Graphing Rates Activation Energy Unit 3 Reaction Rates









- Slides: 27

Graphing Rates & Activation Energy Unit 3: Reaction Rates and Equilibrium

Review Last Day • What was collision theory? • What were the 3 factors that affect the rate of a reaction? • The rate of reaction is determined by which of these factors?

How do reactions take place? • If we consider the following equation, 2 C 2 H 2 + 5 O 2 → 4 CO 2 + 2 H 2 O • This reaction says that 7 molecules (2 C 2 H 2 and 5 O 2) need to collide at once for the reaction to occur. . pretty unlikely • So instead, the reaction probably occurs in two or three steps. . much more likely as less molecules need to colliside properly

How do reactions take place? • Now take a look at this: 2 NO + O 2 → 2 NO 2 • As we saw with Hess’s law, this may not occur all at once, but may have happened in the following steps: 1. 2 NO → N 2 O 2 2. N 2 O 2 + O 2 → 2 NO 2 • The series of steps a reaction undergoes is called the reaction mechanism. • Substances that get cancelled out, like N 2 O 2, are called reaction intermediates. These are typically short lived.

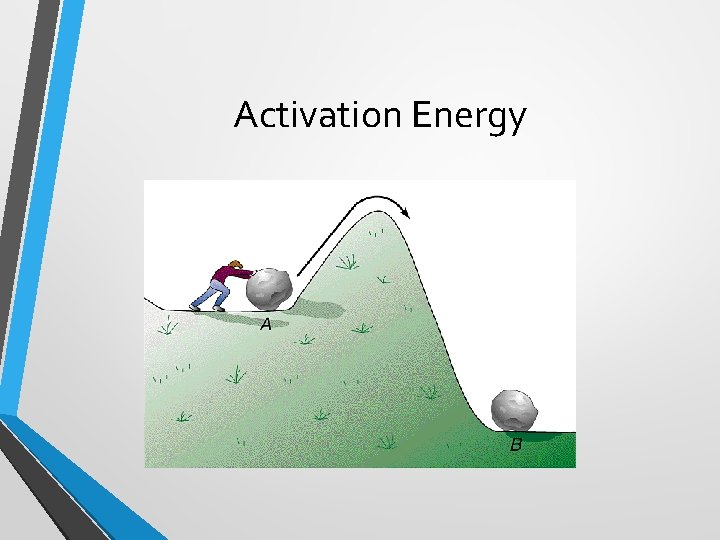
Activation Energy • One thing we said about collisions between molecules is that there must be enough energy between them to make a reaction. • Activation energy (Ea) is the minimum amount of energy for a successful collision. This is often called the threshold energy.

Activation Energy

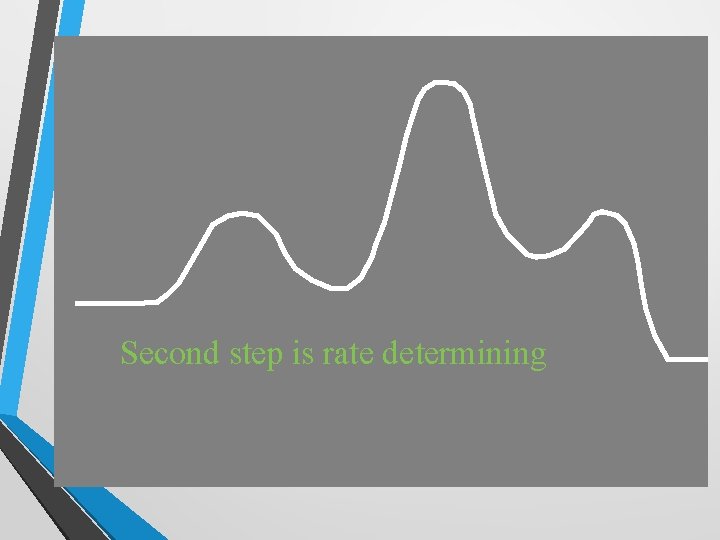
Rate Determining Step • Rate Determining Step – the step that determines the overall rate of a reaction. • Just like a chain is only as strong as its weakest link, the rate of reaction depends on the slowest step. • We call the slowest step in a reaction the rate determining step. If your goal is to speed up a reaction, you want to focus on this step.

