Kinetics Lesson 4 Reaction Mechanisms Reaction Mechanism Most


- Slides: 6

Kinetics Lesson #4 Reaction Mechanisms

Reaction Mechanism • Most chemical reactions do not happen in one step. A balanced equation shows us beginning and end only, but none of the intermediate-steps along the way. • We need to know the reaction mechanism to determine all of these steps. • Reaction mechanism – a series of elementary steps by which a chemical reaction occurs. • Elementary step – a step involving a one-, two-, or three-entity collision that cannot be explained by simpler reactions.

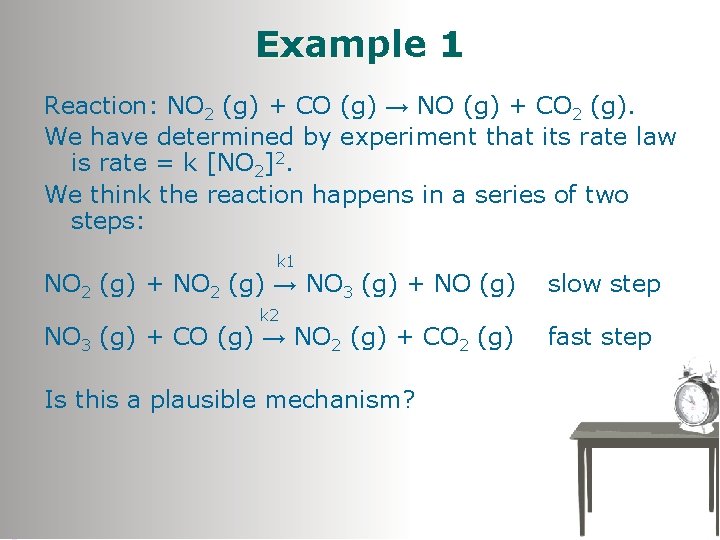
Example 1 Reaction: NO 2 (g) + CO (g) → NO (g) + CO 2 (g). We have determined by experiment that its rate law is rate = k [NO 2]2. We think the reaction happens in a series of two steps: k 1 NO 2 (g) + NO 2 (g) → NO 3 (g) + NO (g) k 2 NO 3 (g) + CO (g) → NO 2 (g) + CO 2 (g) Is this a plausible mechanism? slow step fast step

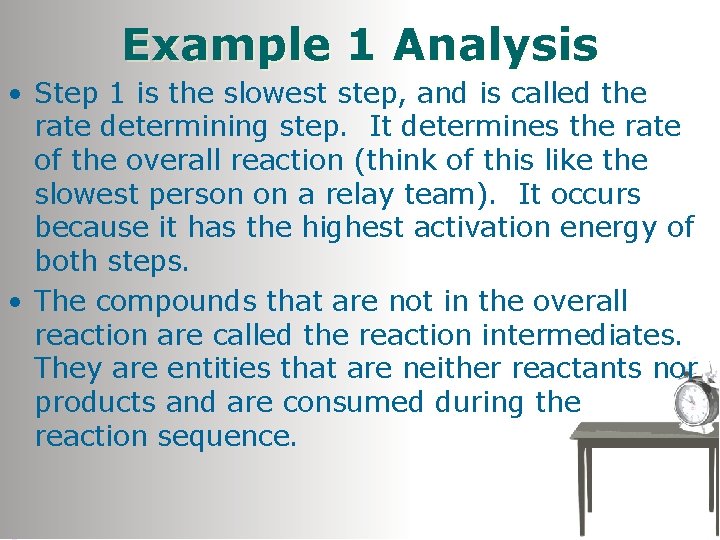
Example 1 Analysis • Step 1 is the slowest step, and is called the rate determining step. It determines the rate of the overall reaction (think of this like the slowest person on a relay team). It occurs because it has the highest activation energy of both steps. • The compounds that are not in the overall reaction are called the reaction intermediates. They are entities that are neither reactants nor products and are consumed during the reaction sequence.

Example 2 Nitryl fluoride gas can be made from nitrogen dioxide and fluorine: 2 NO 2 (g) + F 2 (g) → 2 NO 2 F (g). The experimentally determined rate law is rate = k[NO 2][F 2]. A suggested mechanism for the reaction is: NO 2 (g) + F 2 (g) → NO 2 F (g) + F (g) slow NO 2 (g) + F (g) → NO 2 F (g) fast Does this mechanism satisfy the two requirements for a plausible reaction mechanism?

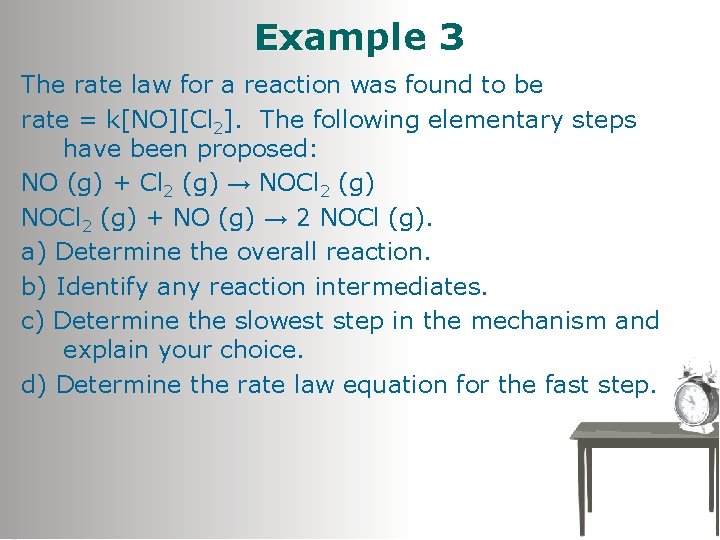
Example 3 The rate law for a reaction was found to be rate = k[NO][Cl 2]. The following elementary steps have been proposed: NO (g) + Cl 2 (g) → NOCl 2 (g) + NO (g) → 2 NOCl (g). a) Determine the overall reaction. b) Identify any reaction intermediates. c) Determine the slowest step in the mechanism and explain your choice. d) Determine the rate law equation for the fast step.