Chapter 12 Chemical Kinetics Chemical Kinetics Studies the



![a. A + b. B products Trial [A] M/S [B] M/S Rate M/S 1 a. A + b. B products Trial [A] M/S [B] M/S Rate M/S 1](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-17.jpg)
![Concentration and Rate Homework Compare Experiments 1 and 2: when [NH 4+] doubles, the Concentration and Rate Homework Compare Experiments 1 and 2: when [NH 4+] doubles, the](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-18.jpg)
![Concentration and Rate Homework Likewise, compare Experiments 5 and 6: when [NO 2 -] Concentration and Rate Homework Likewise, compare Experiments 5 and 6: when [NO 2 -]](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-20.jpg)



![Zero Order Integration 2 NH 3(g) N 2 + 3 H 2 (g) [A]t Zero Order Integration 2 NH 3(g) N 2 + 3 H 2 (g) [A]t](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-28.jpg)
![[NO 2] vs Time • The plot is not a straight line, so the [NO 2] vs Time • The plot is not a straight line, so the](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-32.jpg)
![Determining rxn order Graphing ln [NO 2] vs. t yields: • The plot is Determining rxn order Graphing ln [NO 2] vs. t yields: • The plot is](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-33.jpg)
![Second-Order Processes A graph of 1/[NO 2] vs. t gives this plot. Time (s) Second-Order Processes A graph of 1/[NO 2] vs. t gives this plot. Time (s)](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-34.jpg)















![ZERO ORDER (N=0) [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20 ZERO ORDER (N=0) [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-85.jpg)
![SECOND ORDER (N=2) [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20 SECOND ORDER (N=2) [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-86.jpg)





![WARM-UP #6 The rate law for a reaction is rate = k[A][B]2 Which one WARM-UP #6 The rate law for a reaction is rate = k[A][B]2 Which one](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-104.jpg)
![• FIRST ORDER: or [A]t = concentration of A at any time, t • FIRST ORDER: or [A]t = concentration of A at any time, t](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-106.jpg)
![EXAMPLE #2 Time (s) [AB] (M) 0 50 100 150 200 250 300 350 EXAMPLE #2 Time (s) [AB] (M) 0 50 100 150 200 250 300 350](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-110.jpg)

![WARM-UP #1 A reaction follows the rate law: Rate = k[A]2. Which of the WARM-UP #1 A reaction follows the rate law: Rate = k[A]2. Which of the](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-119.jpg)

![WARM-UP #4 The following reaction is second order in [A] and the rate constant WARM-UP #4 The following reaction is second order in [A] and the rate constant](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-122.jpg)
![WARM-UP #5 The reaction A → B is first order in [A]. Consider the WARM-UP #5 The reaction A → B is first order in [A]. Consider the](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-123.jpg)

- Slides: 134

Chapter 12 Chemical Kinetics

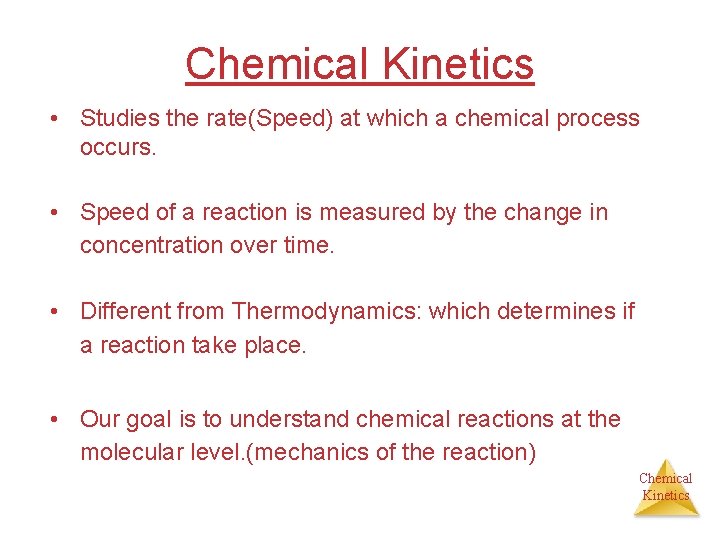
Chemical Kinetics • Studies the rate(Speed) at which a chemical process occurs. • Speed of a reaction is measured by the change in concentration over time. • Different from Thermodynamics: which determines if a reaction take place. • Our goal is to understand chemical reactions at the molecular level. (mechanics of the reaction) Chemical Kinetics

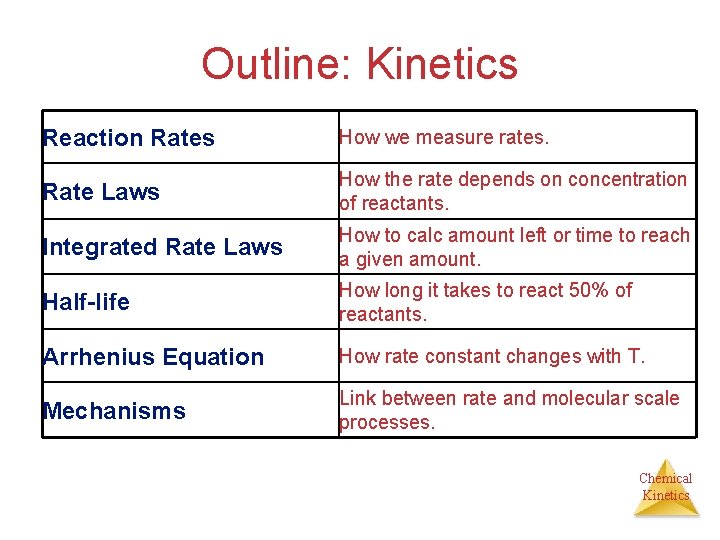
Outline: Kinetics Reaction Rates How we measure rates. Rate Laws How the rate depends on concentration of reactants. Integrated Rate Laws How to calc amount left or time to reach a given amount. Half-life How long it takes to react 50% of reactants. Arrhenius Equation How rate constant changes with T. Mechanisms Link between rate and molecular scale processes. Chemical Kinetics

Reaction Rates • We define the reaction rate as the change in concentration of a reactant or product per unit time. • For the reaction “A B” there are two ways of measuring rate: (1) the speed at which the reactants disappear (2) the speed at which the products appear Chemical Kinetics

Reaction Rates of reactions can be determined by monitoring the change in concentration of either reactants or products as a function of time. Chemical Kinetics

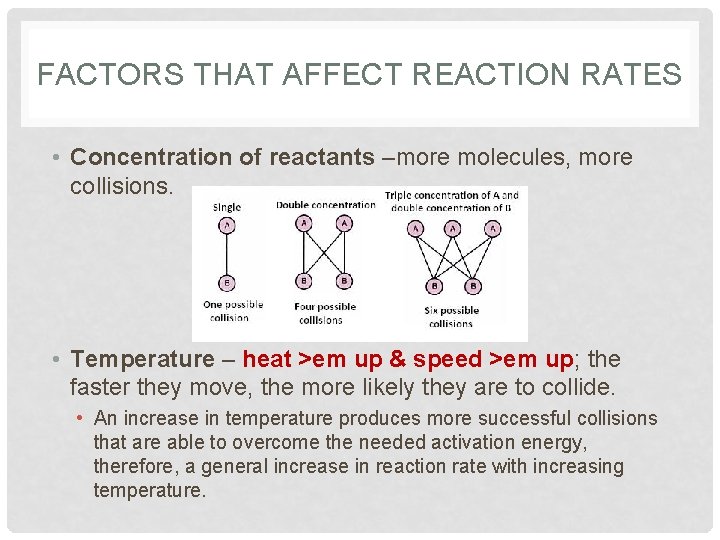
Factors That Affect Reaction Rates • Concentration of Reactants As the concentration of reactants increases, so does the likelihood that reactant molecules will collide. • Temperature At higher temperatures, reactant molecules have more kinetic energy, move faster, and collide more often and with greater energy. • Catalysts Speed rxn by changing mechanism. • Surface area More area for reactants to be in contact with each other. • Pressure of gaseous reactants or Products. Increased number of collisions Chemical Kinetics

Chemical Kinetics

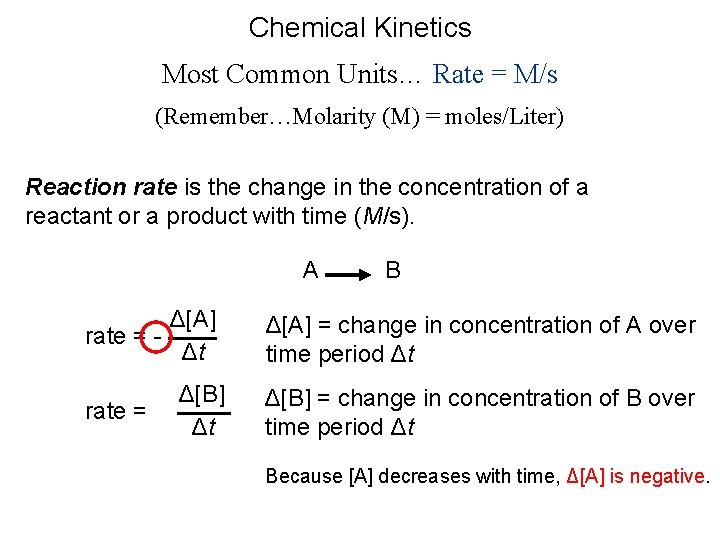
Chemical Kinetics Most Common Units… Rate = M/s (Remember…Molarity (M) = moles/Liter) Reaction rate is the change in the concentration of a reactant or a product with time (M/s). A B Δ[A] rate = Δt rate = Δ[B] Δt Δ[A] = change in concentration of A over time period Δt Δ[B] = change in concentration of B over time period Δt Because [A] decreases with time, Δ[A] is negative.

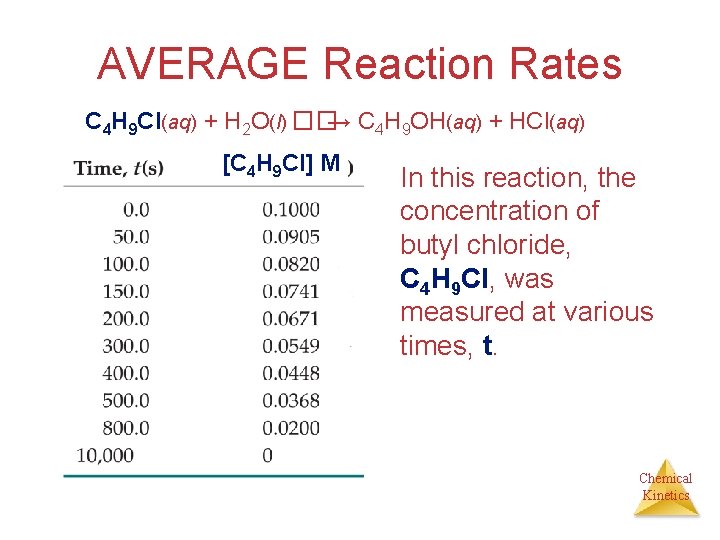
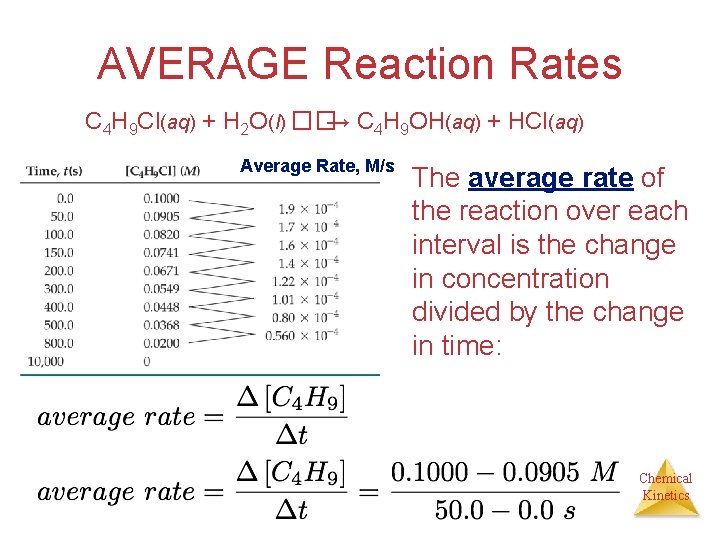
AVERAGE Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) ��→ C 4 H 9 OH(aq) + HCl(aq) [C 4 H 9 Cl] M In this reaction, the concentration of butyl chloride, C 4 H 9 Cl, was measured at various times, t. Chemical Kinetics

AVERAGE Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) ��→ C 4 H 9 OH(aq) + HCl(aq) Average Rate, M/s The average rate of the reaction over each interval is the change in concentration divided by the change in time: Chemical Kinetics

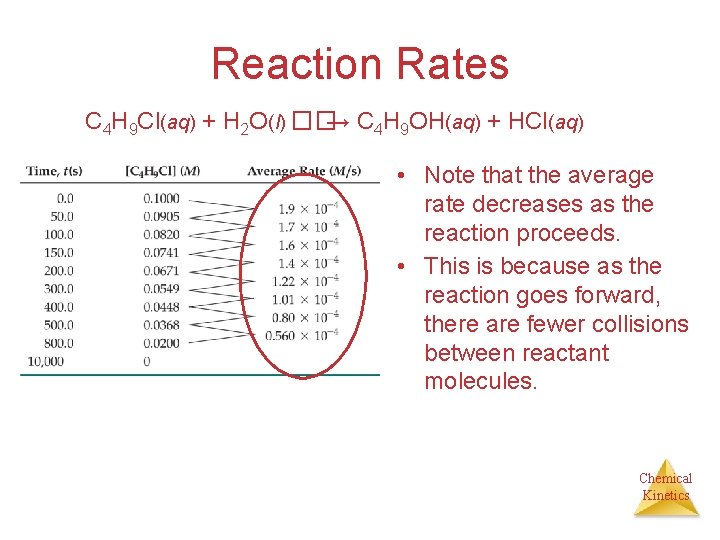
Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) ��→ C 4 H 9 OH(aq) + HCl(aq) • Note that the average rate decreases as the reaction proceeds. • This is because as the reaction goes forward, there are fewer collisions between reactant molecules. Chemical Kinetics