What is potential energy? • As particles approach each other potential energy increases. • That is, if the particles are closer together they have a greater potential to react (or do work) than if they were farther apart. • Once they hit, the potential energy is at a high

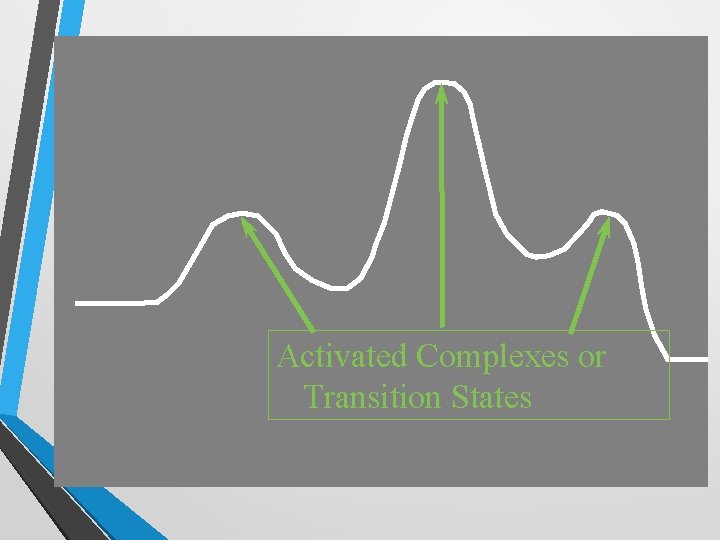
Transition State • If the particles hit correctly (with sufficient speed and orientation) a new ‘molecule’ will form for a very short period. • During this time, the ‘molecule’ has bonds that are both breaking and forming. Therefore, it is extremely unstable (which accounts for its short existence). • This unstable ‘molecule’ is called the activated complex, or transition state.

Transition State • This transition state is a combination of the two molecules coming together. • It is important to note that the molecule is not happy in this shape and wants to change quickly, hence why transition states are so short lived and why the molecule is called “unstable”. The activation energy (Ea) is the amount of energy required to reach the activated complex. •

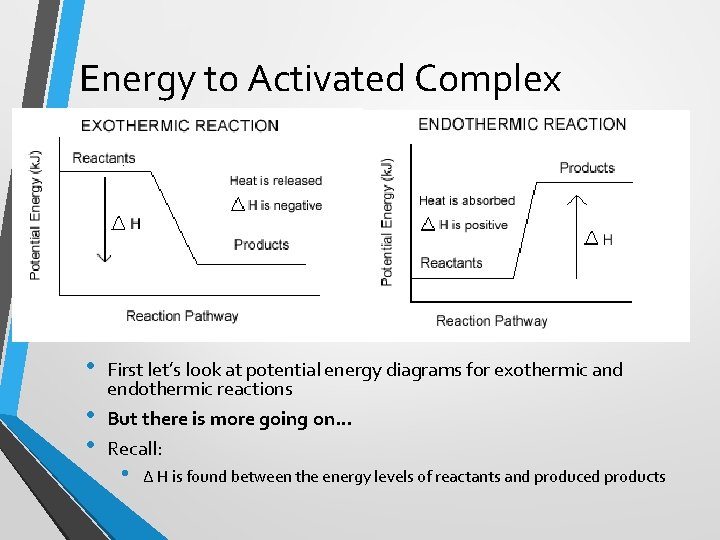
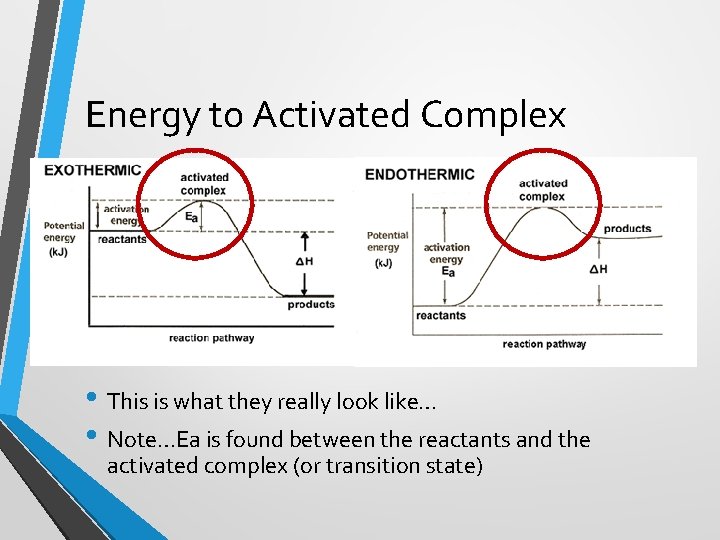
Energy to Activated Complex • • • First let’s look at potential energy diagrams for exothermic and endothermic reactions But there is more going on… Recall: • ∆ H is found between the energy levels of reactants and produced products

