Reactions of Oils and Fats Reactions of Oils












- Slides: 34

Reactions of Oils and Fats

Reactions of Oils and Fats • Hydrolysis • Oxidation • Hydrogenation • Esterification

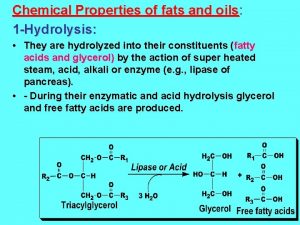
Hydrolysis • Chemical (Autocatalytic) • Enzymatical (Lipase) H 2 C OH 3 fatty acids O O O C - R 1 O O HO - C - R 1 HC OH + = HC O C- R 2 +3 H 20 HO - C - R 2 O H 2 C OH O glycerol H 2 C O C - R 3 R HO - C 3 triacylglycerol H 2 C

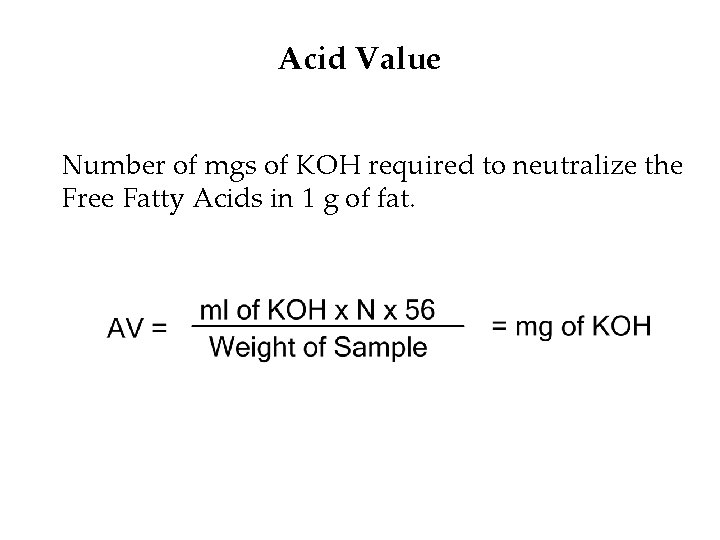
Acid Value Number of mgs of KOH required to neutralize the Free Fatty Acids in 1 g of fat.

Oxidation of Oils and Fats • The reaction of molecular oxygen with organic molecules has for long been a process of considerable interest. • Although a wide variety of organic molecules are susceptible to chemical attack by oxygen, a great deal of attention has recently been focused on lipids because of the remarkable implications of their oxidative damage.

Oxidation of Oils and Fats The results of the oxidation of fats and oils is the • development of objectionable flavors and odors characteristic of the condition known as “oxidative rancidity. ” • Loss of shelf-life, functionality and nutritional value. • Adverse health effects (carcinogenic)

Oxidation of Lipids • Autoxidation of Lipids is the oxidative deterioration of unsaturated fatty acids via an autocatalytic process consisting of a free radical chain mechanism. • The chain of reaction includes – Initiation – Propagation – Termination

What is Free radical? • A free radical is a group with an odd number of unpaired electrons. • They are extremely unstable and immediately react with another molecule to form stable substances.

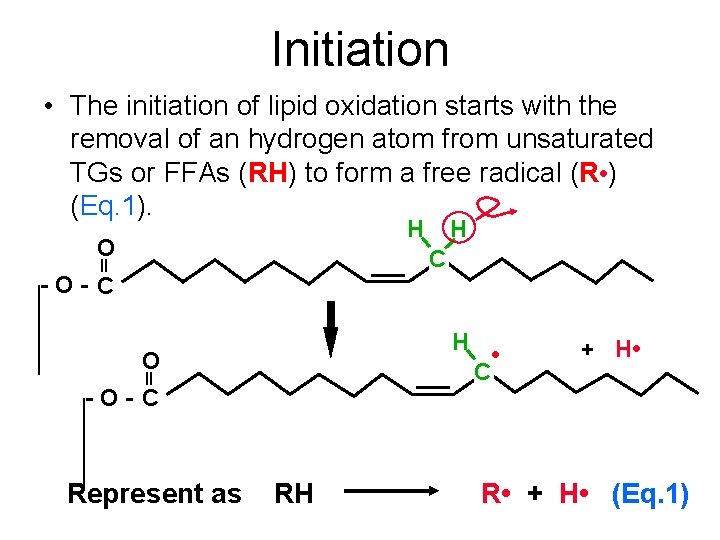
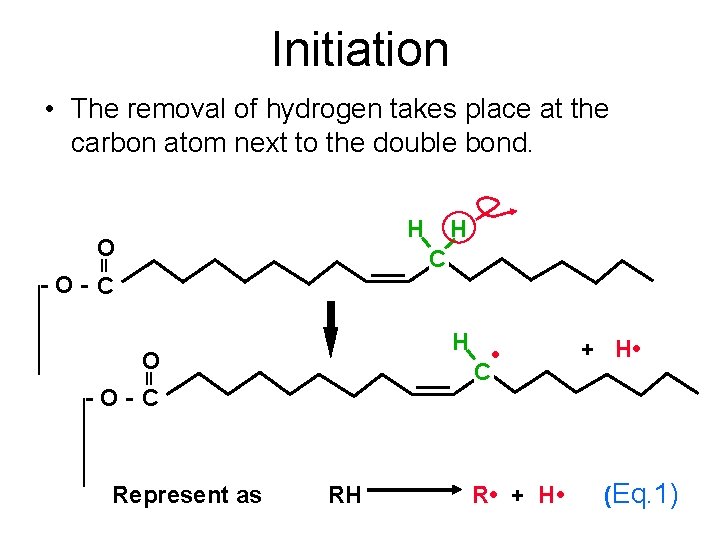
Initiation • The initiation of lipid oxidation starts with the removal of an hydrogen atom from unsaturated TGs or FFAs (RH) to form a free radical (R • ) (Eq. 1). H O H C -O- C H O -O- C Represent as RH • C + H • R • + H • (Eq. 1)

