Fats Oils n n n AL Chemistry Structure






- Slides: 18

Fats & Oils n n n AL Chemistry Structure & Properties Hydrolysis of Fats & Oils Iodine Value Hardening of Vegetable Oil Hydrolytic & Oxidative Rancidity p. 1

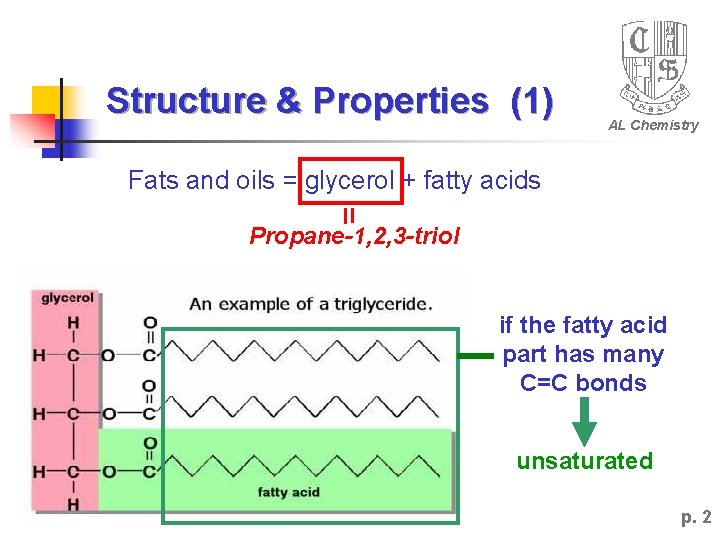
Structure & Properties (1) AL Chemistry Fats and oils = glycerol + fatty acids Propane-1, 2, 3 -triol if the fatty acid part has many C=C bonds unsaturated p. 2

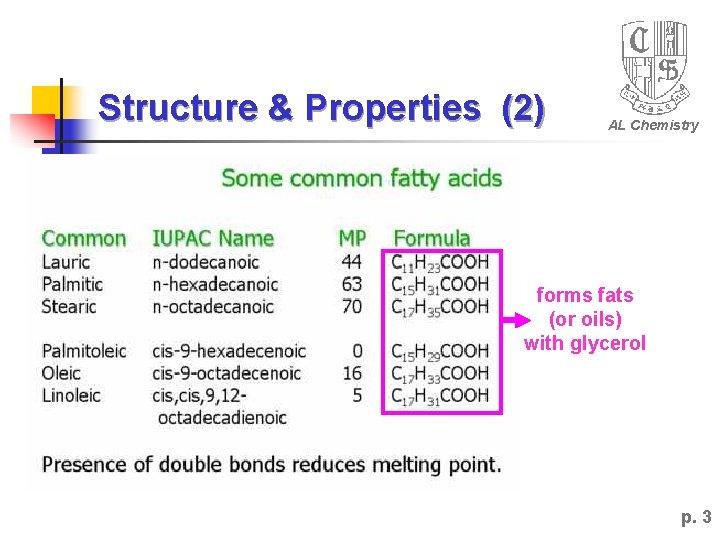
Structure & Properties (2) AL Chemistry forms fats (or oils) with glycerol p. 3