Energy to Activated Complex • This is what they really look like… • Note…Ea is found between the reactants and the activated complex (or transition state)

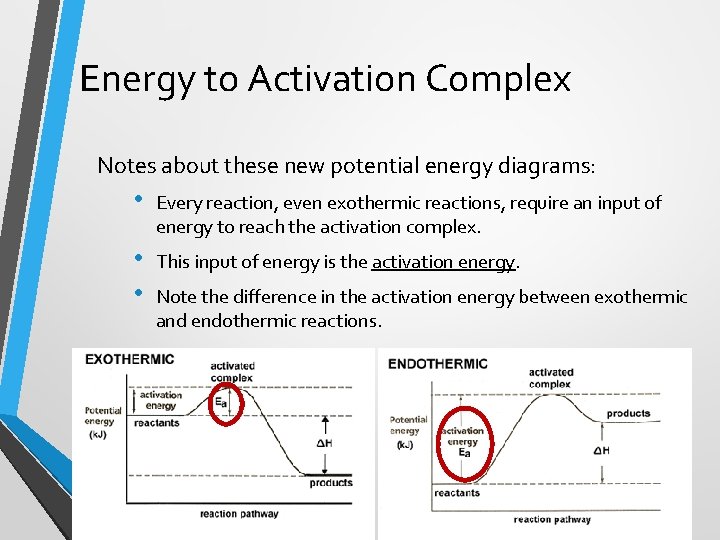
Energy to Activation Complex Notes about these new potential energy diagrams: • Every reaction, even exothermic reactions, require an input of energy to reach the activation complex. • • This input of energy is the activation energy. Note the difference in the activation energy between exothermic and endothermic reactions.

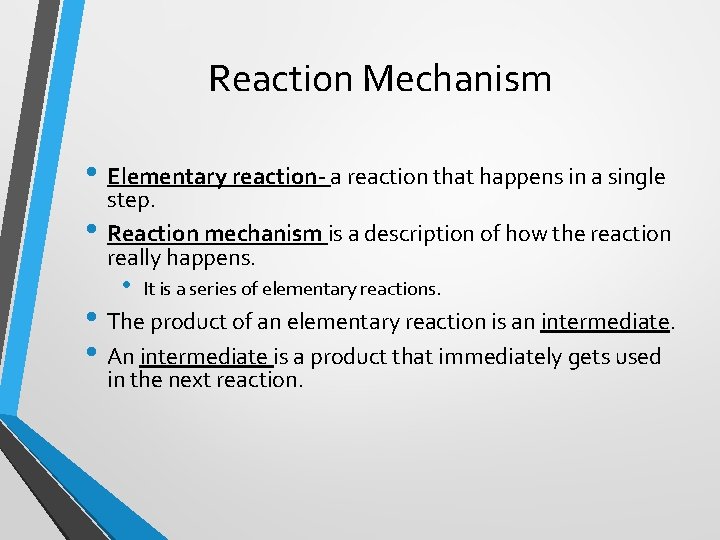
Reaction Mechanism • Elementary reaction- a reaction that happens in a single • step. Reaction mechanism is a description of how the reaction really happens. • It is a series of elementary reactions. • The product of an elementary reaction is an intermediate. • An intermediate is a product that immediately gets used in the next reaction.