Initiation • The removal of hydrogen takes place at the carbon atom next to the double bond. H O H C -O- C H O -O- C Represent as RH • C R • + H • (Eq. 1)

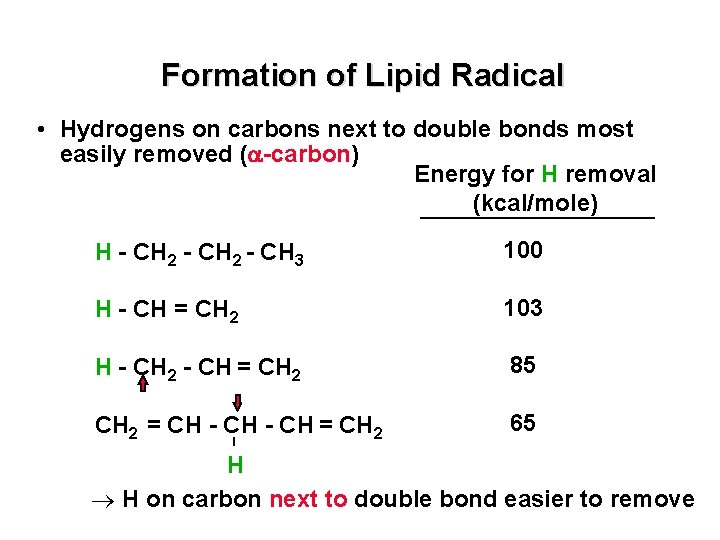
Formation of Lipid Radical • Hydrogens on carbons next to double bonds most easily removed ( -carbon) Energy for H removal (kcal/mole) H - CH 2 - CH 3 100 H - CH = CH 2 103 H - CH 2 - CH = CH 2 85 CH 2 = CH - CH = CH 2 65 H H on carbon next to double bond easier to remove

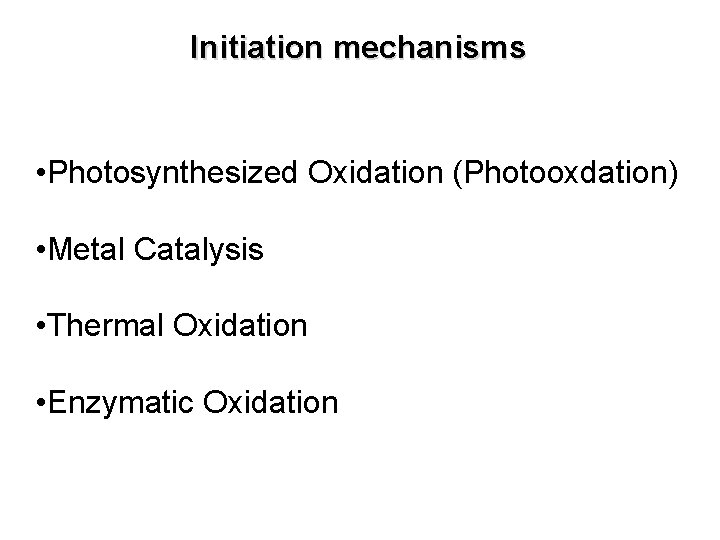
Initiation mechanisms • Photosynthesized Oxidation (Photooxdation) • Metal Catalysis • Thermal Oxidation • Enzymatic Oxidation