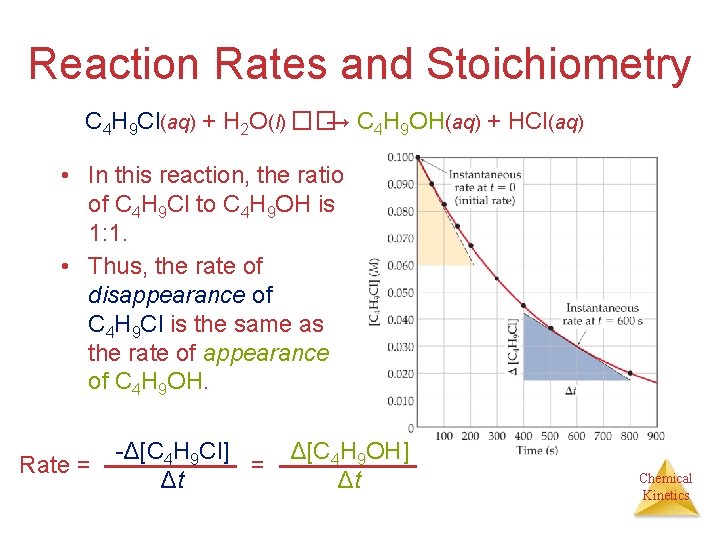
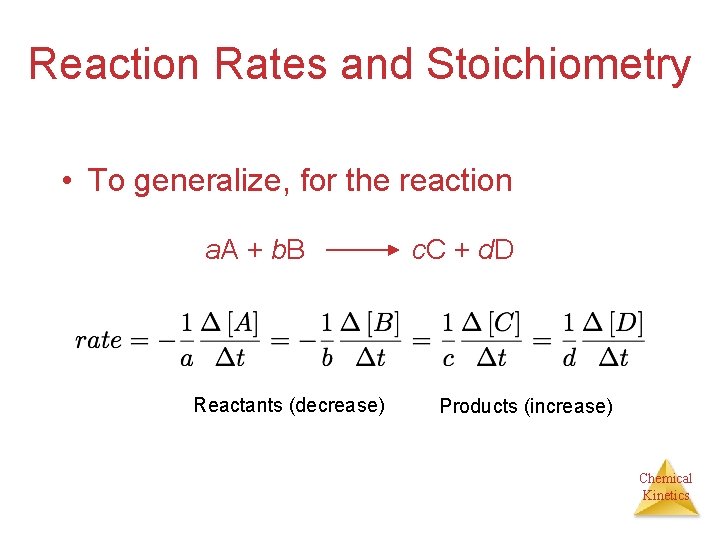
Reaction Rates and Stoichiometry C 4 H 9 Cl(aq) + H 2 O(l) ��→ C 4 H 9 OH(aq) + HCl(aq) • In this reaction, the ratio of C 4 H 9 Cl to C 4 H 9 OH is 1: 1. • Thus, the rate of disappearance of C 4 H 9 Cl is the same as the rate of appearance of C 4 H 9 OH. Rate = -Δ[C 4 H 9 Cl] = Δt Δ[C 4 H 9 OH] Δt Chemical Kinetics

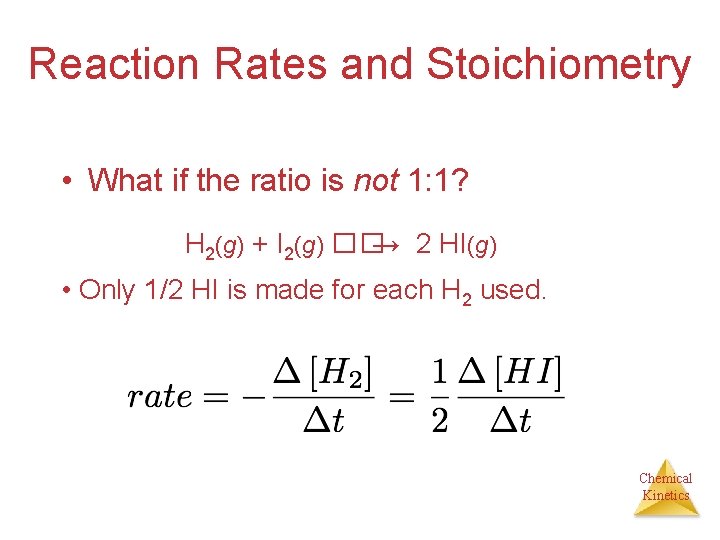
Reaction Rates and Stoichiometry • What if the ratio is not 1: 1? H 2(g) + I 2(g) ��→ 2 HI(g) • Only 1/2 HI is made for each H 2 used. Chemical Kinetics

Reaction Rates and Stoichiometry • To generalize, for the reaction a. A + b. B Reactants (decrease) c. C + d. D Products (increase) Chemical Kinetics

THE RATE LAW USING INITIAL CONCENTRATIONS Each reaction has its own equation that gives its rate as a function of reactant concentrations. Chemical Kinetics

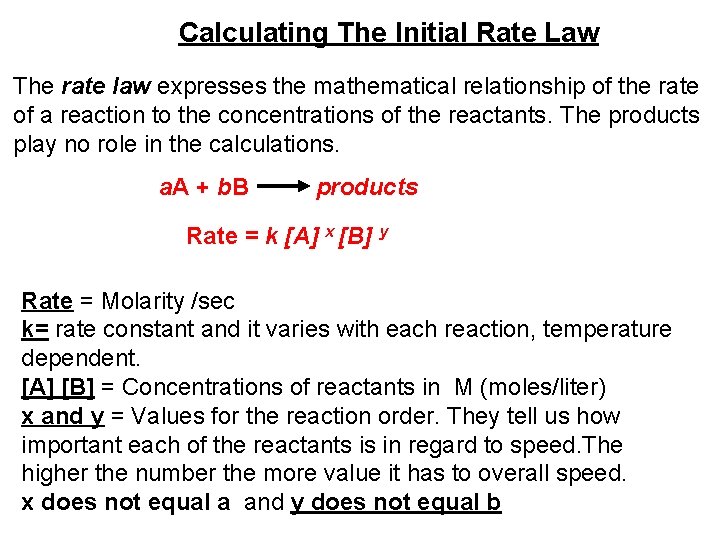
Calculating The Initial Rate Law The rate law expresses the mathematical relationship of the rate of a reaction to the concentrations of the reactants. The products play no role in the calculations. a. A + b. B products Rate = k [A] x [B] y Rate = Molarity /sec k= rate constant and it varies with each reaction, temperature dependent. [A] [B] = Concentrations of reactants in M (moles/liter) x and y = Values for the reaction order. They tell us how important each of the reactants is in regard to speed. The higher the number the more value it has to overall speed. x does not equal a and y does not equal b
![a A b B products Trial A MS B MS Rate MS 1 a. A + b. B products Trial [A] M/S [B] M/S Rate M/S 1](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-17.jpg)
a. A + b. B products Trial [A] M/S [B] M/S Rate M/S 1 0. 100 2. 00 x 10 -3 2 0. 200 . 100 4. 00 x 10 -3 3 0. 200 16. 00 x 10 -3
![Concentration and Rate Homework Compare Experiments 1 and 2 when NH 4 doubles the Concentration and Rate Homework Compare Experiments 1 and 2: when [NH 4+] doubles, the](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-18.jpg)
Concentration and Rate Homework Compare Experiments 1 and 2: when [NH 4+] doubles, the initial rate doubles. Chemical Kinetics

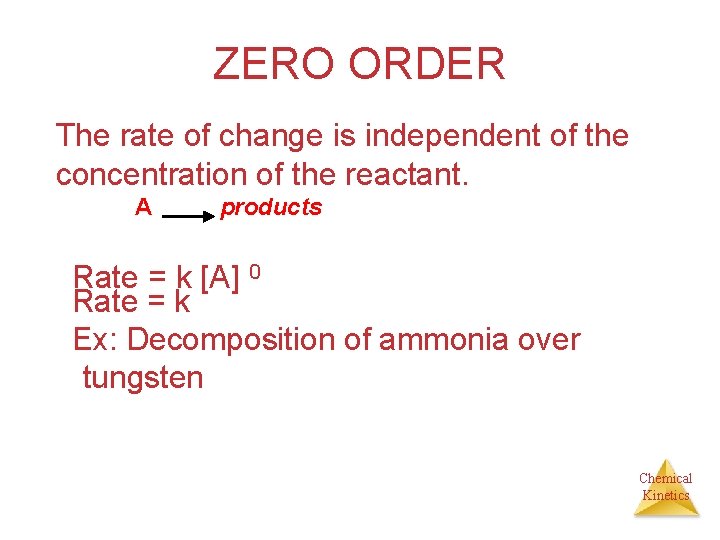
ZERO ORDER The rate of change is independent of the concentration of the reactant. A products Rate = k [A] 0 Rate = k Ex: Decomposition of ammonia over tungsten Chemical Kinetics
![Concentration and Rate Homework Likewise compare Experiments 5 and 6 when NO 2 Concentration and Rate Homework Likewise, compare Experiments 5 and 6: when [NO 2 -]](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-20.jpg)
Concentration and Rate Homework Likewise, compare Experiments 5 and 6: when [NO 2 -] doubles, the initial rate doubles. Chemical Kinetics

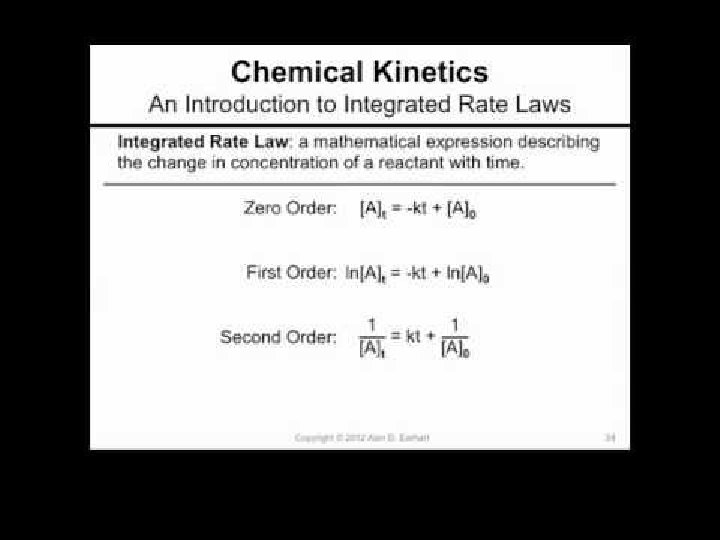
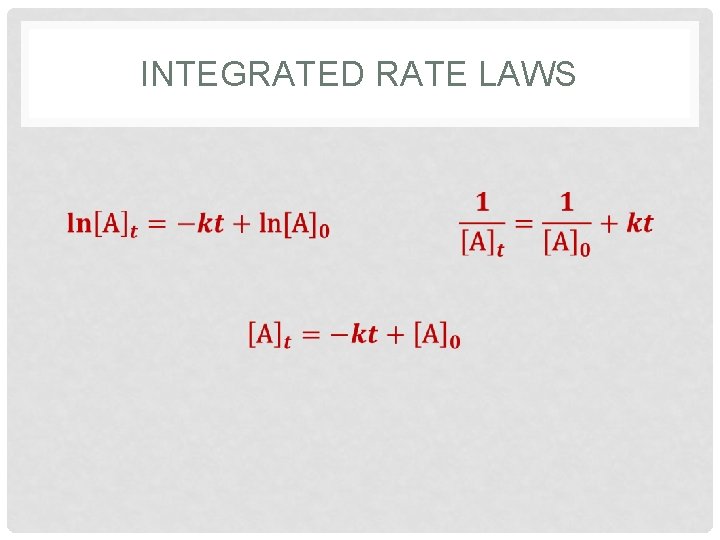
INTEGRATED RATE LAWS Chemical Kinetics

