UNIT 3 LIPIDS Occurrence of Lipids Fat depots












- Slides: 27

UNIT 3 LIPIDS

Occurrence of Lipids? • Fat depots (large amount of fats): – Subcutaneous tissues – Mesenteric tissues – Fatty tissues around the kidney – Yellow bone marrow • Food sources: – Milk, Egg, Meat, Liver 2

OBJECTIVES • Define lipids and its occurrence • State the biological significance of fats • • Define chemical composition of fats Define physical properties of fats Define chemical properties of fats Classify lipids into fatty acids, triglycerides, steroids Define phospholipids Describe the chemistry and functions of cholesterol Explain lipoproteins 3

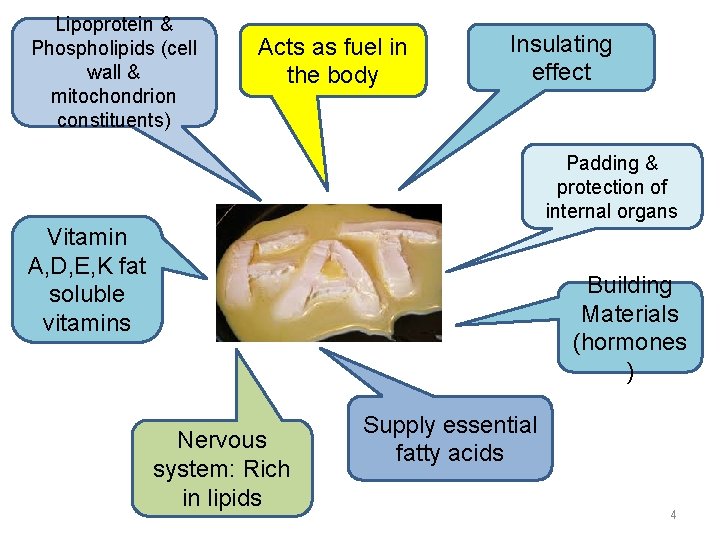
Lipoprotein & Phospholipids (cell wall & mitochondrion constituents) Acts as fuel in the body Insulating effect Padding & protection of internal organs Vitamin A, D, E, K fat soluble vitamins Building Materials (hormones ) Nervous system: Rich in lipids Supply essential fatty acids 4

OBJECTIVES • • Define lipids and its occurrence State the biological significance of fats • Define chemical composition of fats • • • Define physical properties of fats Define chemical properties of fats Classify lipids into fatty acids, triglycerides, steroids Define phospholipids Describe the chemistry and functions of cholesterol Explain lipoproteins 5

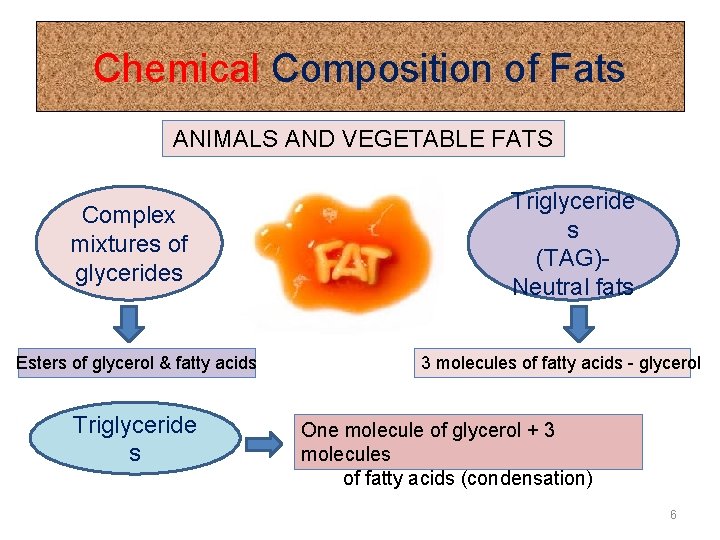
Chemical Composition of Fats ANIMALS AND VEGETABLE FATS Complex mixtures of glycerides Esters of glycerol & fatty acids Triglyceride s (TAG)Neutral fats 3 molecules of fatty acids - glycerol One molecule of glycerol + 3 molecules of fatty acids (condensation) 6