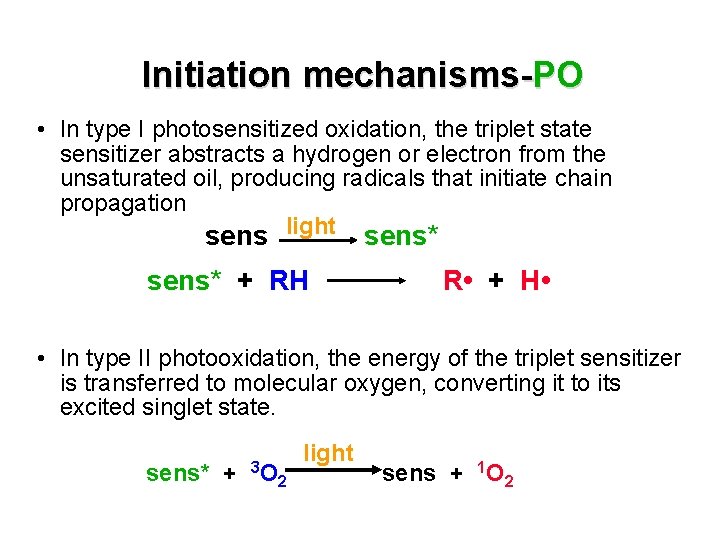
Initiation mechanisms-PO §Light, in the presence of oxygen, promotes oxidation of unsaturated fatty acids. §Photooxidation energy from light is captured aided by sensitizer molecules (pigments: chlorophile) • Light excites these sensitizers to the triplet state that promotes oxidation by type I and type II mechanisms.

Initiation mechanisms-PO • In type I photosensitized oxidation, the triplet state sensitizer abstracts a hydrogen or electron from the unsaturated oil, producing radicals that initiate chain propagation sens light sens* + RH R • + H • • In type II photooxidation, the energy of the triplet sensitizer is transferred to molecular oxygen, converting it to its excited singlet state. sens* + 3 O light 2 sens + 1 O 2

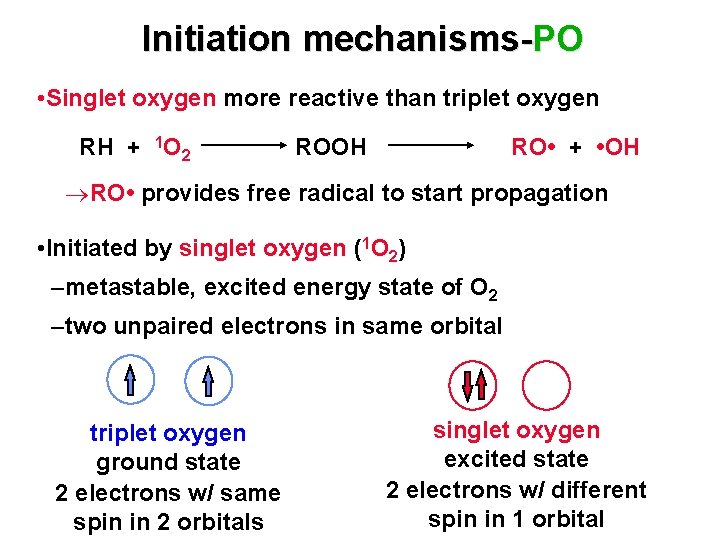
Initiation mechanisms-PO • Singlet oxygen more reactive than triplet oxygen RH + 1 O 2 ROOH RO • + • OH RO • provides free radical to start propagation • Initiated by singlet oxygen (1 O 2) –metastable, excited energy state of O 2 –two unpaired electrons in same orbital triplet oxygen ground state 2 electrons w/ same spin in 2 orbitals singlet oxygen excited state 2 electrons w/ different spin in 1 orbital

Initiation mechanisms-Metal Catalysis • Metal ions (e. g. Fe, Co, Cu) can also initiate reaction – found naturally in foods, from metal equipment RH + M+2 R • + H+ + M+

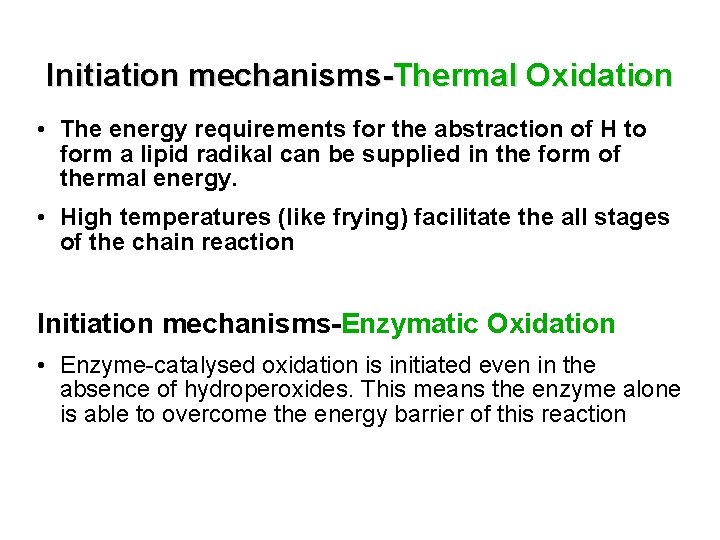
Initiation mechanisms-Thermal Oxidation • The energy requirements for the abstraction of H to form a lipid radikal can be supplied in the form of thermal energy. • High temperatures (like frying) facilitate the all stages of the chain reaction Initiation mechanisms-Enzymatic Oxidation • Enzyme-catalysed oxidation is initiated even in the absence of hydroperoxides. This means the enzyme alone is able to overcome the energy barrier of this reaction