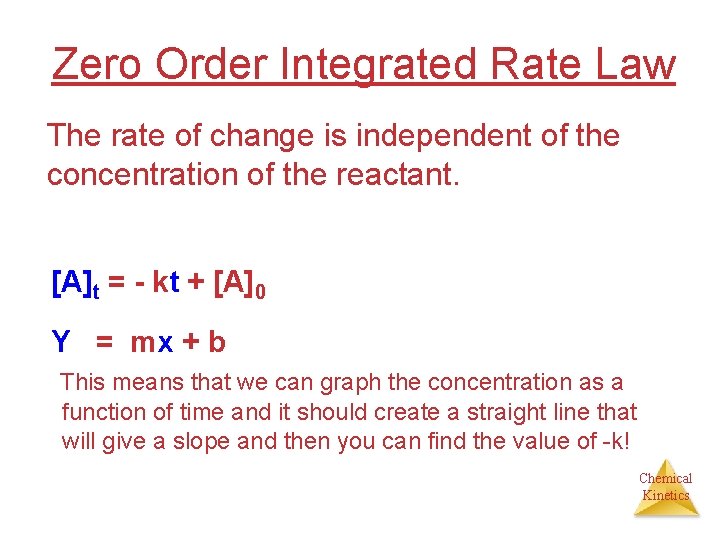
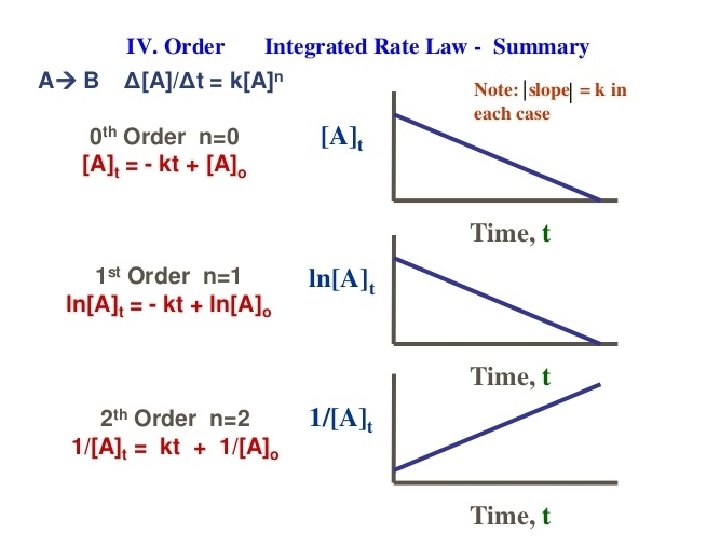
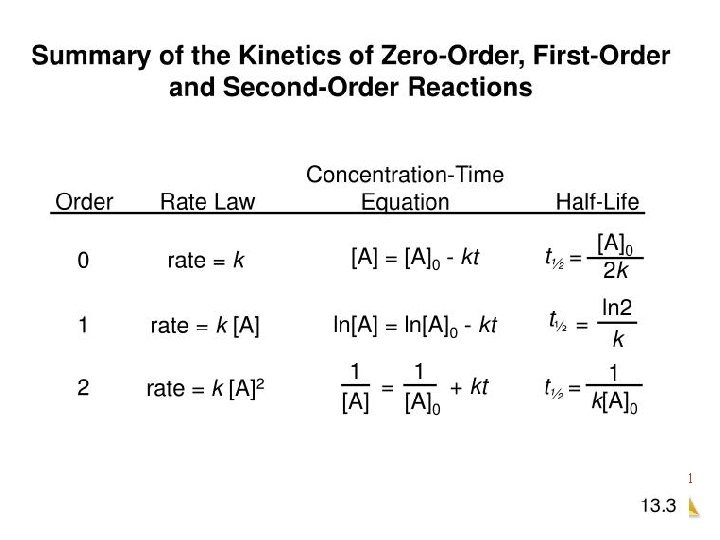
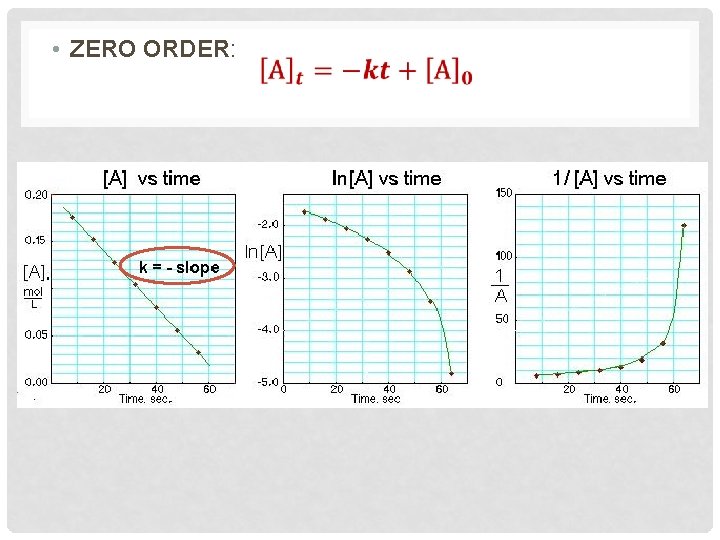
Zero Order Integrated Rate Law The rate of change is independent of the concentration of the reactant. [A]t = - kt + [A]0 Y = mx + b This means that we can graph the concentration as a function of time and it should create a straight line that will give a slope and then you can find the value of -k! Chemical Kinetics

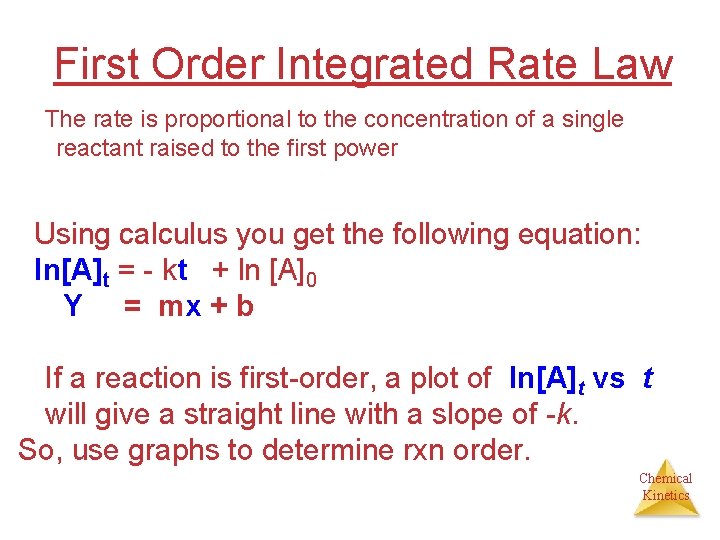
First Order Integrated Rate Law The rate is proportional to the concentration of a single reactant raised to the first power Using calculus you get the following equation: ln[A]t = - kt + ln [A]0 Y = mx + b If a reaction is first-order, a plot of ln[A]t vs t will give a straight line with a slope of -k. So, use graphs to determine rxn order. Chemical Kinetics

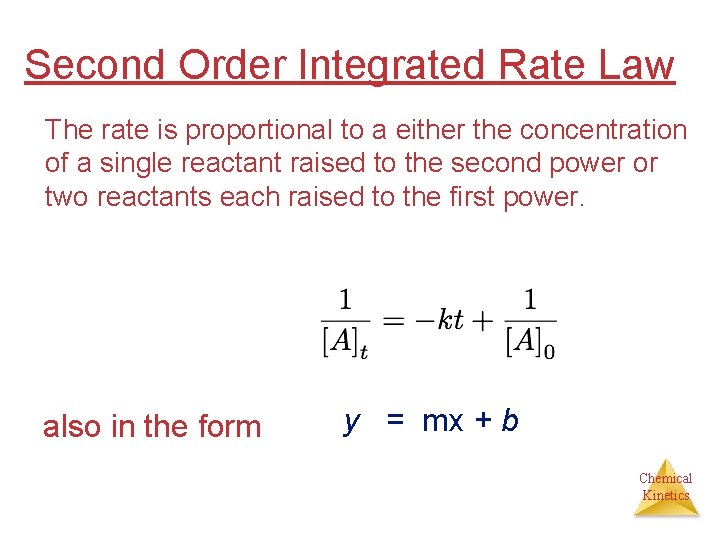
Second Order Integrated Rate Law The rate is proportional to a either the concentration of a single reactant raised to the second power or two reactants each raised to the first power. also in the form y = mx + b Chemical Kinetics

How are these equations helpful? • You now have a technique for determining the order of a reaction by observing graphs created from experimental data of concentration vs time. • If a simple graph of concentration vs time is a straight line then you know that it is a zero order reaction. • If the natural log of concentration vs time is a straight line then you know that it is a first order reaction. • If the reciprocal concentration vs time is a straight line then you know that it is a second order. Chemical Kinetics

The conclusion: In all cases, once you get a straight line fit , you can then get the rate constant from calculating the slope of the line. Slope (m) = ΔY ΔX Chemical Kinetics

Chemical Kinetics
![Zero Order Integration 2 NH 3g N 2 3 H 2 g At Zero Order Integration 2 NH 3(g) N 2 + 3 H 2 (g) [A]t](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-28.jpg)
Zero Order Integration 2 NH 3(g) N 2 + 3 H 2 (g) [A]t = - kt + [A]0 Chemical Kinetics

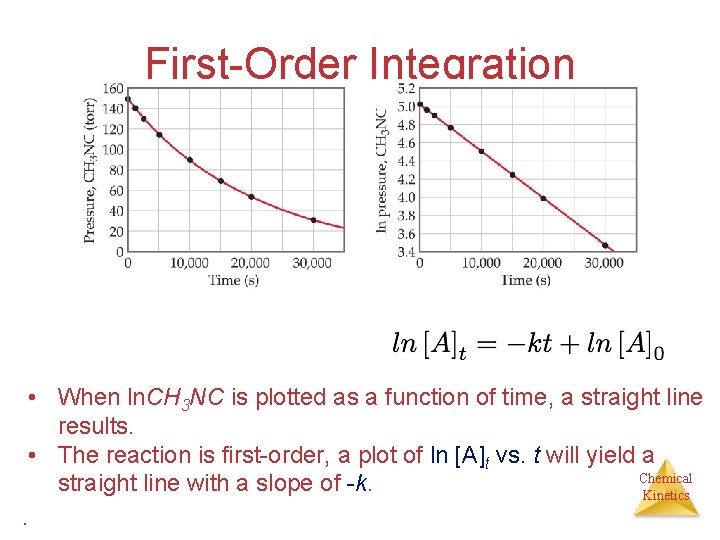
First-Order Integration • When ln. CH 3 NC is plotted as a function of time, a straight line results. • The reaction is first-order, a plot of ln [A]t vs. t will yield a Chemical straight line with a slope of -k. Kinetics.

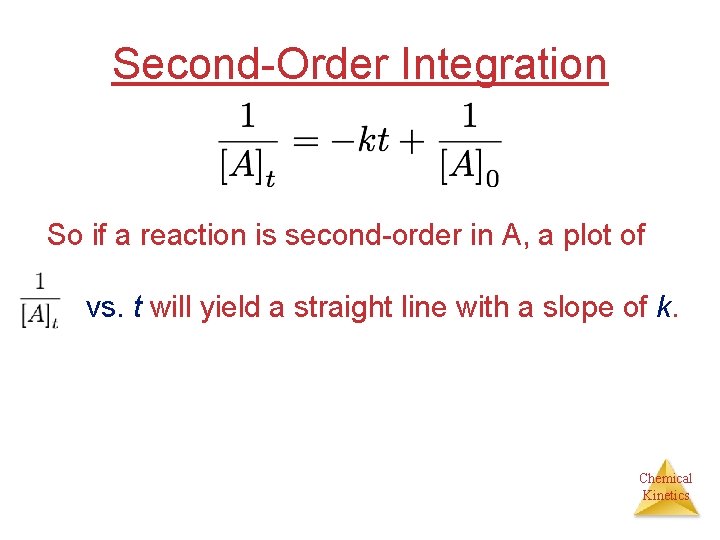
Second-Order Integration So if a reaction is second-order in A, a plot of vs. t will yield a straight line with a slope of k. Chemical Kinetics

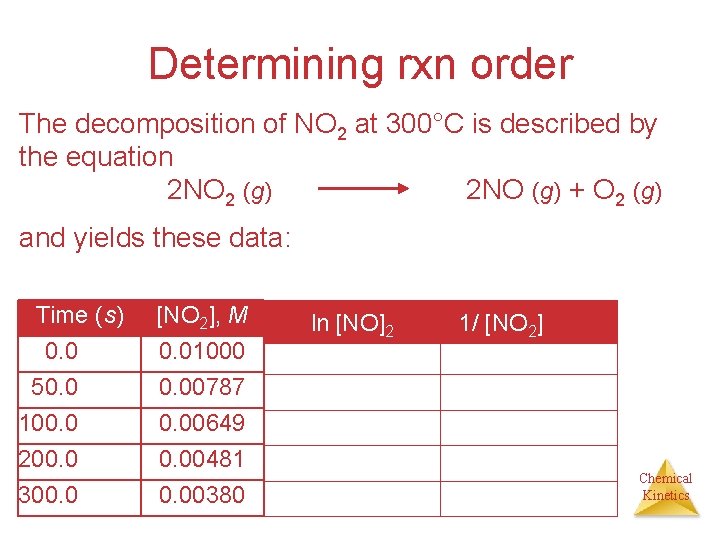
Determining rxn order The decomposition of NO 2 at 300°C is described by the equation 2 NO 2 (g) 2 NO (g) + O 2 (g) and yields these data: Time (s) 0. 0 50. 0 100. 0 200. 0 300. 0 [NO 2], M 0. 01000 0. 00787 0. 00649 0. 00481 0. 00380 ln [NO]2 1/ [NO 2] Chemical Kinetics
![NO 2 vs Time The plot is not a straight line so the [NO 2] vs Time • The plot is not a straight line, so the](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-32.jpg)
[NO 2] vs Time • The plot is not a straight line, so the process is not zero-order. Chemical Kinetics
![Determining rxn order Graphing ln NO 2 vs t yields The plot is Determining rxn order Graphing ln [NO 2] vs. t yields: • The plot is](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-33.jpg)
Determining rxn order Graphing ln [NO 2] vs. t yields: • The plot is not a straight line, so the process is not first-order in [A]. Time (s) 0. 0 50. 0 100. 0 200. 0 300. 0 [NO 2], M 0. 01000 0. 00787 0. 00649 0. 00481 0. 00380 ln [NO 2] -4. 610 -4. 845 -5. 038 -5. 337 -5. 573 Does not fit: Chemical Kinetics
![SecondOrder Processes A graph of 1NO 2 vs t gives this plot Time s Second-Order Processes A graph of 1/[NO 2] vs. t gives this plot. Time (s)](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-34.jpg)
Second-Order Processes A graph of 1/[NO 2] vs. t gives this plot. Time (s) 0. 0 50. 0 100. 0 200. 0 300. 0 [NO 2], M 0. 01000 0. 00787 0. 00649 0. 00481 0. 00380 1/[NO 2] 100 127 154 208 263 • This is a straight line. Therefore, the process is secondorder in [NO 2]. k= 0. 543 Chemical Kinetics

Chemical Kinetics

Half-Life • Half-life is defined as the time required for one-half of a reactant to react. • Half-Life is also defined as the rate of decay of a radioactive substance. A radioactive substance is one that will slowly decay into a more stable form as time goes on. Chemical Kinetics

Determining a Half Life To determine a half life, t½, the time required for the initial concentration of a reactant to be reduced to one-half its initial value, we need to know: ● The order of the reaction or enough information to determine it. ● The rate constant, k, for the reaction or enough information to determine it. ● In some cases, we need to know the initial concentration, [Ao] ● Substitute this information into the equation for the half life of a reaction with this order and solve for t½. Chemical Kinetics