Physical Properties of Fats • Greasy to touch and leaves an oily impression on paper. • Are insoluble in water but soluble in organic solvents. • Have less specific gravity than water (solid fat= 0. 86), (liquid fat = 0. 95) • Pure glycerides are tasteless, odorless, colorless and neutral in reaction (acidicyellow color (hydrolysis & oxidation) 7

Physical Properties of Fats • Flavor of butter is due to the presence of bacterial flora; color of butter, human fat and egg yolk (due to presence of carotene & xanthophil). • Hardness and consistency depends on the amount of saturated and unsaturated fatty acids present. Saturated fatty acids are solid (room temperature) while Unsaturated fatty acids are liquid (room temperature) (e. g. oils) 8

Physical Properties of Fats • Fats have definite melting points. • When liquid fat is placed on water- it spreads uniformly over the surface of water. If the quantity is small – it forms a layer of 1 molecule thickness (effect: to lower surface tension- help transport fat) • Though fat is insoluble in water- can be broken down into minute droplets and dispersed in water (emulsification) 9

OBJECTIVES • • Define lipids and its occurrence State the biological significance of fats Define chemical composition of fats Define physical properties of fats • Define chemical properties of fats • • Classify lipids into fatty acids, triglycerides, steroids Define phospholipids Describe the chemistry and functions of cholesterol Explain lipoproteins 10

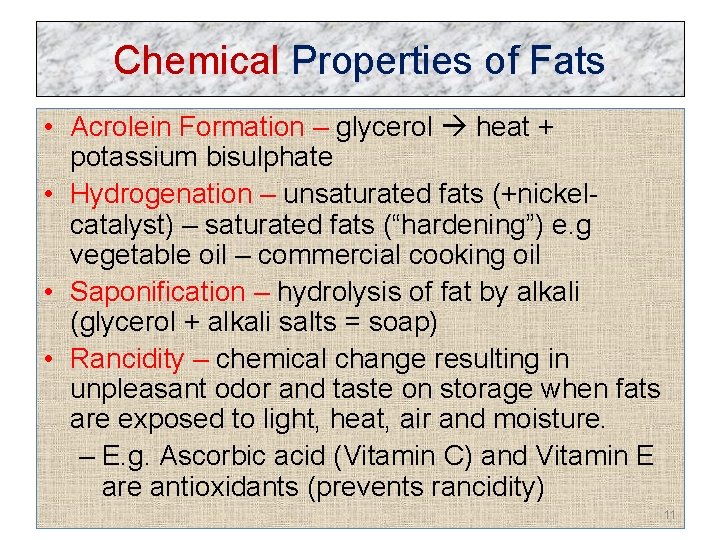
Chemical Properties of Fats • Acrolein Formation – glycerol heat + potassium bisulphate • Hydrogenation – unsaturated fats (+nickelcatalyst) – saturated fats (“hardening”) e. g vegetable oil – commercial cooking oil • Saponification – hydrolysis of fat by alkali (glycerol + alkali salts = soap) • Rancidity – chemical change resulting in unpleasant odor and taste on storage when fats are exposed to light, heat, air and moisture. – E. g. Ascorbic acid (Vitamin C) and Vitamin E are antioxidants (prevents rancidity) 11

OBJECTIVES • • • Define lipids and its occurrence State the biological significance of fats Define chemical composition of fats Define physical properties of fats Define chemical properties of fats • Classify lipids into fatty acids, triglycerides, steroids • • • Define phospholipids Describe the chemistry and functions of cholesterol Explain lipoproteins 12

Essential Fatty Acids (Polyunsaturated Fatty Acids) Lipids Examples • Linoleic acid Polyunsaturated Fatty Acids • Linolenic acid Sources • Linseed • Cotton seeds • Peanuts • Corn oils • Arachidonic acid (not synthesized by the body- must be taken in the diet) • Linoleic acid – the only fatty acid which is absolutely indispensable. 13