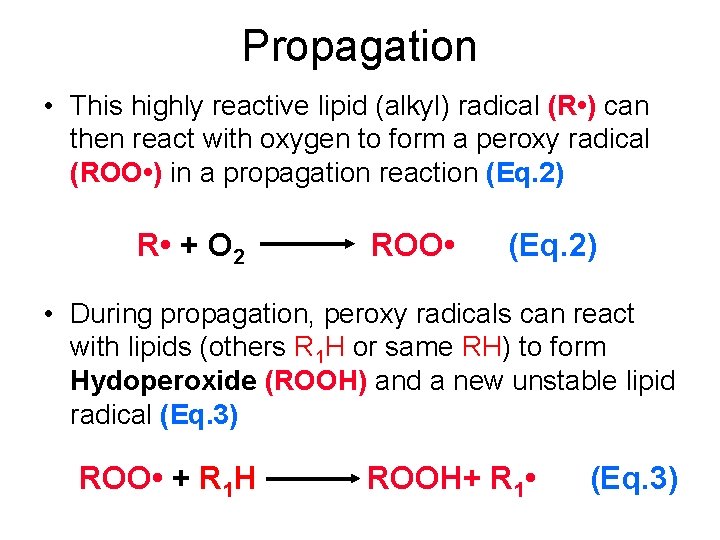
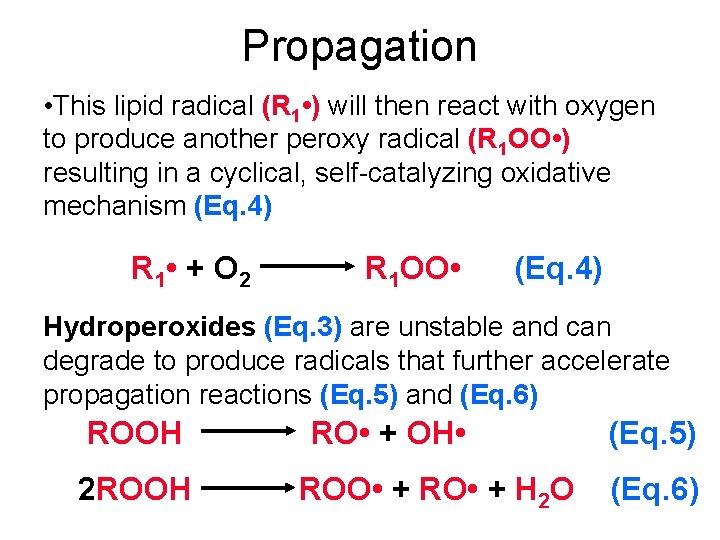
Propagation • This highly reactive lipid (alkyl) radical (R • ) can then react with oxygen to form a peroxy radical (ROO • ) in a propagation reaction (Eq. 2) R • + O 2 ROO • (Eq. 2) • During propagation, peroxy radicals can react with lipids (others R 1 H or same RH) to form Hydoperoxide (ROOH) and a new unstable lipid radical (Eq. 3) ROO • + R 1 H ROOH+ R 1 • (Eq. 3)

Propagation • This lipid radical (R 1 • ) will then react with oxygen to produce another peroxy radical (R 1 OO • ) resulting in a cyclical, self-catalyzing oxidative mechanism (Eq. 4) R 1 • + O 2 R 1 OO • (Eq. 4) Hydroperoxides (Eq. 3) are unstable and can degrade to produce radicals that further accelerate propagation reactions (Eq. 5) and (Eq. 6) ROOH 2 ROOH RO • + OH • ROO • + RO • + H 2 O (Eq. 5) (Eq. 6)

