Reaction Mechanisms Chapter 14 Reaction Mechanisms The sequence




![Fast Initial Step • Because Ratef = Rater , k 1 [NO] [Br 2] Fast Initial Step • Because Ratef = Rater , k 1 [NO] [Br 2]](https://slidetodoc.com/presentation_image_h2/fd1cd579c7826896b4a84ae1e25d1b28/image-13.jpg)
![Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for](https://slidetodoc.com/presentation_image_h2/fd1cd579c7826896b4a84ae1e25d1b28/image-14.jpg)

- Slides: 16

Reaction Mechanisms Chapter 14

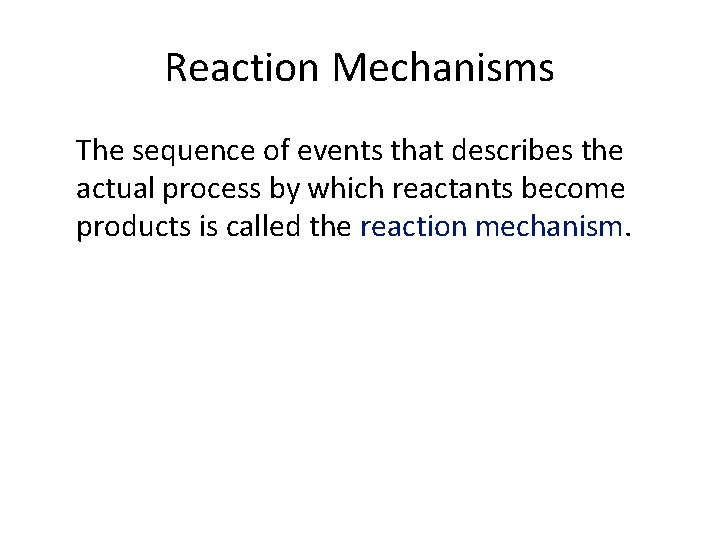
Reaction Mechanisms The sequence of events that describes the actual process by which reactants become products is called the reaction mechanism.

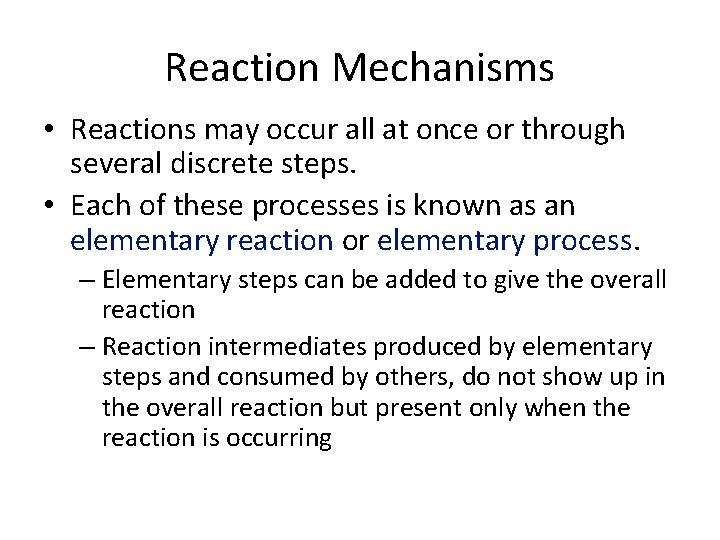
Reaction Mechanisms • Reactions may occur all at once or through several discrete steps. • Each of these processes is known as an elementary reaction or elementary process. – Elementary steps can be added to give the overall reaction – Reaction intermediates produced by elementary steps and consumed by others, do not show up in the overall reaction but present only when the reaction is occurring