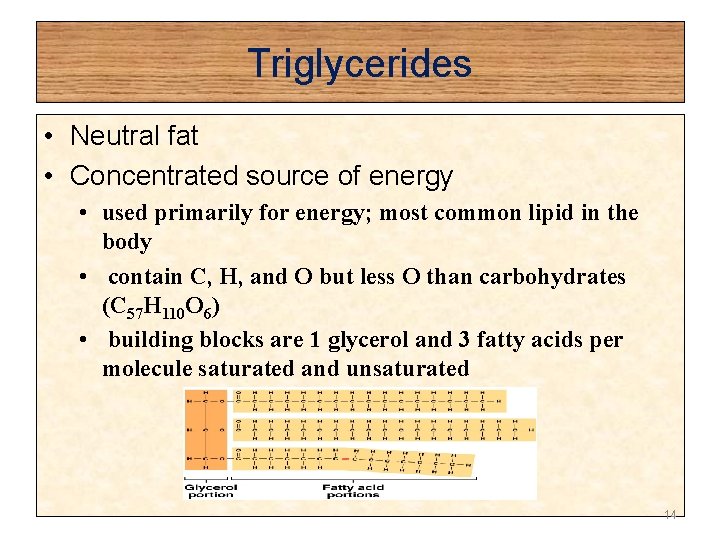
Triglycerides • Neutral fat • Concentrated source of energy • used primarily for energy; most common lipid in the body • contain C, H, and O but less O than carbohydrates (C 57 H 110 O 6) • building blocks are 1 glycerol and 3 fatty acids per molecule saturated and unsaturated 14

What are Steroids? • Are non-saponifiable lipids • Are biological compounds with diverse physiological activities • Are compounds having a cyclopentanoperhydrophenanthrene ring system • Has only a hydroxyl group (-OH) as its functional group (sterol, e. g. cholesterol) 15

OBJECTIVES • • • Define lipids and its occurrence State the biological significance of fats Define chemical composition of fats Define physical properties of fats Define chemical properties of fats Classify lipids into fatty acids, triglycerides, steroids • Define phospholipids • • Describe the chemistry and functions of cholesterol Explain lipoproteins 16

What are Phospholipids? • Lipids containing phosphorus • Are good emulsifying agents • Found in cell membranes and in subcellular structures (lipid & water interaction) 17

OBJECTIVES • • Define lipids and its occurrence State the biological significance of fats Define chemical composition of fats Define physical properties of fats Define chemical properties of fats Classify lipids into fatty acids, triglycerides, steroids Define phospholipids • Describe the chemistry and functions of cholesterol • Explain lipoproteins 18

What is Cholesterol? • Are light yellow crystalline solid • Are soluble in chloroform and other fat solvents • Polyunsaturated acids – lower the plasma cholesterol level • The most abundant lipid in the human body Are synthesized in the liver, adrenal cortex, intestines, testes and skin. Play an important role as a component of biomembranes and has a modulating effect on the fluid state of the membrane. Can be estimated by color reactions (e. g. Liebermann. Burchard reaction) – blue or green color 19

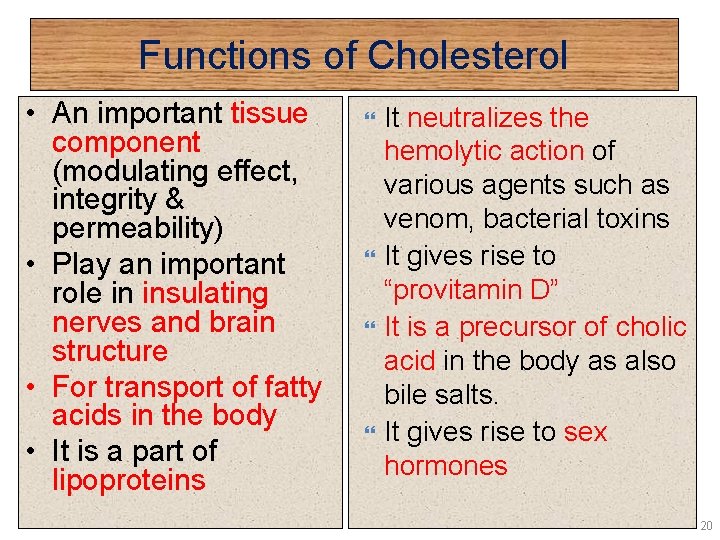
Functions of Cholesterol • An important tissue component (modulating effect, integrity & permeability) • Play an important role in insulating nerves and brain structure • For transport of fatty acids in the body • It is a part of lipoproteins It neutralizes the hemolytic action of various agents such as venom, bacterial toxins It gives rise to “provitamin D” It is a precursor of cholic acid in the body as also bile salts. It gives rise to sex hormones 20