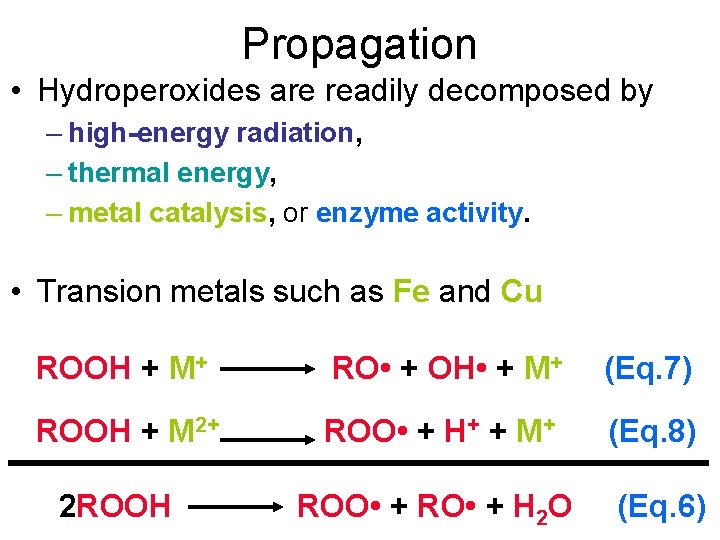
Propagation • Hydroperoxides are readily decomposed by – high-energy radiation, – thermal energy, – metal catalysis, or enzyme activity. • Transion metals such as Fe and Cu ROOH + M+ RO • + OH • + M+ (Eq. 7) ROOH + M 2+ ROO • + H+ + M+ (Eq. 8) ROO • + RO • + H 2 O (Eq. 6) 2 ROOH

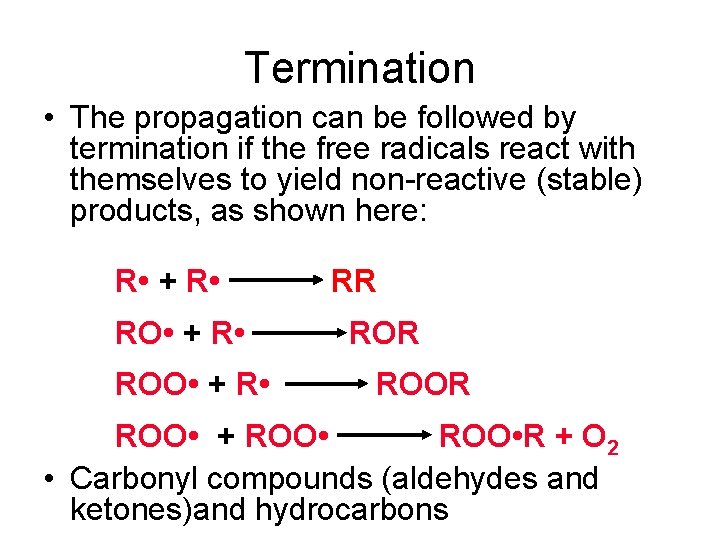
Termination • The propagation can be followed by termination if the free radicals react with themselves to yield non-reactive (stable) products, as shown here: R • + R • ROO • + R • RR ROOR ROO • + ROO • R + O 2 • Carbonyl compounds (aldehydes and ketones)and hydrocarbons

Pentane Formation from Linolenic Acid CH 3 14 (CH 2 )3 CH 2 13 CH 12 11 CH Initiation (metal) CH 3 (CH 2 )3 CH 2 (CH 2 )3 CH CH O CH 2 CH CH CH 12 11 CH CH n COOH 9 CH 10 9 CH CH CH 2 n COOH CH 2 n. COOH + H. 12 11 CH CH O O H CH 2 10 CH + O 2 . CH 2 11 CH O Hydroperoxide Decomposition CH 3 12 . CH 2 Propagation CH 3 CH - H. Propagation CH 3 9 10 CH 2 _ 12 CH 10 CH 9 CH- CH 2 n COOH . OH 11 10 CH 9 CH CH CH 2 n COOH . O CH 3 (CH 2 )3 CH. 2 O + H C 12 11 10 9 CH CH . Termination + H CH 3 (CH 2 )3 Pentane CH 3 CH 2 n COOH

Oxidation Product • Primary Oxidation Products – Hydroperoxides • Secondary Oxidation Products – Aldehydes and ketones

Factors Affecting Autoxidation 1. Energy in the form of heat and light 2. Catalysts (Metal) 3. Double bonds 4. Enzymes 5. Chemical oxidants 6. Oxygen content and types of oxygen 7. Natural antioxidants 8. Phospholipids 9. Free Fatty acids