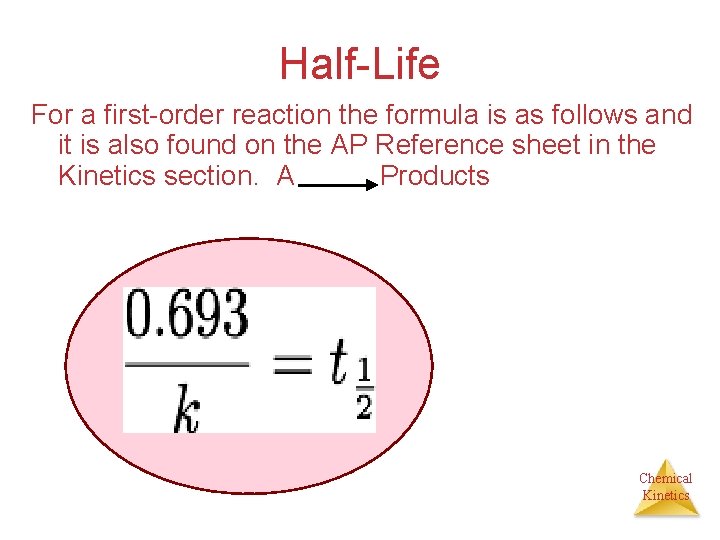
Half-Life For a first-order reaction the formula is as follows and it is also found on the AP Reference sheet in the Kinetics section. A Products Chemical Kinetics

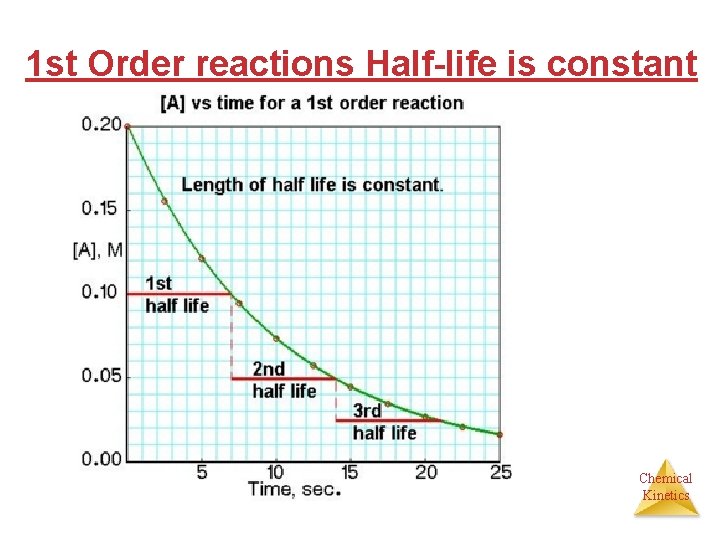
1 st Order reactions Half-life is constant Chemical Kinetics

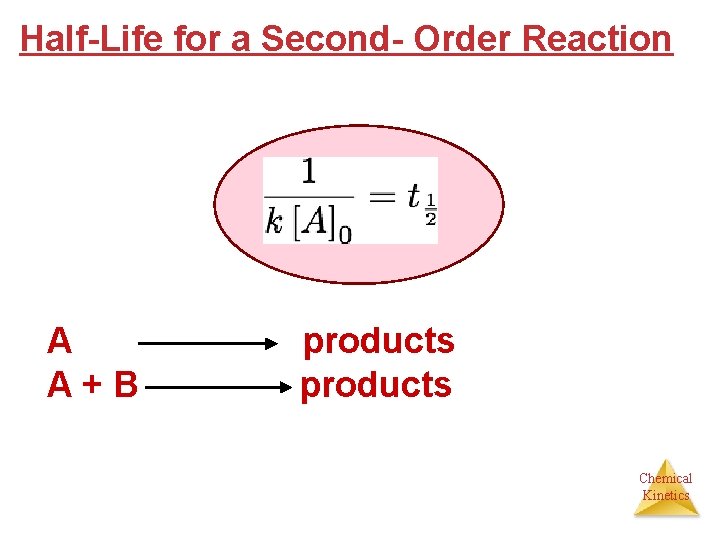
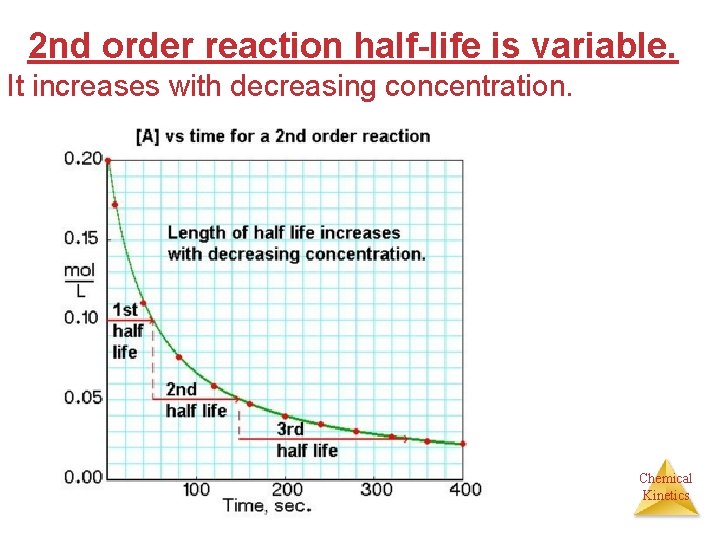
Half-Life for a Second- Order Reaction A products A + B products Chemical Kinetics

2 nd order reaction half-life is variable. It increases with decreasing concentration. Chemical Kinetics

Chemical Kinetics

Finding the Overall Rate Law by Utilizing Reaction Mechanisms Chemical Kinetics

Reaction Mechanisms • Not all reactions occur in one step. • The step by step sequence of 2 or more simple reactions that combine to form the overall reaction. • A chemical mechanism describes in detail exactly what takes place at each stage of an overall chemical reaction. Chemical Kinetics

Reaction Mechanism Terms Complex reaction- overall reaction elementary steps- series of simpler reactions that combine to form the complex reaction. Intermediate-a substance produced in one elementary step and consumed in another. Catalyst-increases the rate but not consumed by the reaction. It remains unchanged. Chemical Kinetics

Multistep Mechanisms • In a multistep process, one of the steps will be slower than all others. • The slowest step is called the ratedetermining step. It will be the one used to find the rate law for the overall reaction. • The overall reaction cannot occur faster than this slowest, rate-determining step Chemical Kinetics

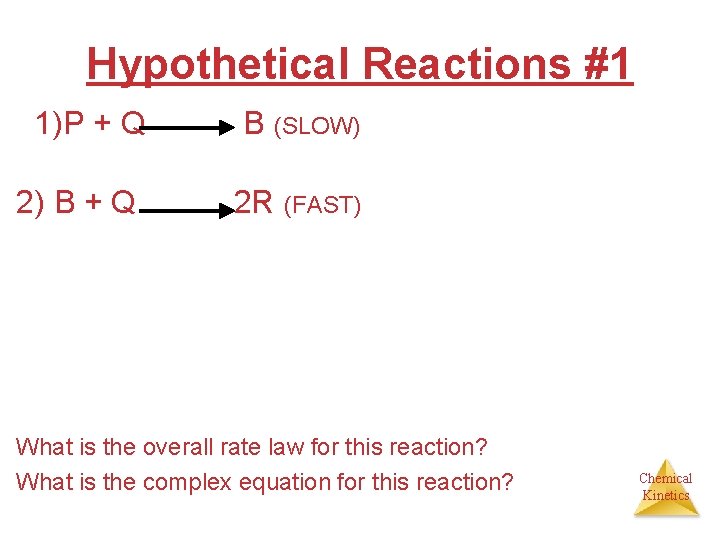
Hypothetical Reactions #1 1)P + Q B (SLOW) 2) B + Q 2 R (FAST) What is the overall rate law for this reaction? What is the complex equation for this reaction? Chemical Kinetics

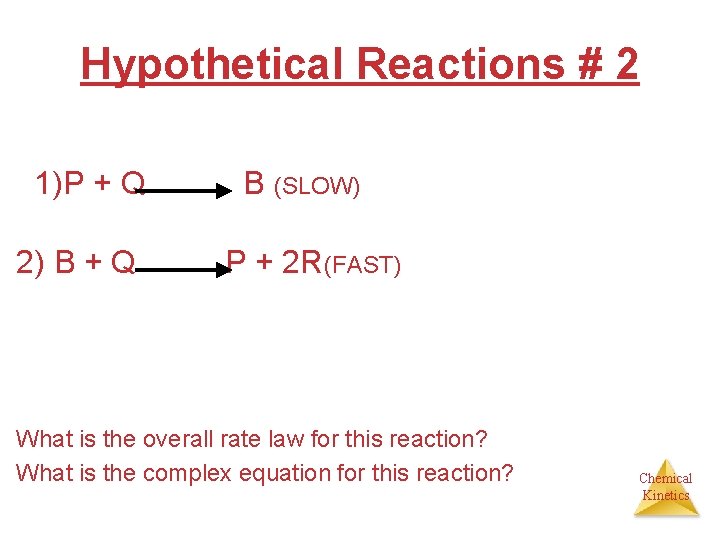
Hypothetical Reactions # 2 1)P + Q B (SLOW) 2) B + Q P + 2 R(FAST) What is the overall rate law for this reaction? What is the complex equation for this reaction? Chemical Kinetics

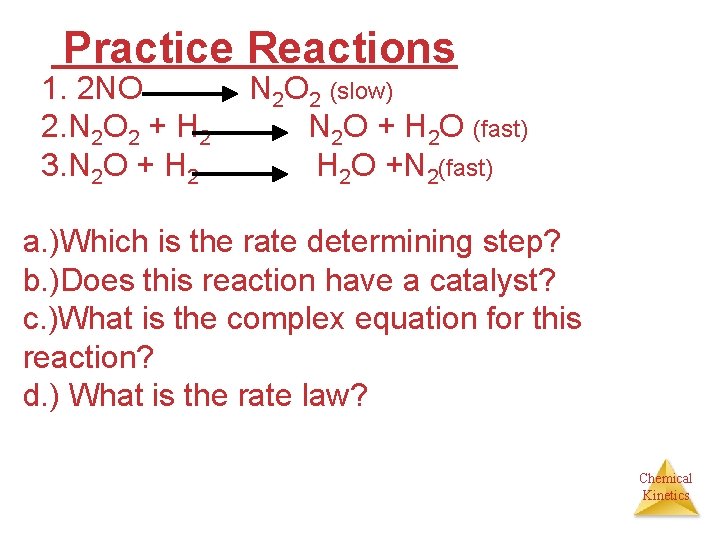
Practice Reactions 1. 2 NO N 2 O 2 (slow) 2. N 2 O 2 + H 2 N 2 O + H 2 O (fast) 3. N 2 O + H 2 H 2 O +N 2(fast) a. )Which is the rate determining step? b. )Does this reaction have a catalyst? c. )What is the complex equation for this reaction? d. ) What is the rate law? Chemical Kinetics

Answers to practice problem A. ) Step one is the rate determining step B. ) No catalyst C. ) 2 NO + 2 H 2 N 2 + 2 H 2 O D. ) rate = k [NO]2 Chemical Kinetics

Please Remember!!!! • YOU CAN ONLY USE THE COEFFICIENT METHOD TO DETERMINE THE RATE LAW OF ELEMENTARY STEPS. • YOU CANNOT USE THE COEFFICIENTS FROM THE COMPLEX EQUATION AND DO THE SAME THING. Chemical Kinetics

UNIT 10 – KINETICS REVIEW AP CHEMISTRY

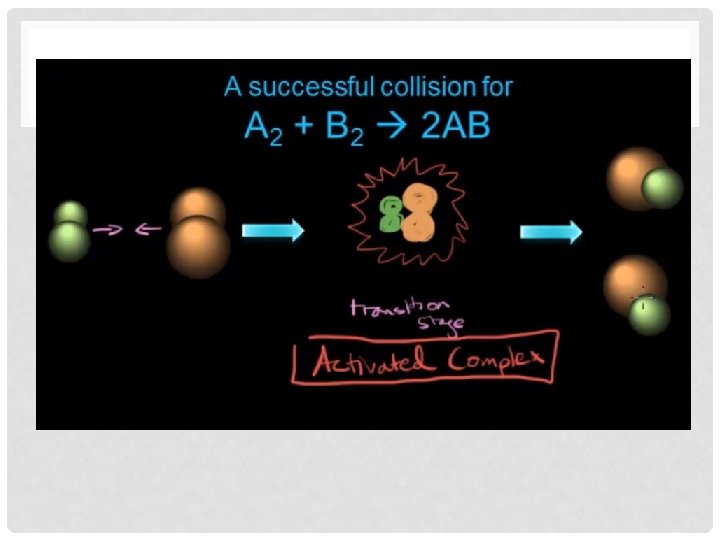
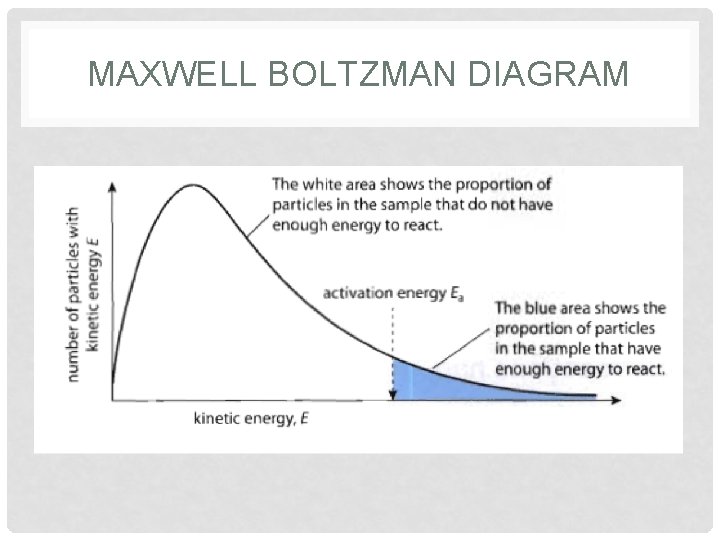
COLLISION THEORY Key Ideas of Collision Theory: • For a chemical reaction to occur, the reacting particles must collide. • Not all collisions are successful (successful = collision results in a reaction)

WHAT DETERMINES IF A COLLISION IS SUCCESSFUL? • Particles need to have proper orientation (direction in which they collide)

WHAT DETERMINES IF A COLLISION IS SUCCESSFUL? • Particles need to have enough energy, or speed • Activation Energy (EA) – Particles need to have a minimum amount of energy when they collide to have a successful collision • EA needed to break existing bonds and form new bonds