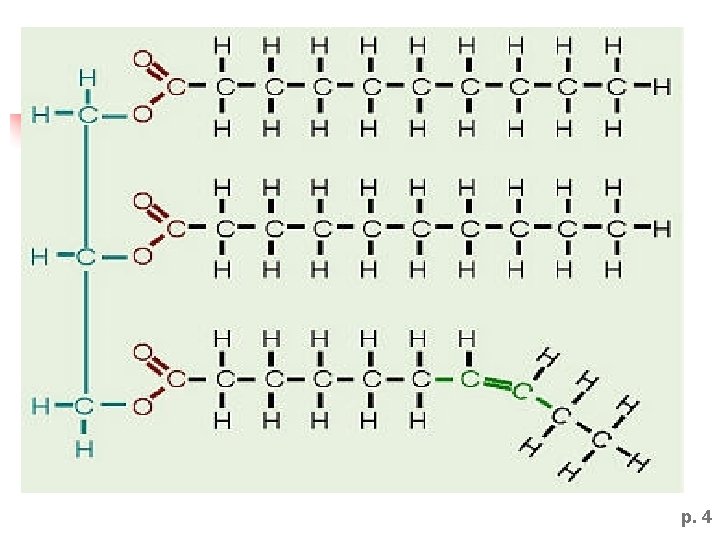
p. 4

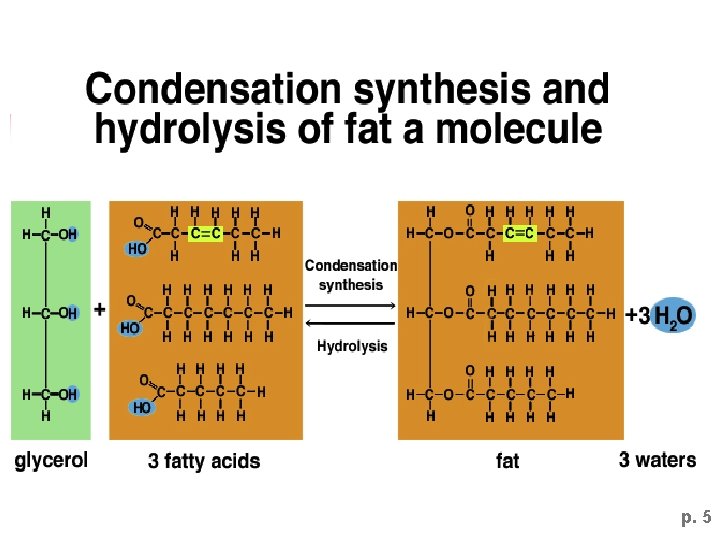
p. 5

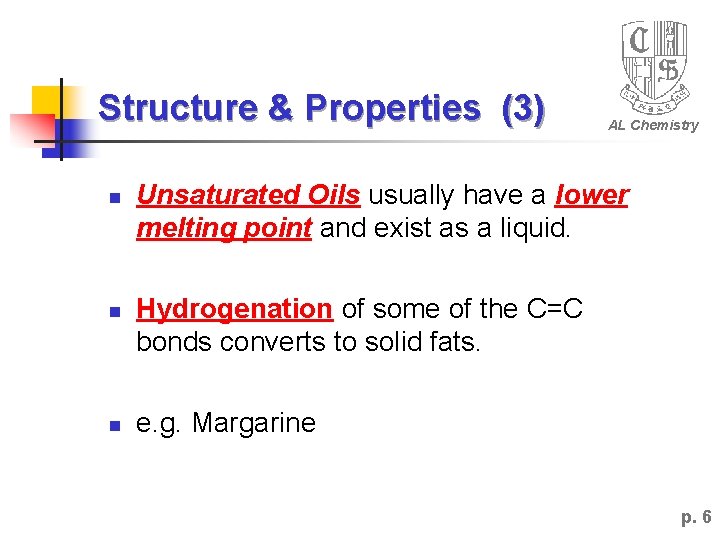
Structure & Properties (3) n n n AL Chemistry Unsaturated Oils usually have a lower melting point and exist as a liquid. Hydrogenation of some of the C=C bonds converts to solid fats. e. g. Margarine p. 6

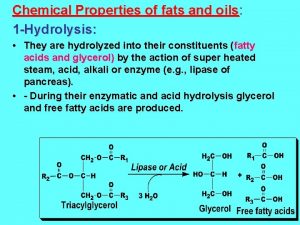
Hydrolysis of Fats & Oils AL Chemistry n Fats and oils are hydrolysed into carboxylic acid and glycerol in human body (acidic medium). n In lab, hydrolysis can be carried out in alkaline medium more effectively, to give carboxylate and glycerol. soap! (saponification) p. 7