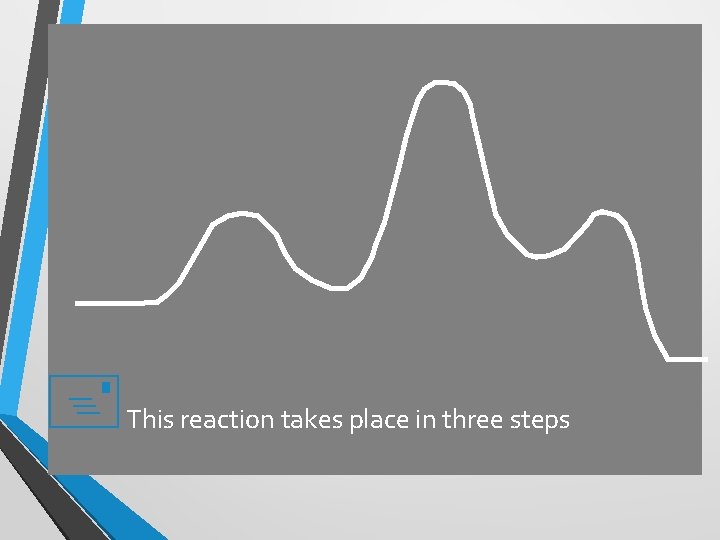
+ This reaction takes place in three steps

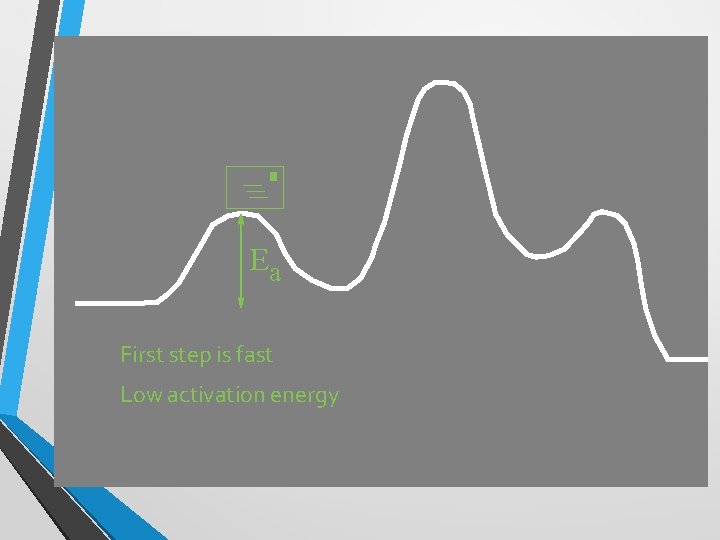
+ Ea First step is fast Low activation energy

+ Ea Second step is slow High activation energy

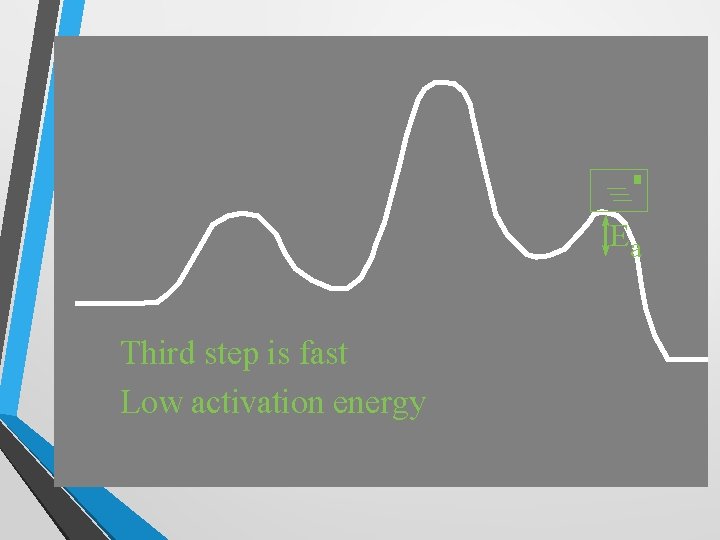
+ Ea Third step is fast Low activation energy