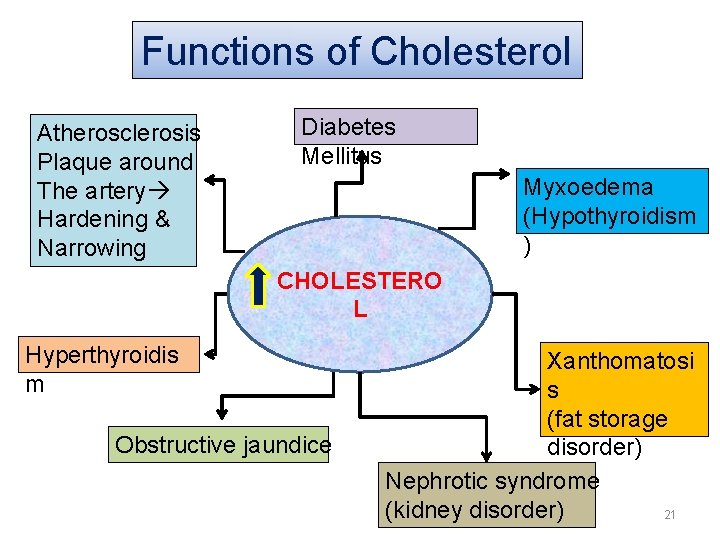
Functions of Cholesterol Atherosclerosis Plaque around The artery Hardening & Narrowing Diabetes Mellitus Myxoedema (Hypothyroidism ) CHOLESTERO L Hyperthyroidis m Obstructive jaundice Xanthomatosi s (fat storage disorder) Nephrotic syndrome (kidney disorder) 21

OBJECTIVES • • Define lipids and its occurrence State the biological significance of fats Define chemical composition of fats Define physical properties of fats Define chemical properties of fats Classify lipids into fatty acids, triglycerides, steroids Define phospholipids Describe the chemistry and functions of cholesterol • Explain lipoproteins 22

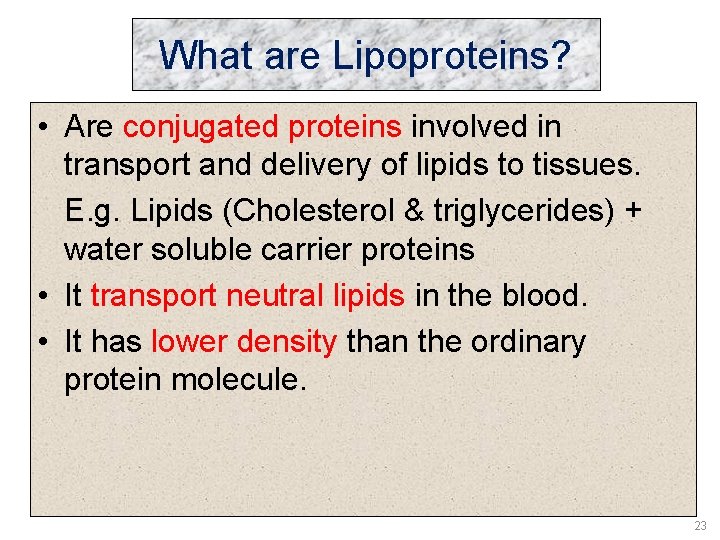
What are Lipoproteins? • Are conjugated proteins involved in transport and delivery of lipids to tissues. E. g. Lipids (Cholesterol & triglycerides) + water soluble carrier proteins • It transport neutral lipids in the blood. • It has lower density than the ordinary protein molecule. 23

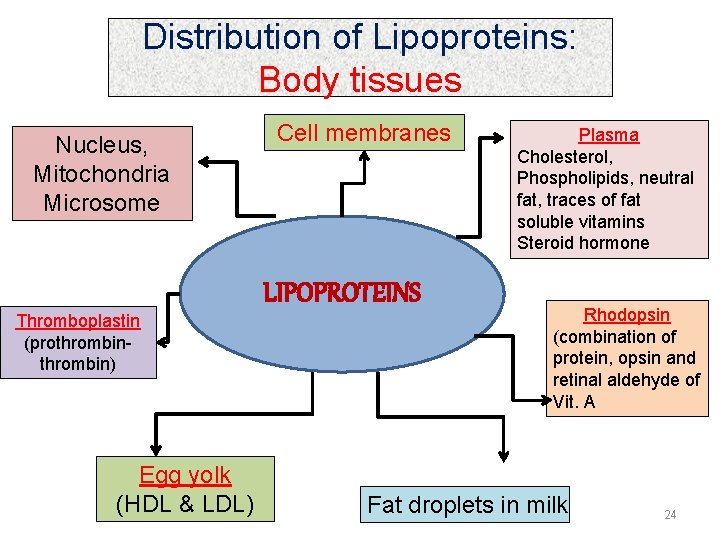
Distribution of Lipoproteins: Body tissues Nucleus, Mitochondria Microsome Thromboplastin (prothrombin) Egg yolk (HDL & LDL) Cell membranes LIPOPROTEINS Plasma Cholesterol, Phospholipids, neutral fat, traces of fat soluble vitamins Steroid hormone Rhodopsin (combination of protein, opsin and retinal aldehyde of Vit. A Fat droplets in milk 24