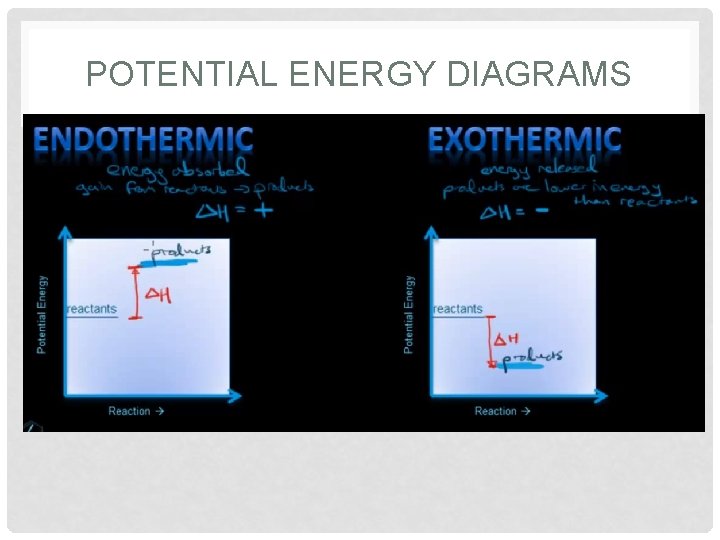
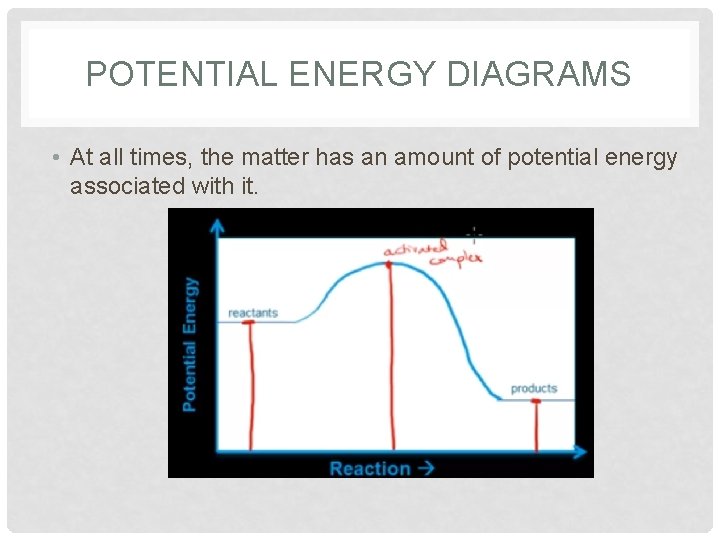
POTENTIAL ENERGY DIAGRAMS

POTENTIAL ENERGY DIAGRAMS • At all times, the matter has an amount of potential energy associated with it.

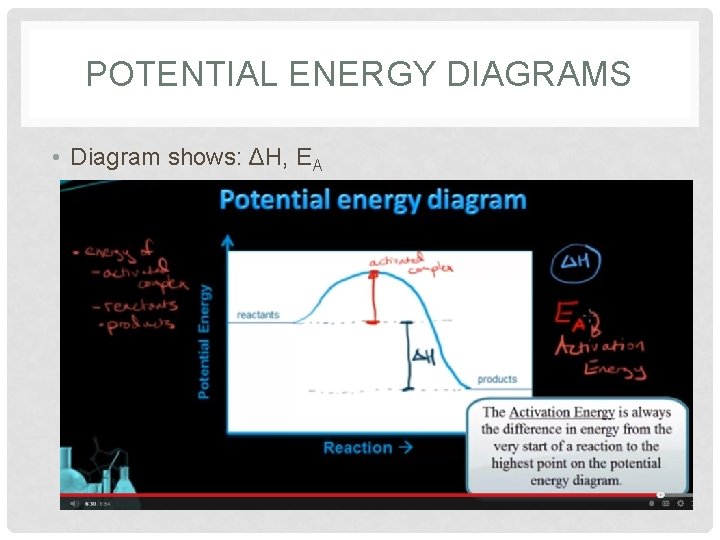
POTENTIAL ENERGY DIAGRAMS • Diagram shows: ΔH, EA

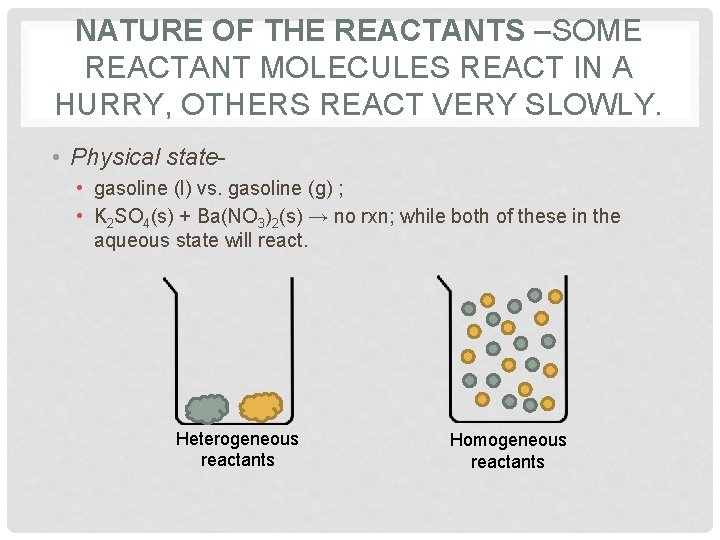
NATURE OF THE REACTANTS –SOME REACTANT MOLECULES REACT IN A HURRY, OTHERS REACT VERY SLOWLY. • Physical state • gasoline (l) vs. gasoline (g) ; • K 2 SO 4(s) + Ba(NO 3)2(s) → no rxn; while both of these in the aqueous state will react. Heterogeneous reactants Homogeneous reactants

FACTORS THAT AFFECT REACTION RATES • Concentration of reactants –more molecules, more collisions. • Temperature – heat >em up & speed >em up; the faster they move, the more likely they are to collide. • An increase in temperature produces more successful collisions that are able to overcome the needed activation energy, therefore, a general increase in reaction rate with increasing temperature.

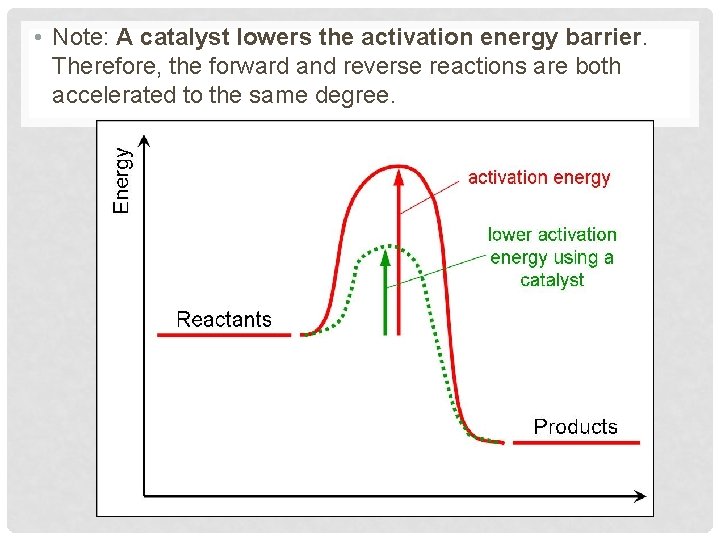
FACTORS THAT AFFECT REACTION RATES Catalysts – accelerate chemical reactions but are not themselves transformed. • Biological catalysts are proteins called enzymes. • A catalyst is a substance that changes the rate of reaction by altering the reaction pathway. Most catalysts work by lowering the activation energy needed for the reaction to proceed, therefore, more collisions are successful and the reaction rate is increased. • Remember! The catalyst is not part of the chemical reaction and is not used up during the reaction. • (May be homogeneous or heterogeneous catalysts. )

• Note: A catalyst lowers the activation energy barrier. Therefore, the forward and reverse reactions are both accelerated to the same degree.

FACTORS THAT AFFECT REACTION RATES Surface area of reactants – exposed surfaces affect speed. • Except for substances in the gaseous state or solution, reactions occur at the boundary, or interface, between two phases. • The greater surface area exposed, the greater chance of collisions between particles, hence, the reaction should proceed at a much faster rate. Ex. coal dust is very explosive as opposed to a piece of charcoal. Solutions are ultimate exposure! Adding an inert gas has NO EFFECT on the rate [or equilibrium] of the reaction.

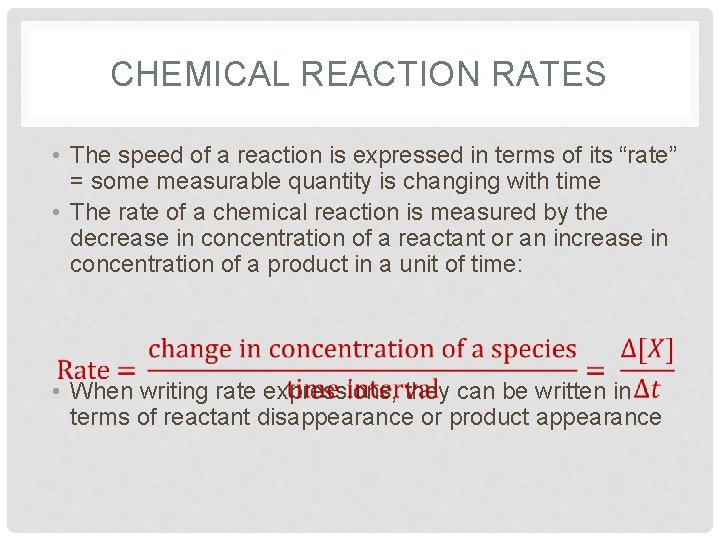
CHEMICAL REACTION RATES • The speed of a reaction is expressed in terms of its “rate” = some measurable quantity is changing with time • The rate of a chemical reaction is measured by the decrease in concentration of a reactant or an increase in concentration of a product in a unit of time: • When writing rate expressions, they can be written in terms of reactant disappearance or product appearance

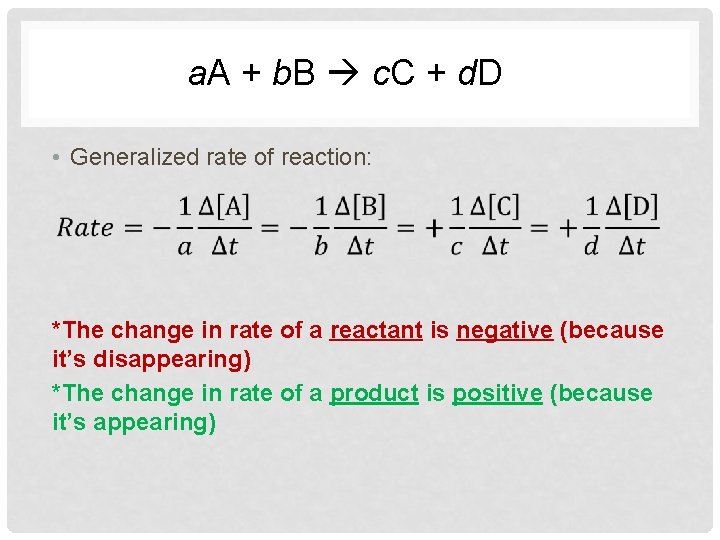
a. A + b. B c. C + d. D • Generalized rate of reaction: *The change in rate of a reactant is negative (because it’s disappearing) *The change in rate of a product is positive (because it’s appearing)

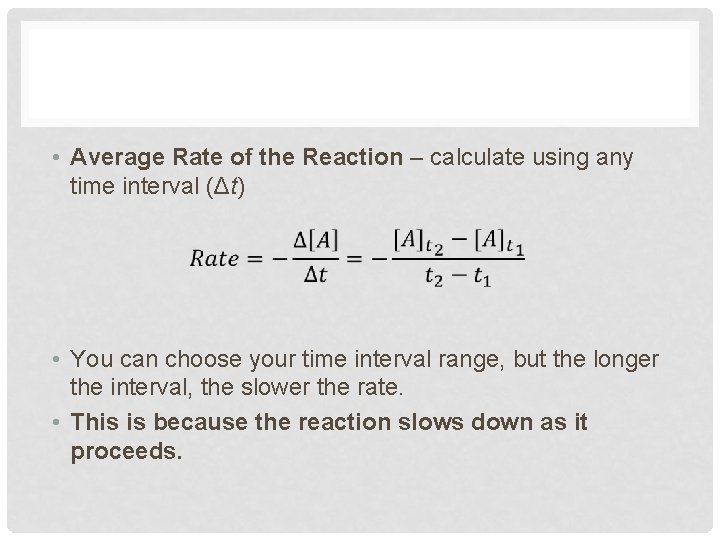
• Average Rate of the Reaction – calculate using any time interval (Δt) • You can choose your time interval range, but the longer the interval, the slower the rate. • This is because the reaction slows down as it proceeds.

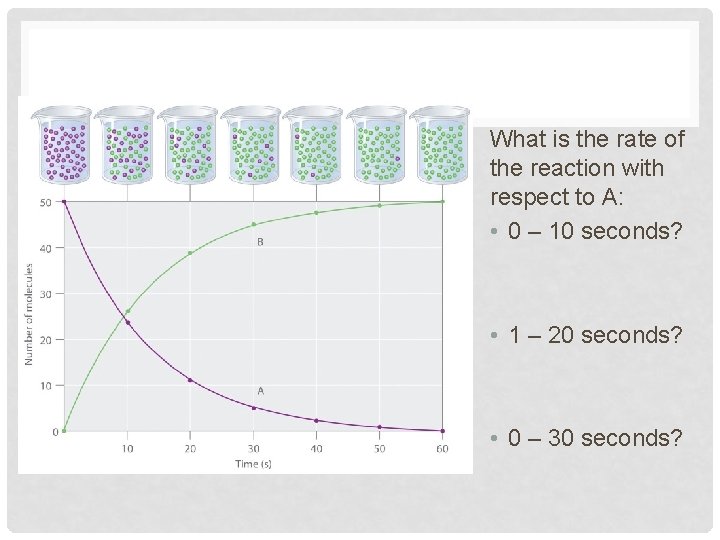
What is the rate of the reaction with respect to A: • 0 – 10 seconds? • 1 – 20 seconds? • 0 – 30 seconds?