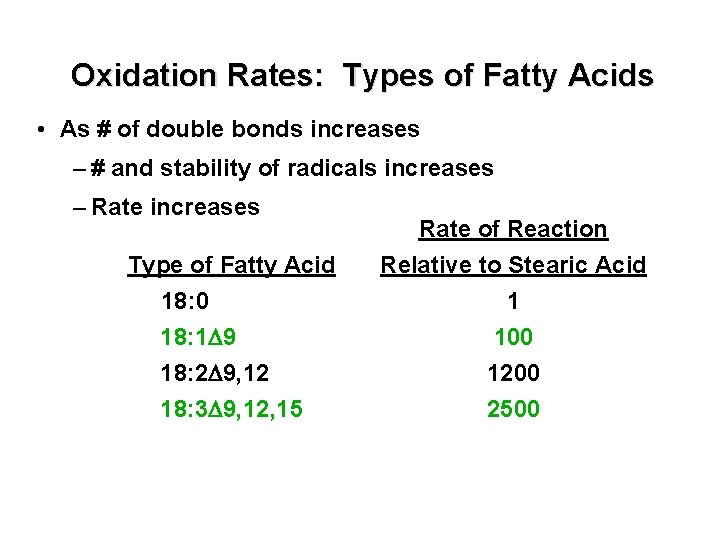
Oxidation Rates: Types of Fatty Acids • As # of double bonds increases – # and stability of radicals increases – Rate increases Type of Fatty Acid 18: 0 18: 1 D 9 18: 2 D 9, 12 18: 3 D 9, 12, 15 Rate of Reaction Relative to Stearic Acid 1 100 1200 2500

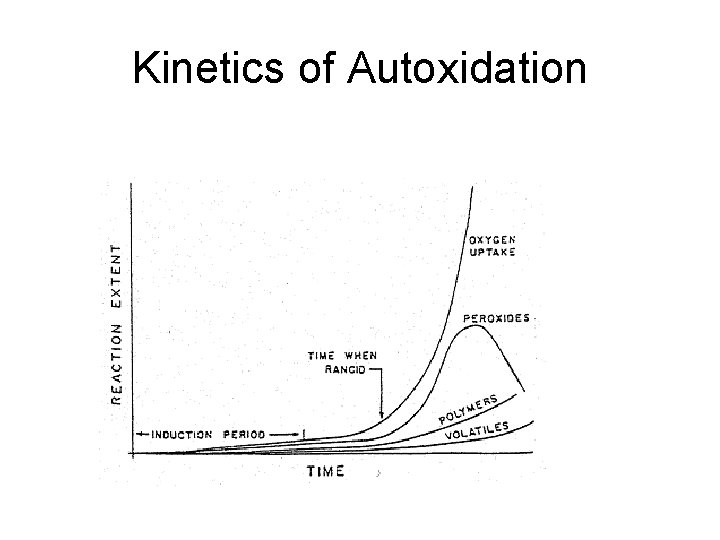
Kinetics of Autoxidation

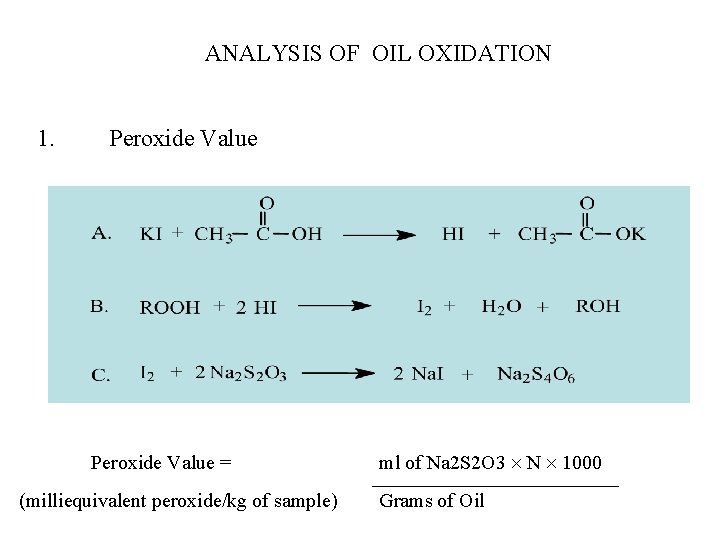
ANALYSIS OF OIL OXIDATION 1. Peroxide Value = (milliequivalent peroxide/kg of sample) ml of Na 2 S 2 O 3 N 1000 Grams of Oil