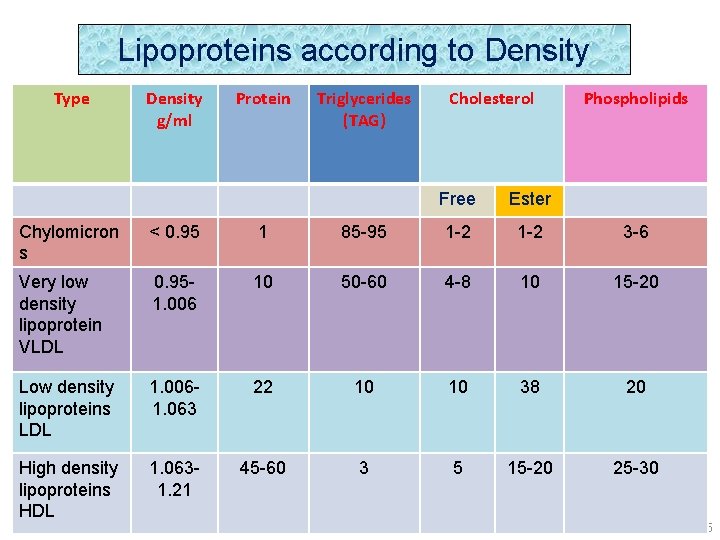
Lipoproteins according to Density Type Density g/ml Protein Triglycerides (TAG) Cholesterol Free Ester Phospholipids Chylomicron s < 0. 95 1 85 -95 1 -2 3 -6 Very low density lipoprotein VLDL 0. 951. 006 10 50 -60 4 -8 10 15 -20 Low density lipoproteins LDL 1. 0061. 063 22 10 10 38 20 High density lipoproteins HDL 1. 0631. 21 45 -60 3 5 15 -20 25 -30 25

EVALUATION • • • Give at least 3 importance of lipids. What are the 4 major groups of lipids? Give 2 examples of lipids? What are the functions of cholesterol? Differentiate steroid from cholesterol? Name 3 physical properties of fats? 26

SPECIAL THANKS TO: Sir Norman, Sir Joven, Ms. Grace 27
 A brick manufacturer has two depots
A brick manufacturer has two depots Trans fat vs cis fat
Trans fat vs cis fat Examples of fat
Examples of fat Co occurrence matrix example
Co occurrence matrix example Animal texture
Animal texture Partial failure
Partial failure Gypsum occurrence
Gypsum occurrence Express action or state of being
Express action or state of being Occurrence of silicon
Occurrence of silicon Oep and aep catastrophe
Oep and aep catastrophe Cardinality and connectivity
Cardinality and connectivity Irony in an occurrence at owl creek bridge
Irony in an occurrence at owl creek bridge An occurrence at owl creek bridge stream of consciousness
An occurrence at owl creek bridge stream of consciousness Personification in an occurrence at owl creek bridge
Personification in an occurrence at owl creek bridge There will come soft rains climax
There will come soft rains climax It is an occurrence of harmony
It is an occurrence of harmony Occurrence sampling
Occurrence sampling Abstraction-occurrence design pattern
Abstraction-occurrence design pattern Occurrence of zinc
Occurrence of zinc Dangerous occurrence
Dangerous occurrence Come to occurrence
Come to occurrence An occurrence at owl creek bridge comprehension questions
An occurrence at owl creek bridge comprehension questions Unit 6 review questions
Unit 6 review questions Heterogeneous lipids
Heterogeneous lipids What are lipids
What are lipids Ethanol emulsion test for lipids
Ethanol emulsion test for lipids Fats and lipids
Fats and lipids Tom dayspring lipids
Tom dayspring lipids