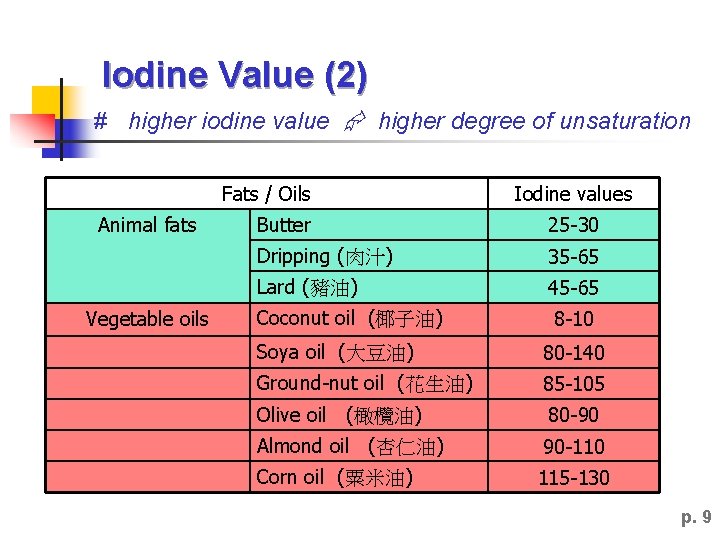
Iodine Value (1) AL Chemistry ► To measure the degree of unsaturation of a fat or vegetable oil. ► It is defined as the number of grams of iodine that reacts with 100 grams of fats/oils. ► Vegetable has a high iodine value than fats. p. 8

Iodine Value (2) # higher iodine value higher degree of unsaturation Fats / Oils Animal fats Vegetable oils Iodine values Butter 25 -30 Dripping (肉汁) 35 -65 Lard (豬油) 45 -65 Coconut oil (椰子油) 8 -10 Soya oil (大豆油) 80 -140 Ground-nut oil (花生油) 85 -105 Olive oil (橄欖油) 80 -90 Almond oil (杏仁油) 90 -110 Corn oil (粟米油) 115 -130 p. 9

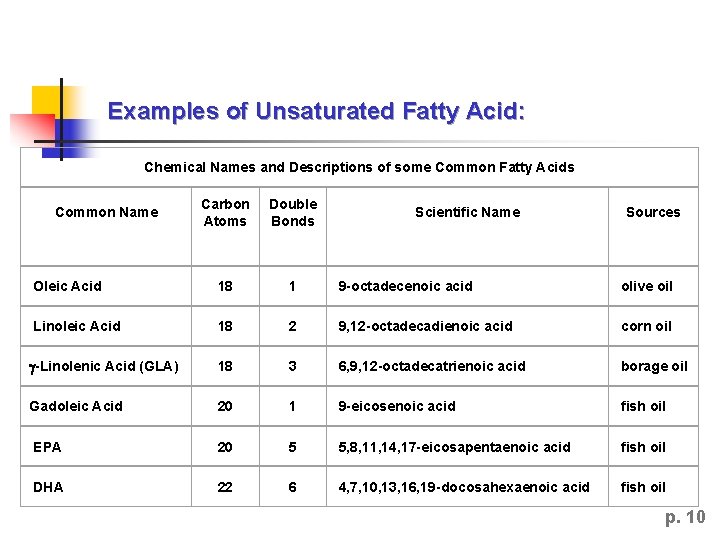
Examples of Unsaturated Fatty Acid: Chemical Names and Descriptions of some Common Fatty Acids Carbon Atoms Double Bonds Oleic Acid 18 1 9 -octadecenoic acid olive oil Linoleic Acid 18 2 9, 12 -octadecadienoic acid corn oil g-Linolenic Acid (GLA) 18 3 6, 9, 12 -octadecatrienoic acid borage oil Gadoleic Acid 20 1 9 -eicosenoic acid fish oil EPA 20 5 5, 8, 11, 14, 17 -eicosapentaenoic acid fish oil DHA 22 6 4, 7, 10, 13, 16, 19 -docosahexaenoic acid fish oil Common Name Scientific Name Sources p. 10