Second step is rate determining

Intermediates are present

Activated Complexes or Transition States

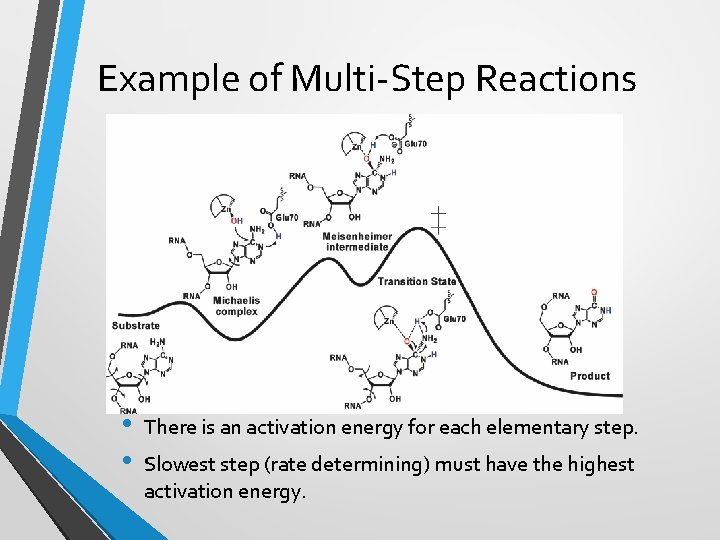
Example of Multi-Step Reactions • • There is an activation energy for each elementary step. Slowest step (rate determining) must have the highest activation energy.

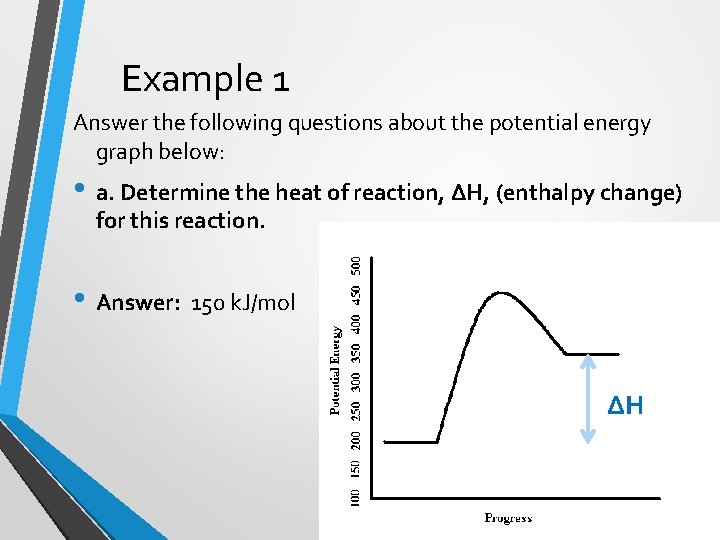
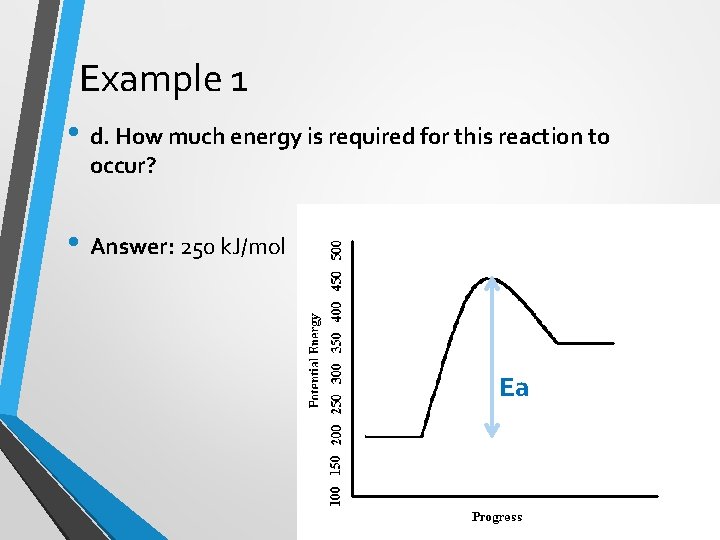
Example 1 Answer the following questions about the potential energy graph below: • a. Determine the heat of reaction, ΔH, (enthalpy change) for this reaction. • Answer: 150 k. J/mol ∆H