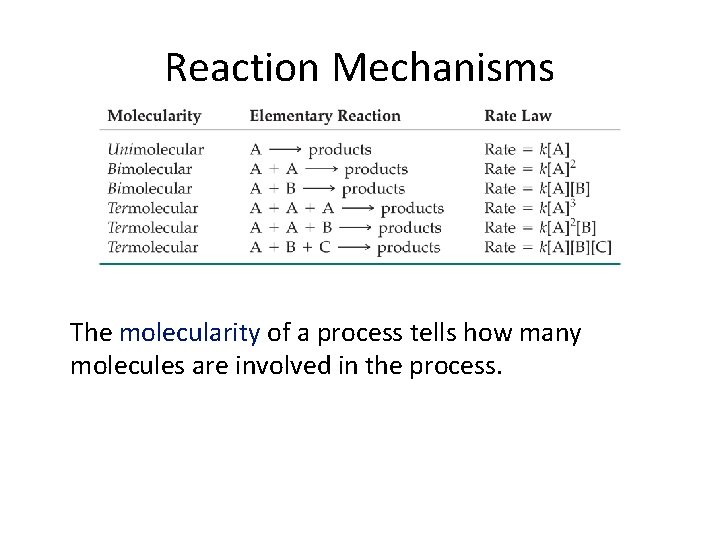
Reaction Mechanisms The molecularity of a process tells how many molecules are involved in the process.

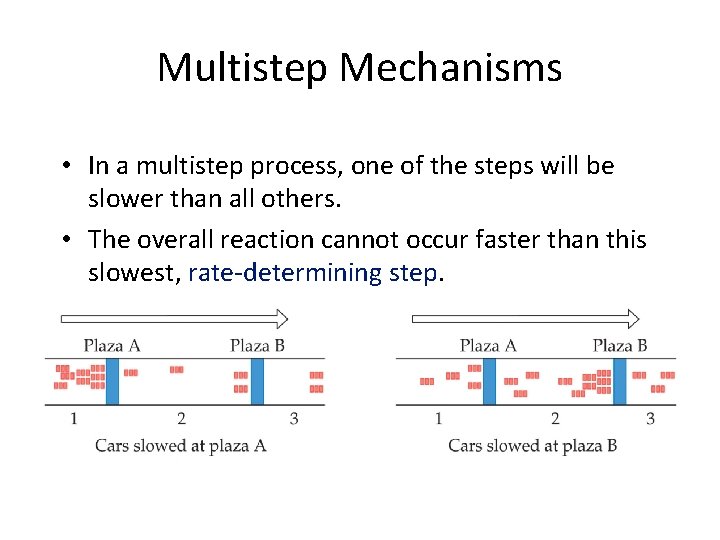
Multistep Mechanisms • In a multistep process, one of the steps will be slower than all others. • The overall reaction cannot occur faster than this slowest, rate-determining step.

• For reactions in which each elementary step is irreversible, the rate of the reaction is set by the slowest elementary step – Called the rate limiting step – Reaction intermediates, which are formed during the reaction but are not present in the overall reaction, play an important role in multistep reactions

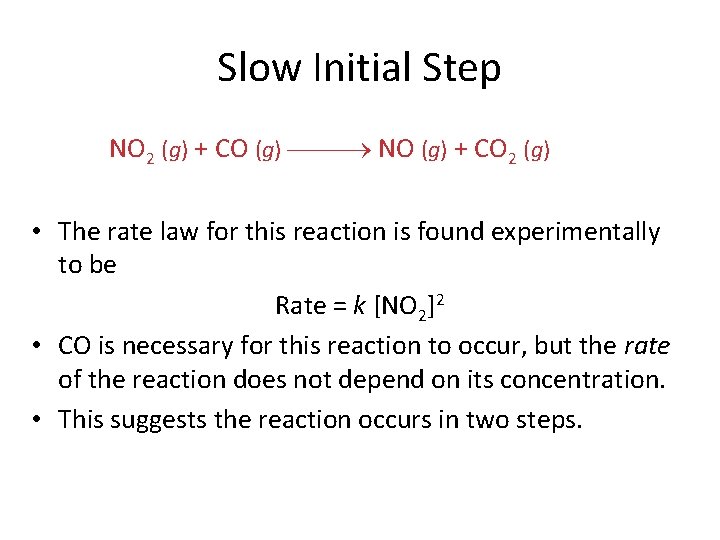
Slow Initial Step NO 2 (g) + CO (g) NO (g) + CO 2 (g) • The rate law for this reaction is found experimentally to be Rate = k [NO 2]2 • CO is necessary for this reaction to occur, but the rate of the reaction does not depend on its concentration. • This suggests the reaction occurs in two steps.