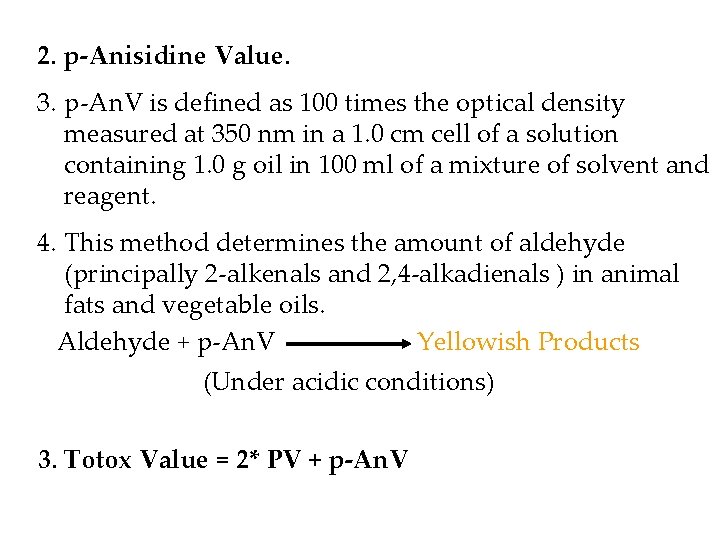
2. p-Anisidine Value. 3. p-An. V is defined as 100 times the optical density measured at 350 nm in a 1. 0 cm cell of a solution containing 1. 0 g oil in 100 ml of a mixture of solvent and reagent. 4. This method determines the amount of aldehyde (principally 2 -alkenals and 2, 4 -alkadienals ) in animal fats and vegetable oils. Aldehyde + p-An. V Yellowish Products (Under acidic conditions) 3. Totox Value = 2* PV + p-An. V

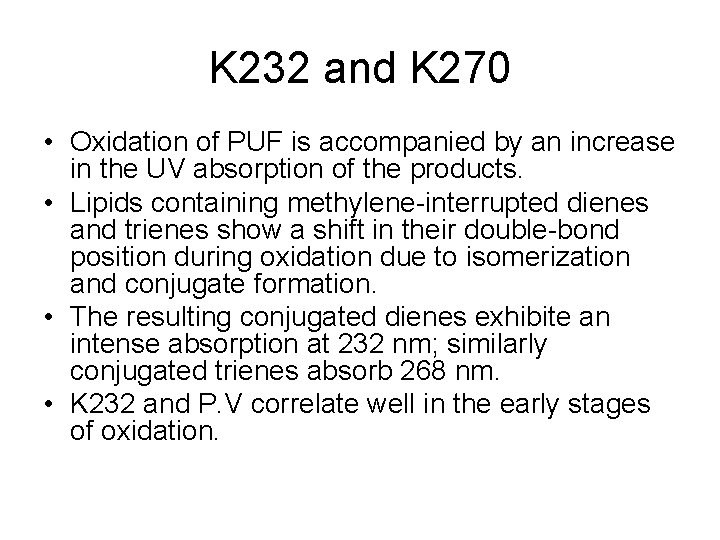
K 232 and K 270 • Oxidation of PUF is accompanied by an increase in the UV absorption of the products. • Lipids containing methylene-interrupted dienes and trienes show a shift in their double-bond position during oxidation due to isomerization and conjugate formation. • The resulting conjugated dienes exhibite an intense absorption at 232 nm; similarly conjugated trienes absorb 268 nm. • K 232 and P. V correlate well in the early stages of oxidation.

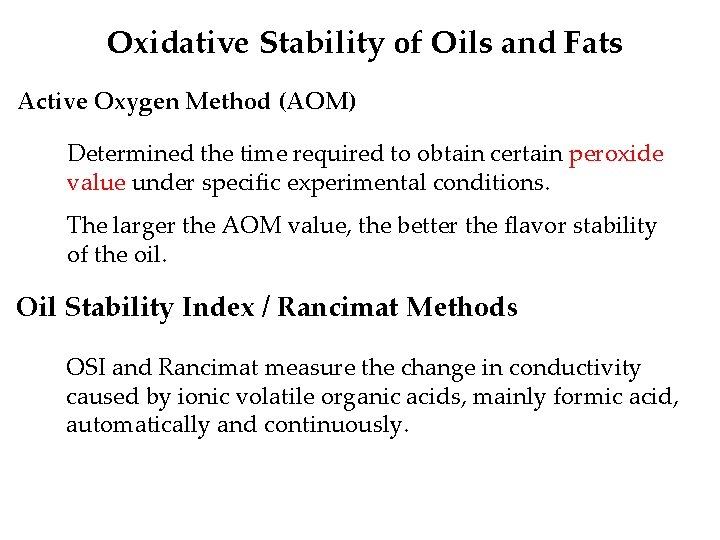
Oxidative Stability of Oils and Fats Active Oxygen Method (AOM) Determined the time required to obtain certain peroxide value under specific experimental conditions. The larger the AOM value, the better the flavor stability of the oil. Oil Stability Index / Rancimat Methods OSI and Rancimat measure the change in conductivity caused by ionic volatile organic acids, mainly formic acid, automatically and continuously.