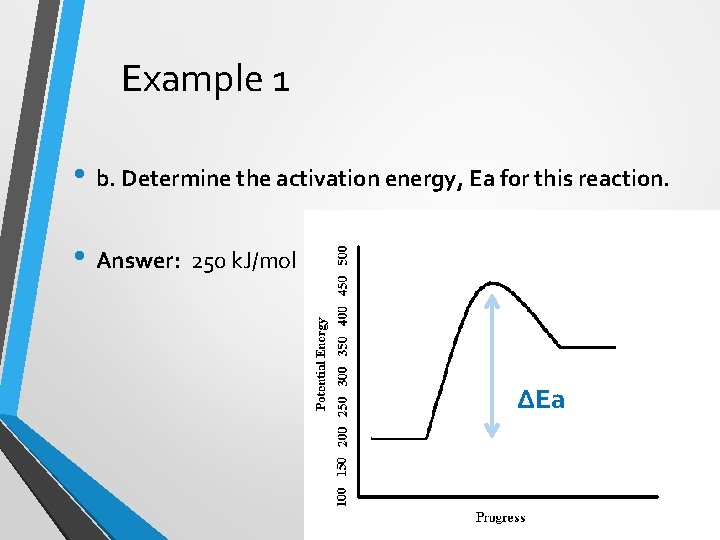
Example 1 • b. Determine the activation energy, Ea for this reaction. • Answer: 250 k. J/mol ∆Ea

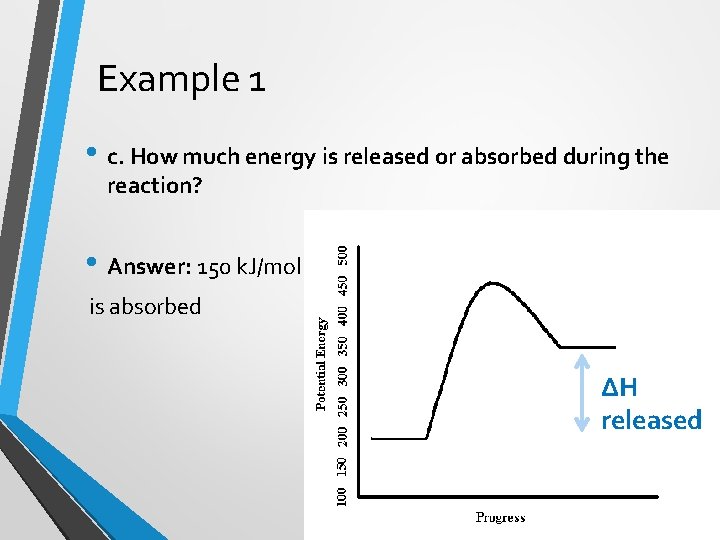
Example 1 • c. How much energy is released or absorbed during the reaction? • Answer: 150 k. J/mol is absorbed ∆H released

Example 1 • d. How much energy is required for this reaction to occur? • Answer: 250 k. J/mol Ea

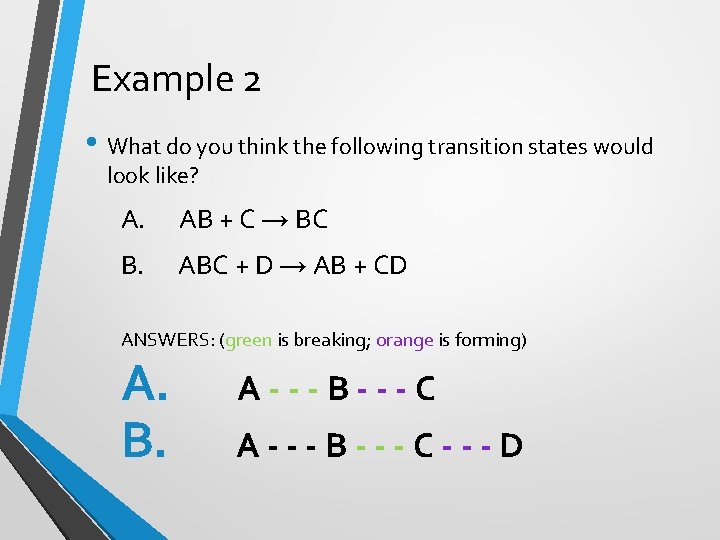
Example 2 • What do you think the following transition states would look like? A. AB + C → BC B. ABC + D → AB + CD ANSWERS: (green is breaking; orange is forming) A. B. A---B---C---D