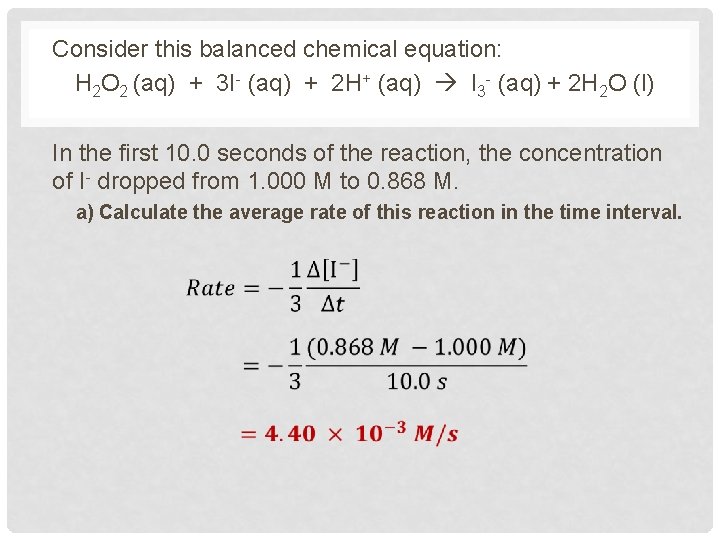
Consider this balanced chemical equation: H 2 O 2 (aq) + 3 I- (aq) + 2 H+ (aq) I 3 - (aq) + 2 H 2 O (l) In the first 10. 0 seconds of the reaction, the concentration of I- dropped from 1. 000 M to 0. 868 M. a) Calculate the average rate of this reaction in the time interval.

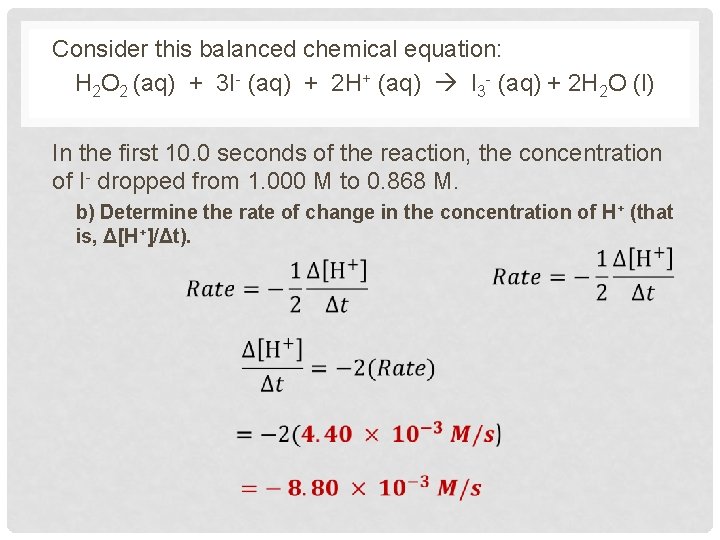
Consider this balanced chemical equation: H 2 O 2 (aq) + 3 I- (aq) + 2 H+ (aq) I 3 - (aq) + 2 H 2 O (l) In the first 10. 0 seconds of the reaction, the concentration of I- dropped from 1. 000 M to 0. 868 M. b) Determine the rate of change in the concentration of H+ (that is, Δ[H+]/Δt).

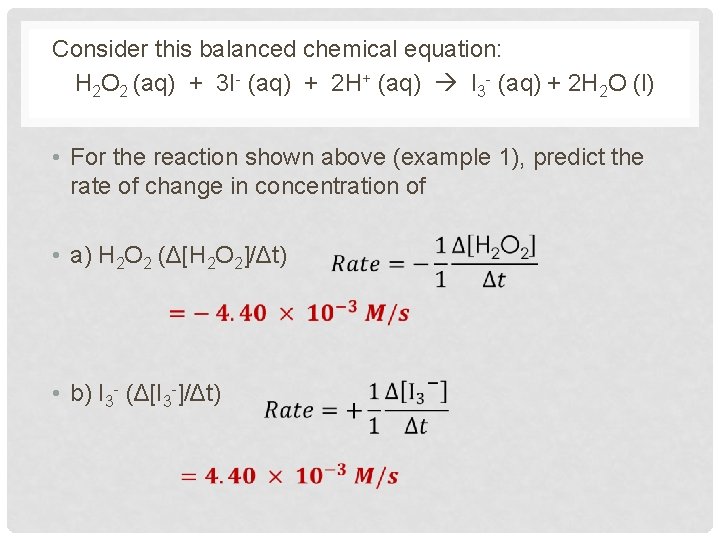
Consider this balanced chemical equation: H 2 O 2 (aq) + 3 I- (aq) + 2 H+ (aq) I 3 - (aq) + 2 H 2 O (l) • For the reaction shown above (example 1), predict the rate of change in concentration of • a) H 2 O 2 (Δ[H 2 O 2]/Δt) • b) I 3 - (Δ[I 3 -]/Δt)

GROUP PRACTICE: EXPRESSING REACTION RATES In groups, a) express the rate of reaction in terms of the change in concentration for each of the reactants and products, b) When the first reactant is decreasing at a rate of 0. 100 M/s, how fast are the other reactants decreasing? How fast are the products increasing?

GROUP PRACTICE: EXPRESSING REACTION RATES 1. 2 NO 2(g) + F 2(g) 2 NO 2 F(g) 2. CH 3 Cl(g) + 2 Cl 2(g) CCl 4(g) + 3 HCl(g) 3. 2 H 2 O 2(aq) 2 H 2 O(l) + O 2(g) 4. NO 2(g) NO(g) + ½ O 2(g) 5. Cl 2(g) + 3 F 2(g) 2 Cl. F 3(g)

WARM-UP #1 • For the reaction A + 2 B C under a given set of conditions, the initial rate is 0. 100 M/s. What is Δ[B]/Δt under the same conditions? a) -0. 0500 M/s b) -1. 000 M/s c) -0. 200 M/s

WARM-UP #2 • Dinitrogen monoxide decomposes into nitrogen and oxygen when heated. The initial rate of the reaction is 0. 022 M/s. What is the initial rate of change of the concentration of N 2 O (that is, Δ[N 2 O]/Δt)? 2 N 2 O (g) 2 N 2 (g) + O 2 (g) a) -0. 022 M/s b) -0. 011 M/s c) -0. 044 M/s d) +0. 022 M/s

WARM-UP #3 A burning splint will burn more vigorously in pure oxygen than in air because A. oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than it is in air. B. oxygen is a catalyst for combustion. C. oxygen is a product of combustion. D. nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature. E. nitrogen is a reactant in combustion and its low concentration in pure oxygen catalyzes the combustion.

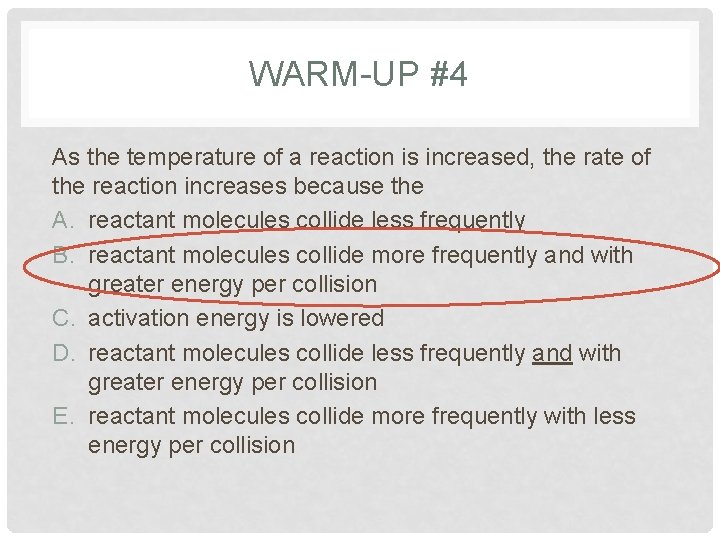
WARM-UP #4 As the temperature of a reaction is increased, the rate of the reaction increases because the A. reactant molecules collide less frequently B. reactant molecules collide more frequently and with greater energy per collision C. activation energy is lowered D. reactant molecules collide less frequently and with greater energy per collision E. reactant molecules collide more frequently with less energy per collision

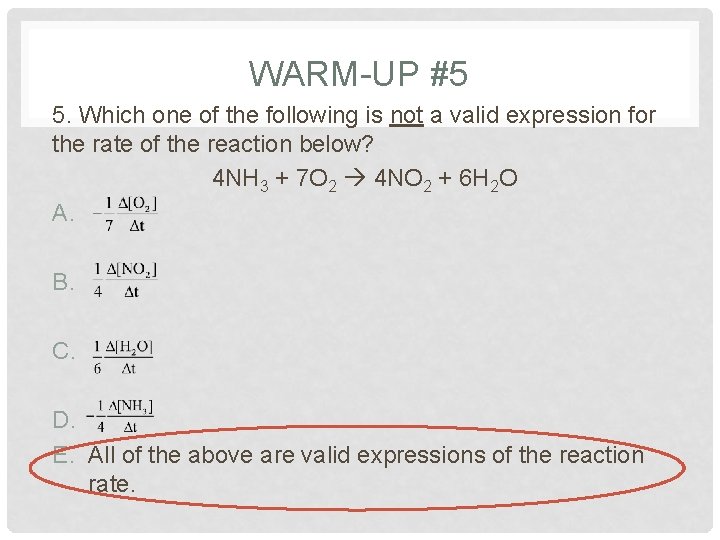
WARM-UP #5 5. Which one of the following is not a valid expression for the rate of the reaction below? 4 NH 3 + 7 O 2 4 NO 2 + 6 H 2 O A. B. C. D. E. All of the above are valid expressions of the reaction rate.

THE RATE LAW • The rate of a reaction often depends on the concentration of one or more of the reactants. A Products • We can express the relationship between the rate of the reaction and the concentration of the react: k = rate constant (“proportionality constant”) n = reaction order

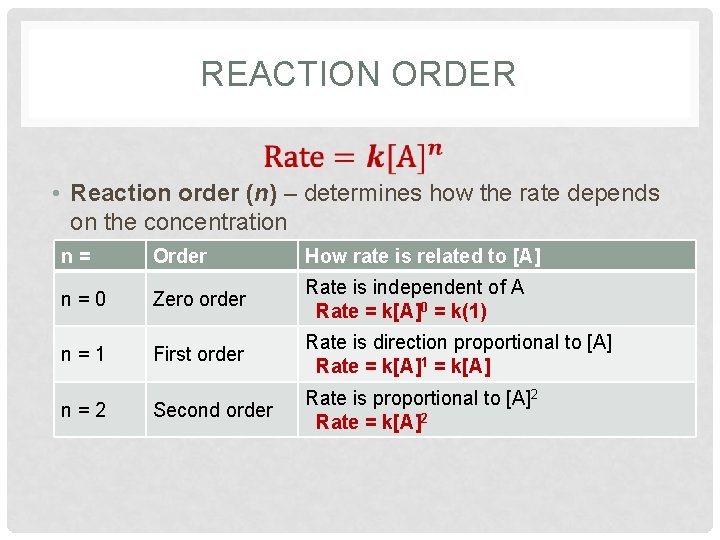
REACTION ORDER • Reaction order (n) – determines how the rate depends on the concentration n = Order How rate is related to [A] n = 0 Zero order Rate is independent of A Rate = k[A]0 = k(1) n = 1 First order Rate is direction proportional to [A] Rate = k[A]1 = k[A] Second order Rate is proportional to [A]2 Rate = k[A]2 n = 2

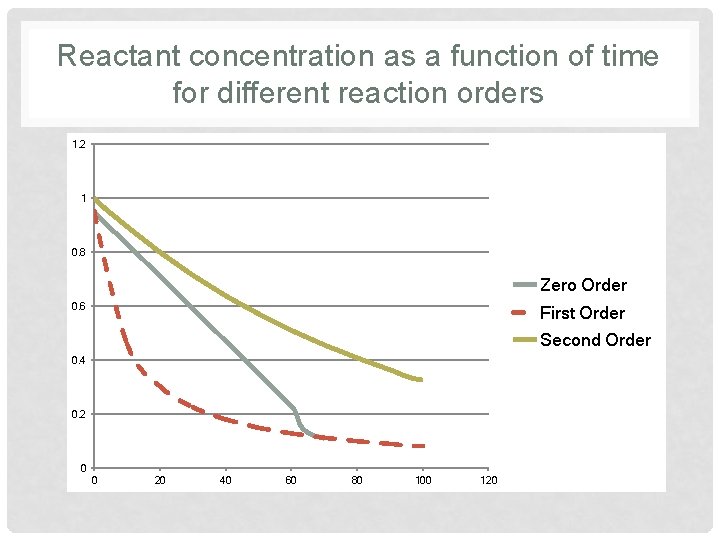
Reactant concentration as a function of time for different reaction orders 1. 2 1 0. 8 Zero Order 0. 6 First Order Second Order 0. 4 0. 2 0 0 20 40 60 80 100 120

DETERMINING THE ORDER OF A REACTION • The order of a reaction can be determined ONLY by experiment! • Method of initial rates – initial rates measured for different initial reactant concentrations • Rate measured for a short period of time at beginning of the experiment • Use to determine the effect of concentration for each reactant on the rate.