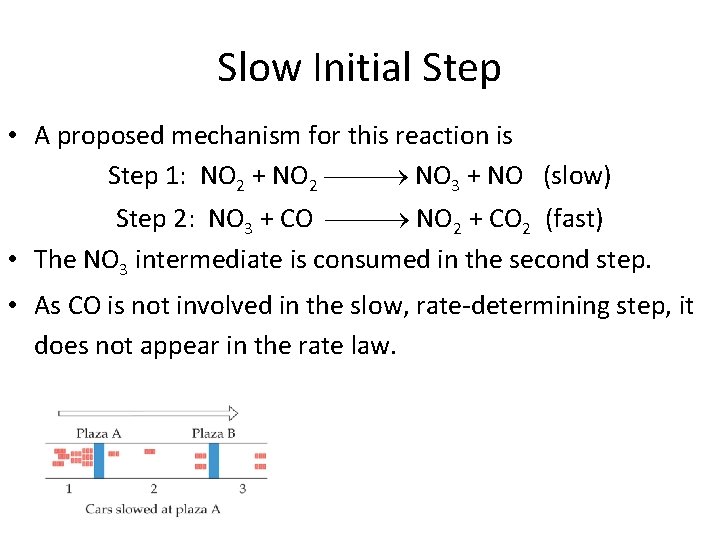
Slow Initial Step • A proposed mechanism for this reaction is Step 1: NO 2 + NO 2 NO 3 + NO (slow) Step 2: NO 3 + CO NO 2 + CO 2 (fast) • The NO 3 intermediate is consumed in the second step. • As CO is not involved in the slow, rate-determining step, it does not appear in the rate law.

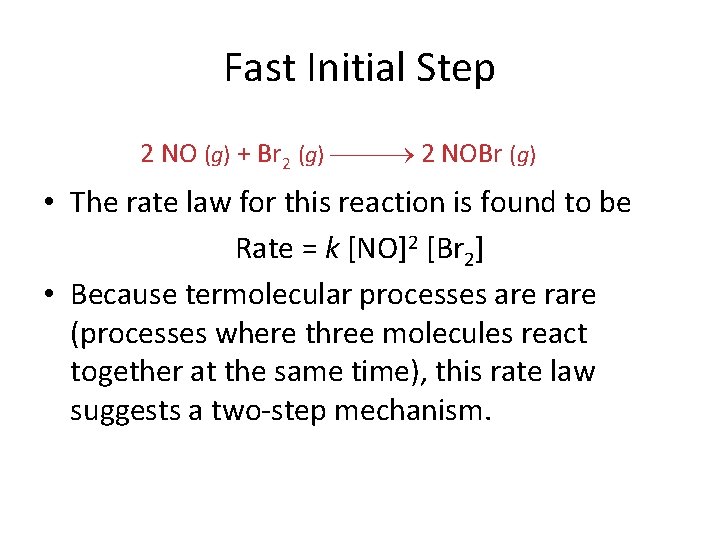
Fast Initial Step 2 NO (g) + Br 2 (g) 2 NOBr (g) • The rate law for this reaction is found to be Rate = k [NO]2 [Br 2] • Because termolecular processes are rare (processes where three molecules react together at the same time), this rate law suggests a two-step mechanism.