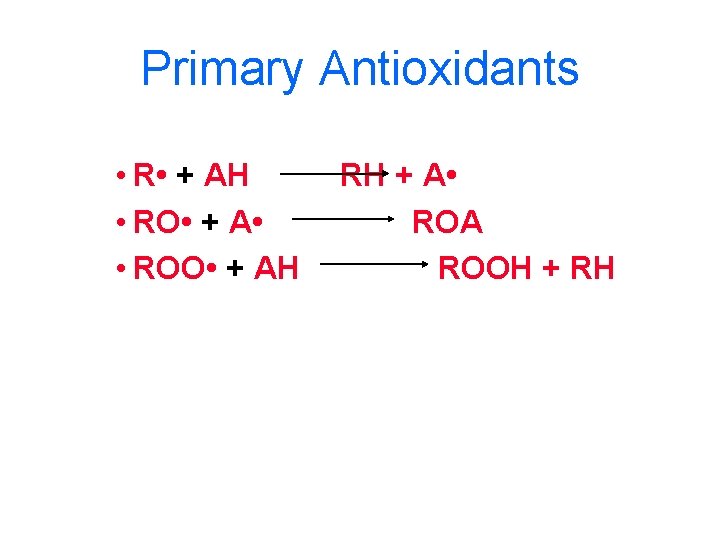
Antioxidants • Primary Antioxidants – Chain-breaking antioxidants are free radical acceptors that delay or inhibite the initiation step or interrupt the propagation step of autoxidation. • Secondary Antioxidants – Act through numerous possible mechanisms, but they do not convert free radicals to more stable products.

Primary Antioxidants • R • + AH • RO • + A • • ROO • + AH RH + A • ROA ROOH + RH

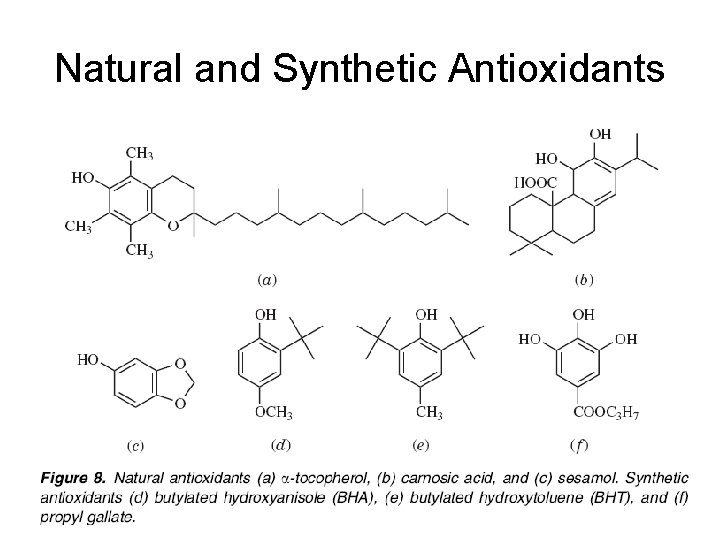
Natural and Synthetic Antioxidants

Secondary Antioxidants • Chelators: citric acid, EDTA • Oxygen Scavengers and Reducing Agents: Ascorbic acid, ascorbyl palmitate, • Singlet Oxygen Quenchers: Caretenoids (beta-carotene, lycopene, lutein) – Deplete singlet oxygen’s excess energy and dissipate the energy in the form of heat.
 Are lipids long term energy storage
Are lipids long term energy storage Fats, oils, and waxes
Fats, oils, and waxes Rancidity meaning
Rancidity meaning Biomolecule
Biomolecule Fats spreads and oils
Fats spreads and oils Non polar molecules that include fats oils and cholesterol
Non polar molecules that include fats oils and cholesterol Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Redox reaction examples
Redox reaction examples Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Section 8-1 carbohydrates fats and proteins answer key
Section 8-1 carbohydrates fats and proteins answer key Saturated vs unsaturated fatty acids
Saturated vs unsaturated fatty acids Section 8-1 carbohydrates fats and proteins answer key
Section 8-1 carbohydrates fats and proteins answer key Venn diagram of saturated and unsaturated fats
Venn diagram of saturated and unsaturated fats Fats and lipids
Fats and lipids Solid fats and added sugars
Solid fats and added sugars Fats and lipids
Fats and lipids Venn diagram saturated and unsaturated fats
Venn diagram saturated and unsaturated fats Diet chart for diabetes
Diet chart for diabetes What do carbohydrates and fats have in common brainpop
What do carbohydrates and fats have in common brainpop Use of volatile oils in pharmacy
Use of volatile oils in pharmacy Volatile oils pharmacognosy
Volatile oils pharmacognosy Professor brian peskin
Professor brian peskin New age oils
New age oils Thieves cleaner packets
Thieves cleaner packets Dr mary bove
Dr mary bove Hydrogenation of oils
Hydrogenation of oils Owensboro grain prices
Owensboro grain prices Cholesterol hydrophobic or hydrophilic
Cholesterol hydrophobic or hydrophilic Waxes
Waxes Lipids 3 functions
Lipids 3 functions Sudan iv test
Sudan iv test Fats meaning
Fats meaning The most concentrated source of energy is
The most concentrated source of energy is