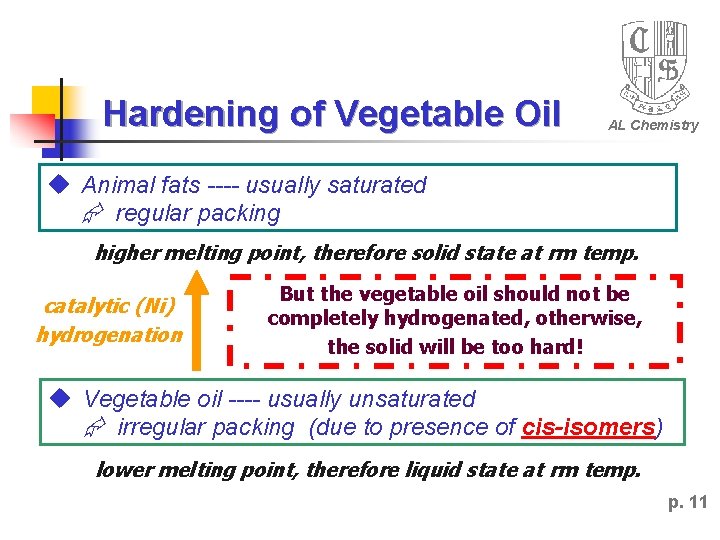
Hardening of Vegetable Oil AL Chemistry Animal fats ---- usually saturated regular packing higher melting point, therefore solid state at rm temp. catalytic (Ni) hydrogenation But the vegetable oil should not be completely hydrogenated, otherwise, the solid will be too hard! Vegetable oil ---- usually unsaturated irregular packing (due to presence of cis-isomers) lower melting point, therefore liquid state at rm temp. p. 11

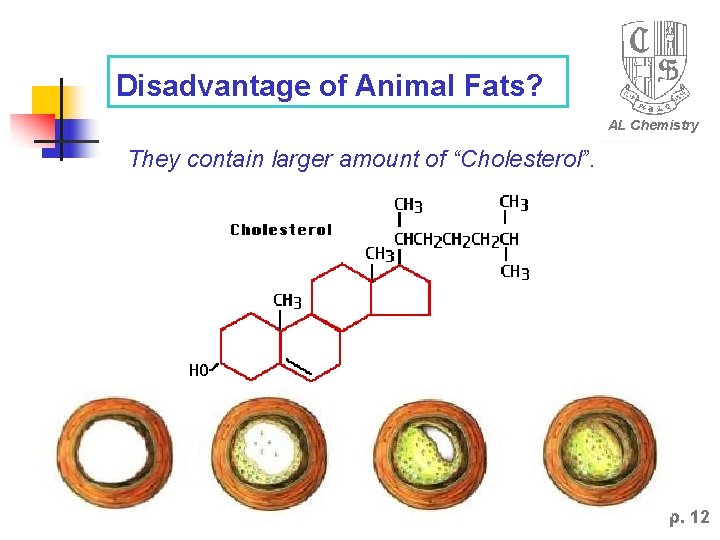
Disadvantage of Animal Fats? AL Chemistry They contain larger amount of “Cholesterol”. p. 12

Hydrolytic and Oxidative Rancidity (酸敗) of Fats & Oils: AL Chemistry Hydrolytic and Oxidative Reactions of triglyceride molecules give out unpleasant odour……. due to volatile and foul smelling RCHO & Fatty Acids Hydrolytic Rancidity occurs due to the presence of moisture in the oils. p. 13

Oxidative Rancidity (1) : AL Chemistry Oxidative Rancidity occurs under exposure to air (oxygen) Very common for those with high iodine value ► high degree of unsaturation --- more susceptible to oxidation! free radical mechanism!! p. 14