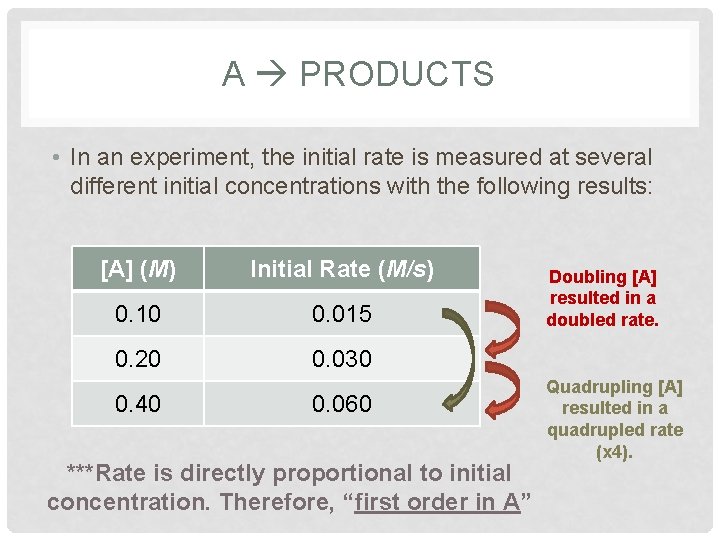
A PRODUCTS • In an experiment, the initial rate is measured at several different initial concentrations with the following results: [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20 0. 030 0. 40 0. 060 ***Rate is directly proportional to initial concentration. Therefore, “first order in A” Doubling [A] resulted in a doubled rate. Quadrupling [A] resulted in a quadrupled rate (x 4).

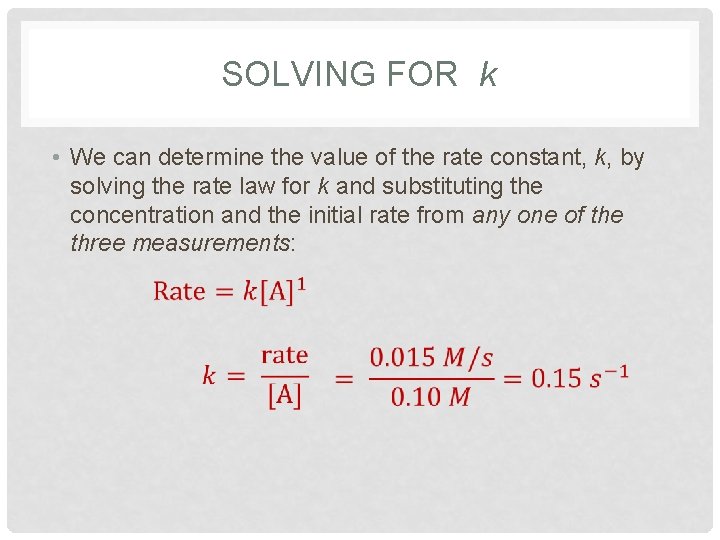
SOLVING FOR k • We can determine the value of the rate constant, k, by solving the rate law for k and substituting the concentration and the initial rate from any one of the three measurements:
![ZERO ORDER N0 A M Initial Rate Ms 0 10 0 015 0 20 ZERO ORDER (N=0) [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-85.jpg)
ZERO ORDER (N=0) [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20 0. 015 0. 40 0. 015 • Zero order reaction, the initial rate is independent of the reactant concentration – the rate is the same at all measured initial concentrations!
![SECOND ORDER N2 A M Initial Rate Ms 0 10 0 015 0 20 SECOND ORDER (N=2) [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-86.jpg)
SECOND ORDER (N=2) [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20 0. 060 0. 40 0. 240 • Second order reaction, the initial rate quadruples for a doubling of the reactant concentration – the relationship is quadradic!

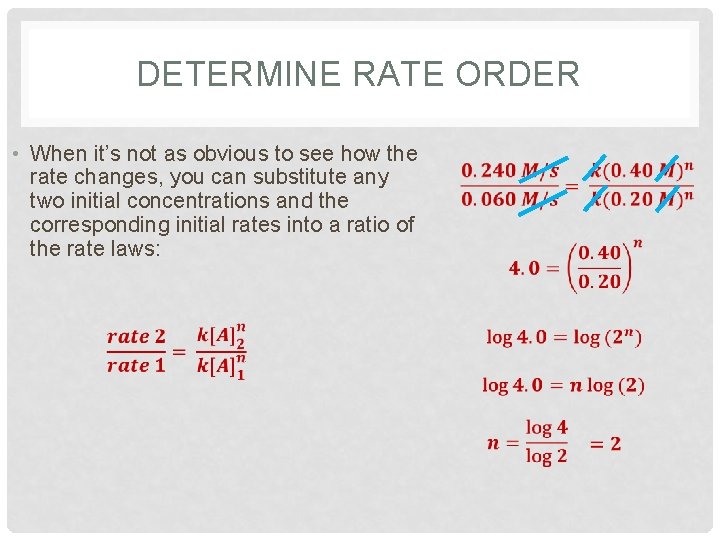
DETERMINE RATE ORDER • When it’s not as obvious to see how the rate changes, you can substitute any two initial concentrations and the corresponding initial rates into a ratio of the rate laws:

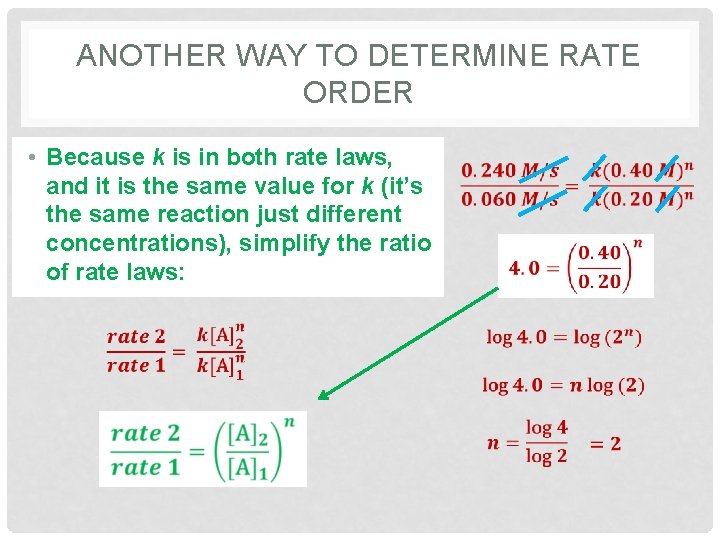
ANOTHER WAY TO DETERMINE RATE ORDER • Because k is in both rate laws, and it is the same value for k (it’s the same reaction just different concentrations), simplify the ratio of rate laws:

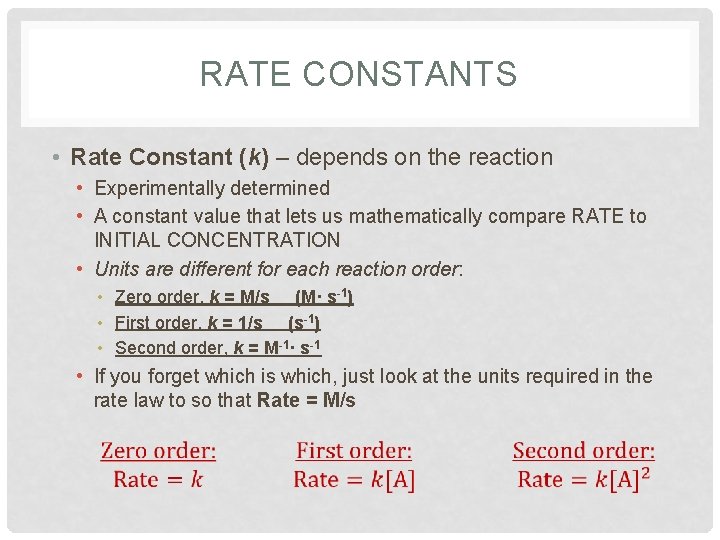
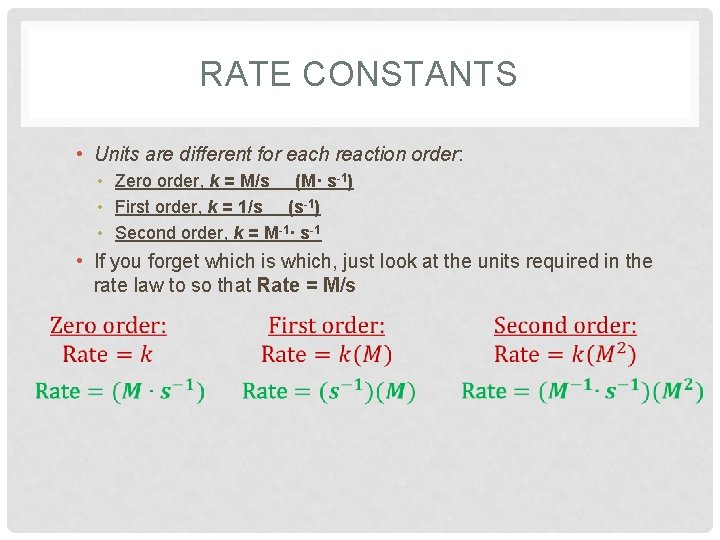
RATE CONSTANTS • Rate Constant (k) – depends on the reaction • Experimentally determined • A constant value that lets us mathematically compare RATE to INITIAL CONCENTRATION • Units are different for each reaction order: • Zero order, k = M/s (M· s-1) • First order, k = 1/s (s-1) • Second order, k = M-1· s-1 • If you forget which is which, just look at the units required in the rate law to so that Rate = M/s

RATE CONSTANTS • Units are different for each reaction order: • Zero order, k = M/s (M· s-1) • First order, k = 1/s (s-1) • Second order, k = M-1· s-1 • If you forget which is which, just look at the units required in the rate law to so that Rate = M/s

• This reaction is first order in N 2 O 5: N 2 O 5(g) NO 3(g) + NO 2(g) • The rate constant for the reaction at a certain temperature is 0. 053/s. • Calculate the rate for the reaction when [N 2 O 5] = 0. 055 M. • What would the rate of the reaction be at the concentration indicated in part a if the reaction were second order? Zero order?

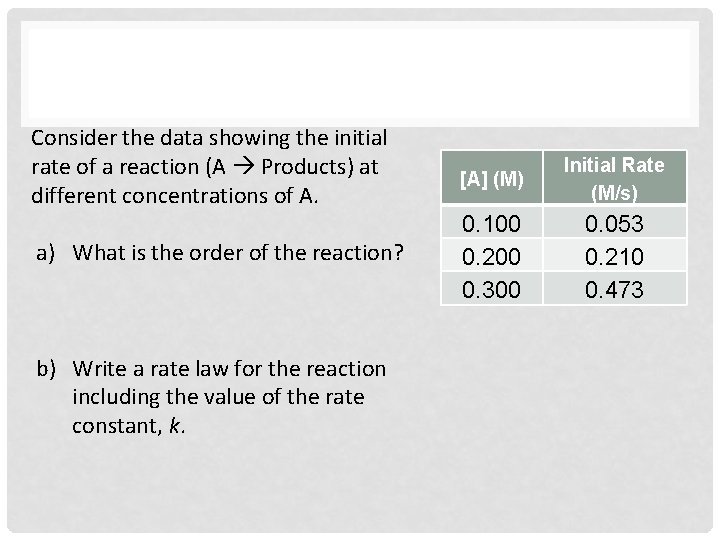
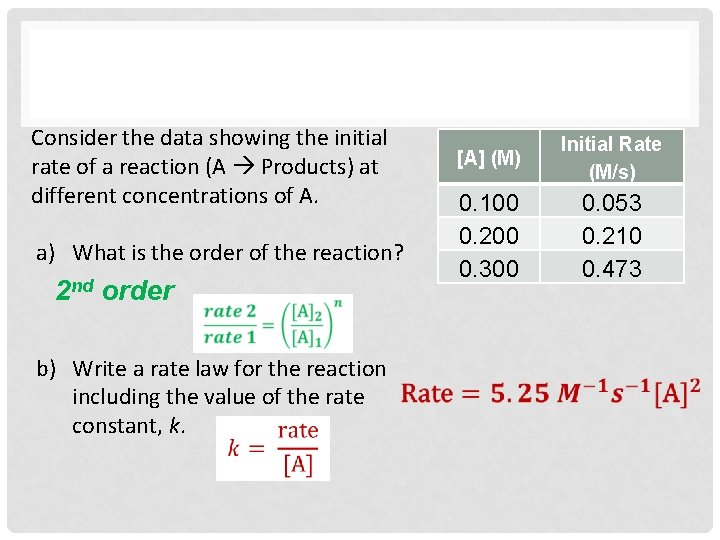
Consider the data showing the initial rate of a reaction (A Products) at different concentrations of A. a) What is the order of the reaction? b) Write a rate law for the reaction including the value of the rate constant, k. [A] (M) Initial Rate (M/s) 0. 100 0. 200 0. 300 0. 053 0. 210 0. 473

Consider the data showing the initial rate of a reaction (A Products) at different concentrations of A. a) What is the order of the reaction? 2 nd order b) Write a rate law for the reaction including the value of the rate constant, k. [A] (M) Initial Rate (M/s) 0. 100 0. 200 0. 300 0. 053 0. 210 0. 473

REACTION ORDER FOR MULTIPLE REACTANTS • For the generic reaction: a. A + b. B c. C + d. D • Each reactant has its own reaction order. • The overall order is the sum of the exponents: overall order = m + n

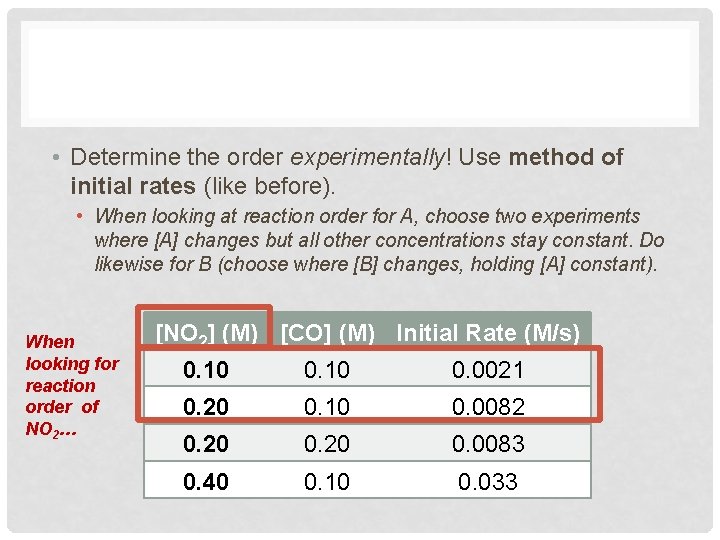
• Determine the order experimentally! Use method of initial rates (like before). • When looking at reaction order for A, choose two experiments where [A] changes but all other concentrations stay constant. Do likewise for B (choose where [B] changes, holding [A] constant). When looking for reaction order of NO 2… [NO 2] (M) [CO] (M) Initial Rate (M/s) 0. 10 0. 0021 0. 20 0. 10 0. 0082 0. 20 0. 0083 0. 40 0. 10 0. 033