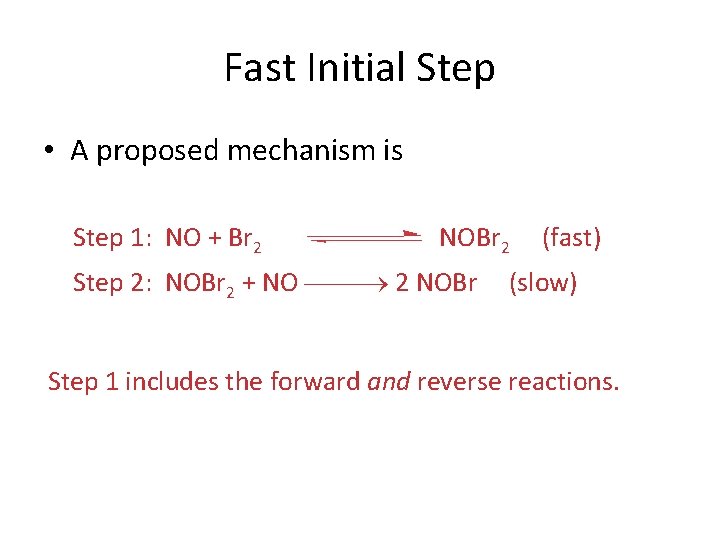
Fast Initial Step • A proposed mechanism is Step 1: NO + Br 2 NOBr 2 Step 2: NOBr 2 + NO 2 NOBr (fast) (slow) Step 1 includes the forward and reverse reactions.

Fast Initial Step • The rate of the overall reaction depends upon the rate of the slow step. • The rate law for that step would be Rate = k 2 [NOBr 2] [NO] • But how can we find [NOBr 2]?

Fast Initial Step • NOBr 2 can react two ways: – With NO to form NOBr – By decomposition to reform NO and Br 2 • The reactants and products of the first step are in equilibrium with each other. • Therefore, Ratef = Rater
![Fast Initial Step Because Ratef Rater k 1 NO Br 2 Fast Initial Step • Because Ratef = Rater , k 1 [NO] [Br 2]](https://slidetodoc.com/presentation_image_h2/fd1cd579c7826896b4a84ae1e25d1b28/image-13.jpg)
Fast Initial Step • Because Ratef = Rater , k 1 [NO] [Br 2] = k− 1 [NOBr 2] • Solving for [NOBr 2] gives us k 1 [NO] [Br 2] = [NOBr 2] k− 1
![Fast Initial Step Substituting this expression for NOBr 2 in the rate law for Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for](https://slidetodoc.com/presentation_image_h2/fd1cd579c7826896b4a84ae1e25d1b28/image-14.jpg)
Fast Initial Step Substituting this expression for [NOBr 2] in the rate law for the rate-determining step gives Rate = k 2 k 1 [NO] [Br 2] [NO] k− 1 = k [NO]2 [Br 2]

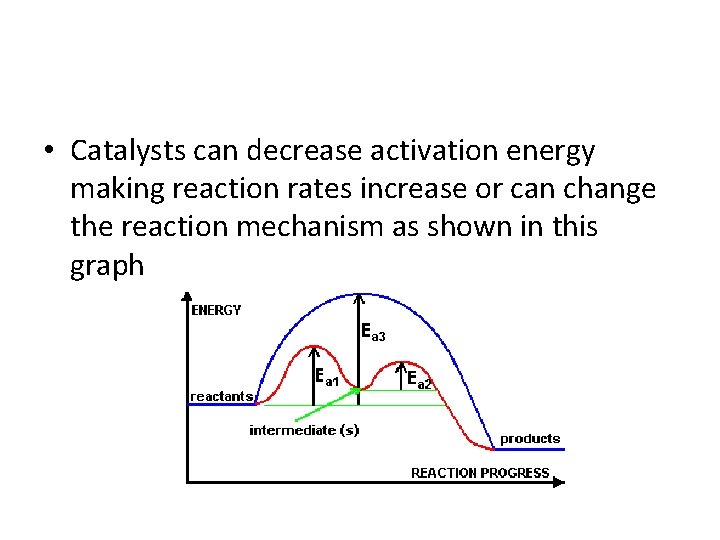
Reaction Coordinate (energy) diagrams • Shows the progress of a reaction from reactants to products it shows intermediate steps as well

• Catalysts can decrease activation energy making reaction rates increase or can change the reaction mechanism as shown in this graph