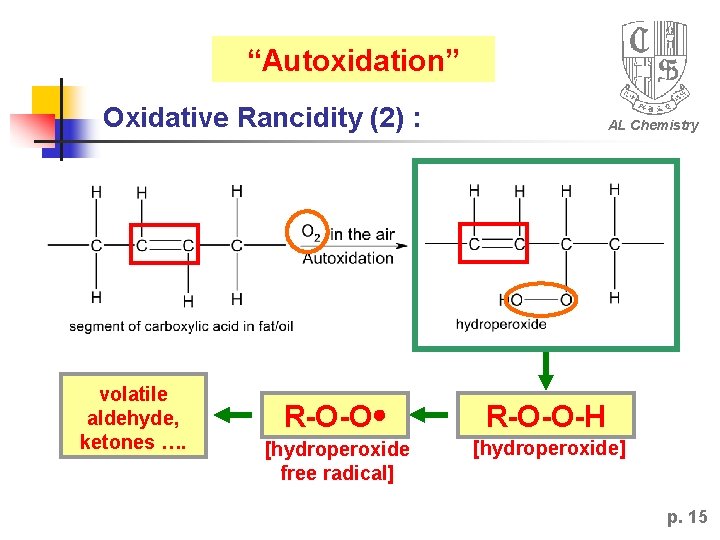
“Autoxidation” Oxidative Rancidity (2) : volatile aldehyde, ketones …. AL Chemistry R-O-O-H [hydroperoxide free radical] [hydroperoxide] p. 15

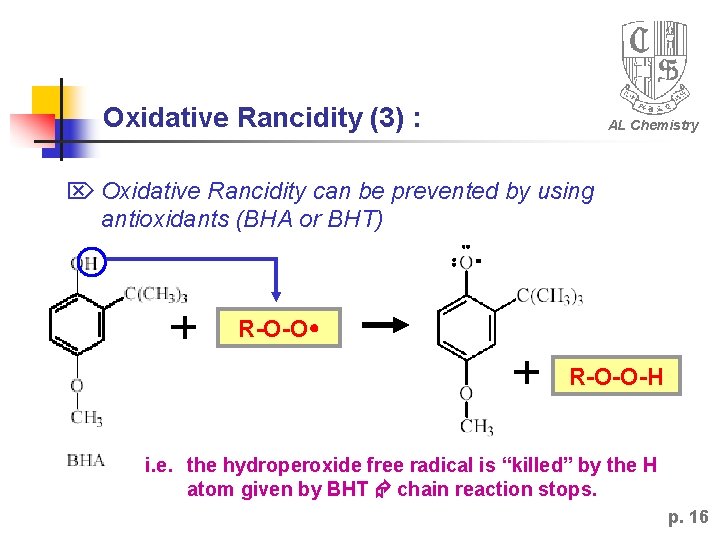
Oxidative Rancidity (3) : AL Chemistry Oxidative Rancidity can be prevented by using antioxidants (BHA or BHT) R-O-O-H i. e. the hydroperoxide free radical is “killed” by the H atom given by BHT chain reaction stops. p. 16

Oxidative Rancidity (4) : AL Chemistry If the triglyceride molecules do not contain any unsaturation carboxylic acid chain …. . no hydroperoxide will be generated, and therefore oxidative rancidity does not occur. p. 17

HKALE …. AL Chemistry 1997: Vegetable oil (iodine value, hydrogenation and saponification) 1998: Animal Fats and Vegetable oil (iodine value, hardening, saponification and rancidity) 2001: Vegetable oil [unsaturated oil] (principle of anti-rancidity by BHT) p. 18
 Compound lipids definition
Compound lipids definition Are lipids long term energy storage
Are lipids long term energy storage Unsaturated fats
Unsaturated fats Which biomolecule group includes fats oils and waxes
Which biomolecule group includes fats oils and waxes Fats spreads and oils
Fats spreads and oils Non polar molecules that include fats oils and cholesterol
Non polar molecules that include fats oils and cholesterol Volatile oils - pharmacognosy
Volatile oils - pharmacognosy Ketone volatile oils
Ketone volatile oils Brian peskin
Brian peskin New age oils
New age oils Essential oils
Essential oils Carmen bove
Carmen bove Hydrogenation of oils
Hydrogenation of oils Owensboro grain prices
Owensboro grain prices Section 8-1 carbohydrates fats and proteins answer key
Section 8-1 carbohydrates fats and proteins answer key Subunits of fat
Subunits of fat Simple fats
Simple fats Function of lipids
Function of lipids Fats quiz brainpop answers
Fats quiz brainpop answers