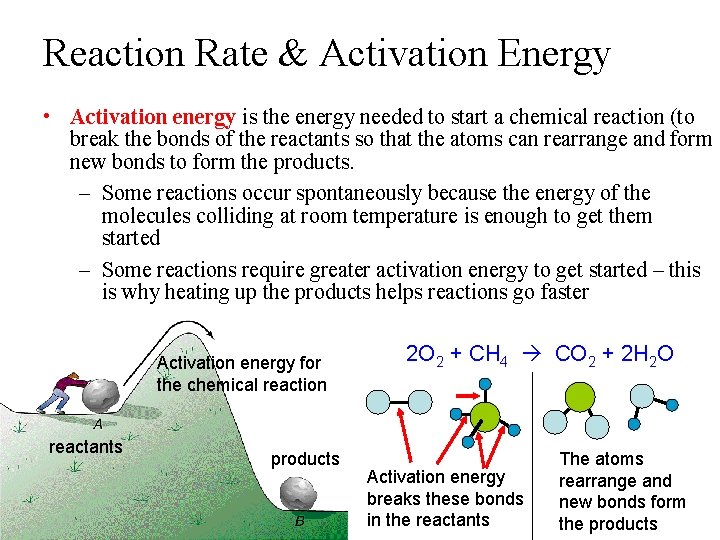
Reaction Rate & Activation Energy • Activation energy is the energy needed to start a chemical reaction (to break the bonds of the reactants so that the atoms can rearrange and form new bonds to form the products. – Some reactions occur spontaneously because the energy of the molecules colliding at room temperature is enough to get them started – Some reactions require greater activation energy to get started – this is why heating up the products helps reactions go faster Activation energy for the chemical reaction reactants products 2 O 2 + CH 4 CO 2 + 2 H 2 O Activation energy breaks these bonds in the reactants The atoms rearrange and new bonds form the products

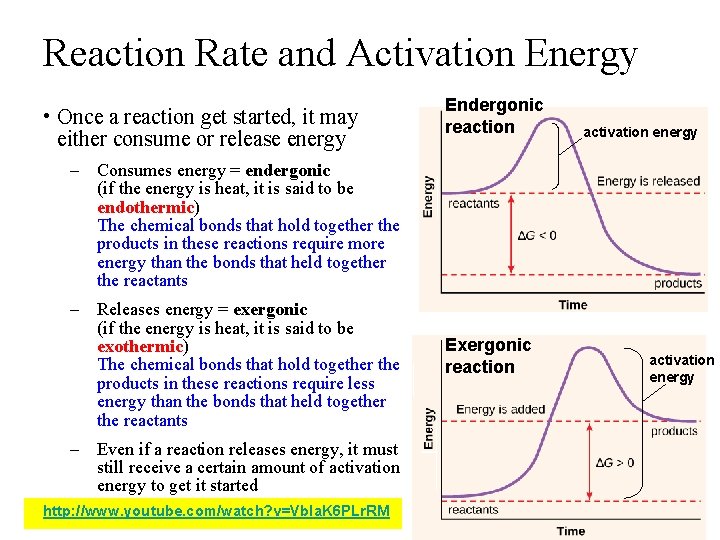
Reaction Rate and Activation Energy • Once a reaction get started, it may either consume or release energy Endergonic reaction activation energy – Consumes energy = endergonic (if the energy is heat, it is said to be endothermic) The chemical bonds that hold together the products in these reactions require more energy than the bonds that held together the reactants – Releases energy = exergonic (if the energy is heat, it is said to be exothermic) The chemical bonds that hold together the products in these reactions require less energy than the bonds that held together the reactants – Even if a reaction releases energy, it must still receive a certain amount of activation energy to get it started http: //www. youtube. com/watch? v=Vb. Ia. K 6 PLr. RM Exergonic reaction activation energy

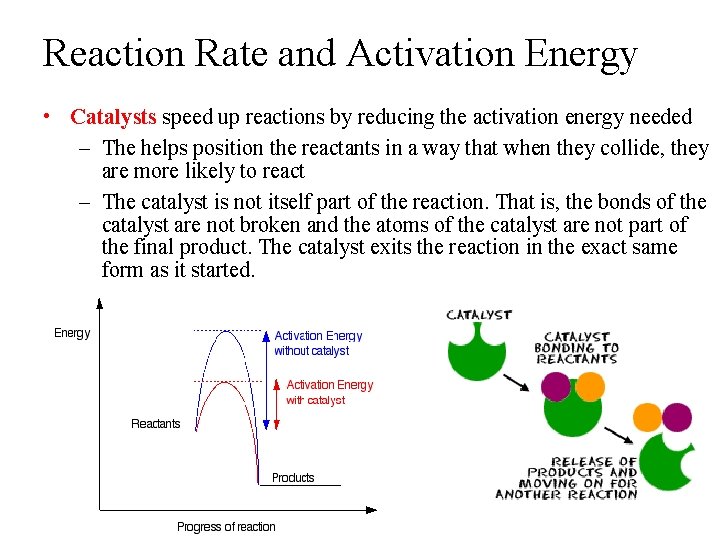
Reaction Rate and Activation Energy • Catalysts speed up reactions by reducing the activation energy needed – The helps position the reactants in a way that when they collide, they are more likely to react – The catalyst is not itself part of the reaction. That is, the bonds of the catalyst are not broken and the atoms of the catalyst are not part of the final product. The catalyst exits the reaction in the exact same form as it started.

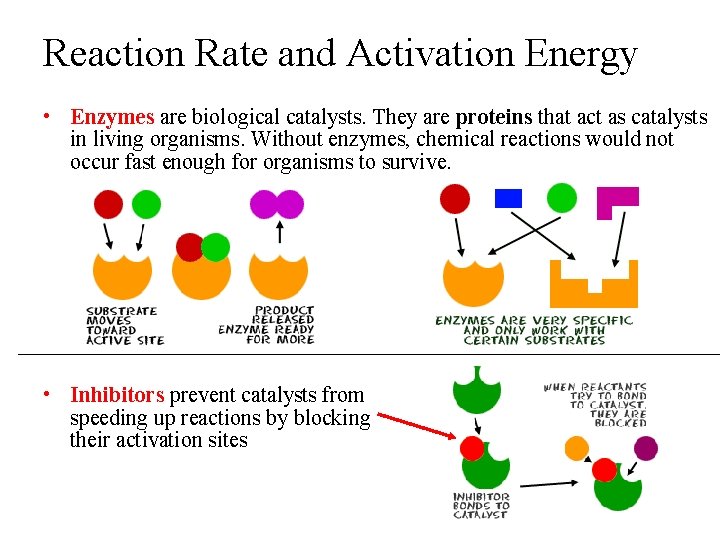
Reaction Rate and Activation Energy • Enzymes are biological catalysts. They are proteins that act as catalysts in living organisms. Without enzymes, chemical reactions would not occur fast enough for organisms to survive. • Inhibitors prevent catalysts from speeding up reactions by blocking their activation sites