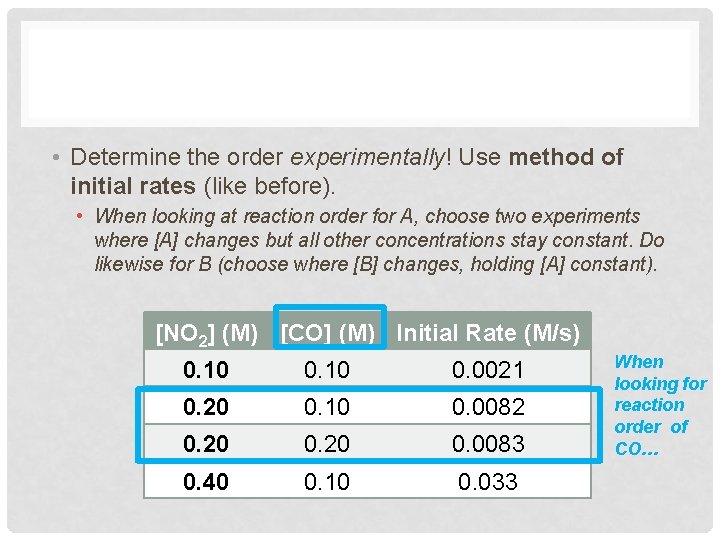
• Determine the order experimentally! Use method of initial rates (like before). • When looking at reaction order for A, choose two experiments where [A] changes but all other concentrations stay constant. Do likewise for B (choose where [B] changes, holding [A] constant). [NO 2] (M) [CO] (M) Initial Rate (M/s) 0. 10 0. 0021 0. 20 0. 10 0. 0082 0. 20 0. 0083 0. 40 0. 10 0. 033 When looking for reaction order of CO…

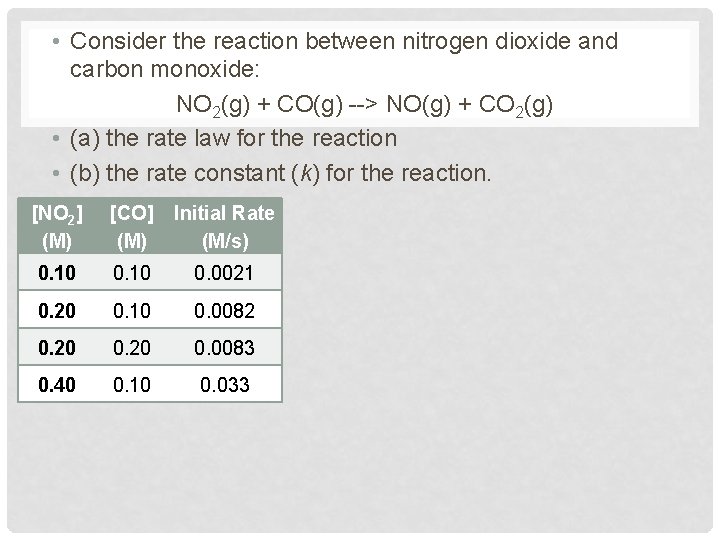
• Consider the reaction between nitrogen dioxide and carbon monoxide: NO 2(g) + CO(g) --> NO(g) + CO 2(g) • (a) the rate law for the reaction • (b) the rate constant (k) for the reaction. [NO 2] (M) [CO] Initial Rate (M) (M/s) 0. 10 0. 0021 0. 20 0. 10 0. 0082 0. 20 0. 0083 0. 40 0. 10 0. 033

A reaction in which A, B, and C reacto to form products is first order in A, second order in B, and zero order in C. a) Write a rate law for the reaction. Rate = k[A][B]2 b) What is the overall order of the reaction? By what factor does the reaction rate change. . . a) if [A] is doubled ? b) if [B] is doubled? c) if [C] is doubled? 3 rd order 2 (doubled) 4 (quadrupled) 1 (no change) d) By what factor does the reaction rate change if the concentration of all three reactants are doubled? 8 (doubled, then quadrupled)

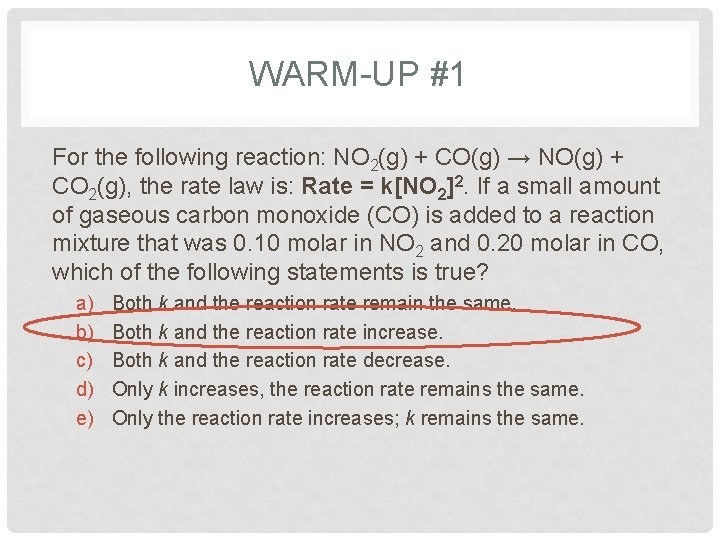
WARM-UP #1 For the following reaction: NO 2(g) + CO(g) → NO(g) + CO 2(g), the rate law is: Rate = k[NO 2]2. If a small amount of gaseous carbon monoxide (CO) is added to a reaction mixture that was 0. 10 molar in NO 2 and 0. 20 molar in CO, which of the following statements is true? a) b) c) d) e) Both k and the reaction rate remain the same. Both k and the reaction rate increase. Both k and the reaction rate decrease. Only k increases, the reaction rate remains the same. Only the reaction rate increases; k remains the same.

WARM-UP #2 Changes in which of the factors affect both rate and rate constant? I- temperature II- concentration A. I only B. II only C. Both I and II D. Neither I or II

WARM-UP #3 A reaction was found to be second order in carbon monoxide concentration. The rate of the reaction _____ if the [CO] is doubled, with everything else kept the same. A. doubles B. remains unchanged C. triples D. increases by a factor of 4 E. is reduced by a factor of 2

WARM-UP #4 The overall order of a reaction is 2. The units of the rate constant for the reaction are _____. A. M/s B. M-1 s-1 C. 1/s D. 1/M E. s/M 2

WARM-UP #5 If the rate law for the reaction, 2 A + 3 B products is first order in A and second order in B, then the rate law is R = A. k[A][B] B. k[A]2[B]3 C. k[A][B]2 D. k[A]2[B] E. k[A]2[B]2
![WARMUP 6 The rate law for a reaction is rate kAB2 Which one WARM-UP #6 The rate law for a reaction is rate = k[A][B]2 Which one](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-104.jpg)
WARM-UP #6 The rate law for a reaction is rate = k[A][B]2 Which one of the following statements is false? A. The reaction is first order in A. B. The reaction is second order in B. C. The reaction is second order overall. D. k is the reaction rate constant E. If [B] is doubled, the reaction rate will increase by a factor of 4.

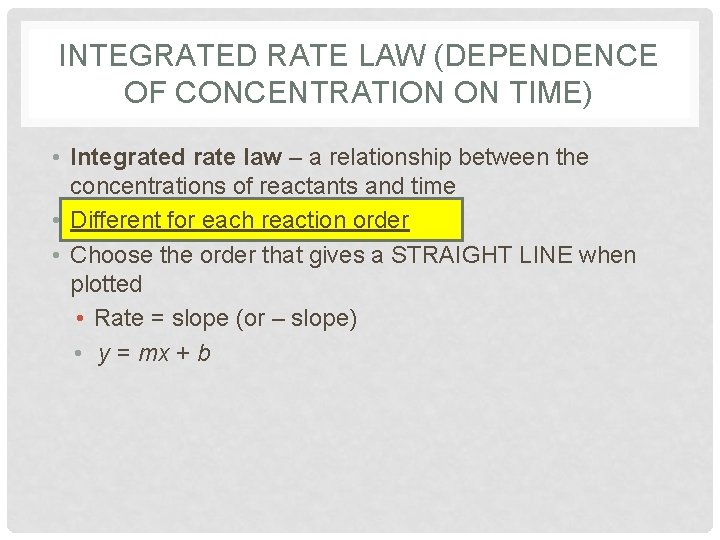
INTEGRATED RATE LAW (DEPENDENCE OF CONCENTRATION ON TIME) • Integrated rate law – a relationship between the concentrations of reactants and time • Different for each reaction order • Choose the order that gives a STRAIGHT LINE when plotted • Rate = slope (or – slope) • y = mx + b
![FIRST ORDER or At concentration of A at any time t • FIRST ORDER: or [A]t = concentration of A at any time, t](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-106.jpg)
• FIRST ORDER: or [A]t = concentration of A at any time, t [A]0 = initial concentration of A (time zero) k = rate constant

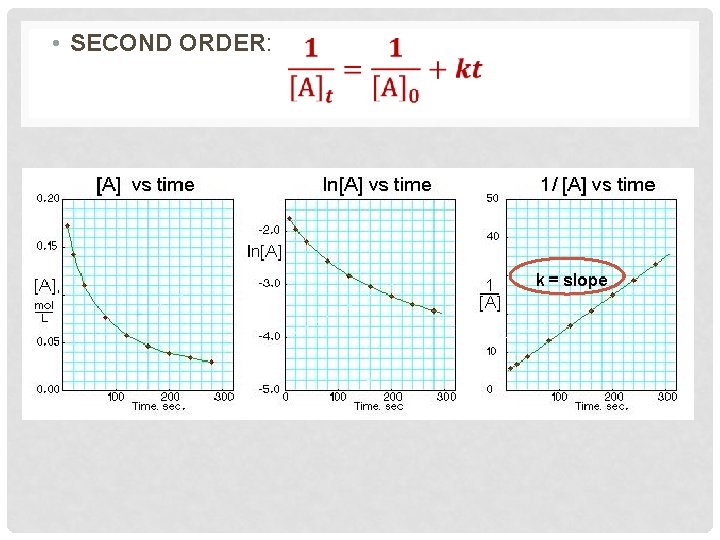
• SECOND ORDER:

• ZERO ORDER:

EXAMPLE #1 • Indicate the order of reaction consistent with each observation: • A plot of the concentration of the reactant versus time yields a straight line. • A plot of the inverse of the concentration versus time yields a straight line. • A plot of the natural log of the concentration of the reactant versus time yields a straight line.
![EXAMPLE 2 Time s AB M 0 50 100 150 200 250 300 350 EXAMPLE #2 Time (s) [AB] (M) 0 50 100 150 200 250 300 350](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-110.jpg)
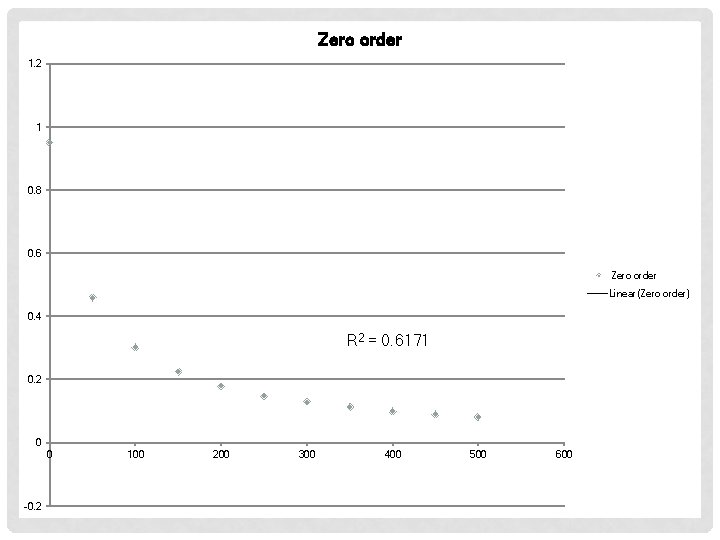
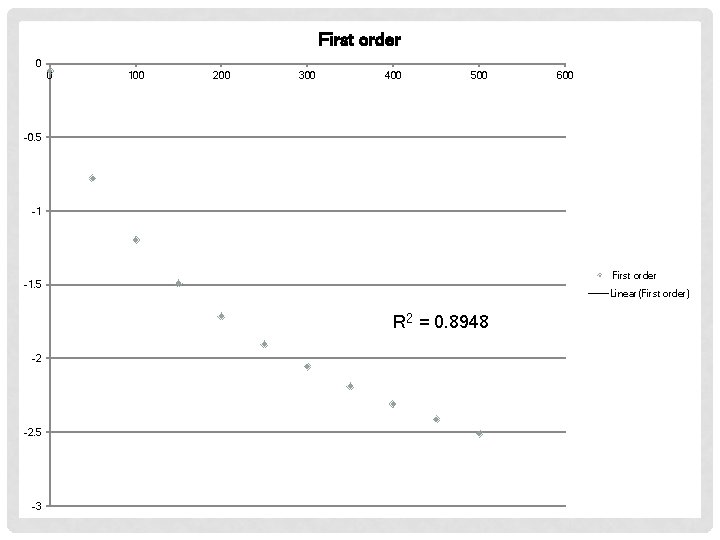
EXAMPLE #2 Time (s) [AB] (M) 0 50 100 150 200 250 300 350 400 450 500 0. 950 0. 459 0. 302 0. 225 0. 180 0. 149 0. 128 0. 112 0. 0994 0. 0812 First order Second Order

Zero order 1. 2 1 0. 8 0. 6 Zero order Linear(Zero order) 0. 4 R 2 = 0. 6171 0. 2 0 0 -0. 2 100 200 300 400 500 600

First order 0 0 100 200 300 400 500 600 -0. 5 -1 First order -1. 5 Linear(First order) R 2 = 0. 8948 -2 -2. 5 -3

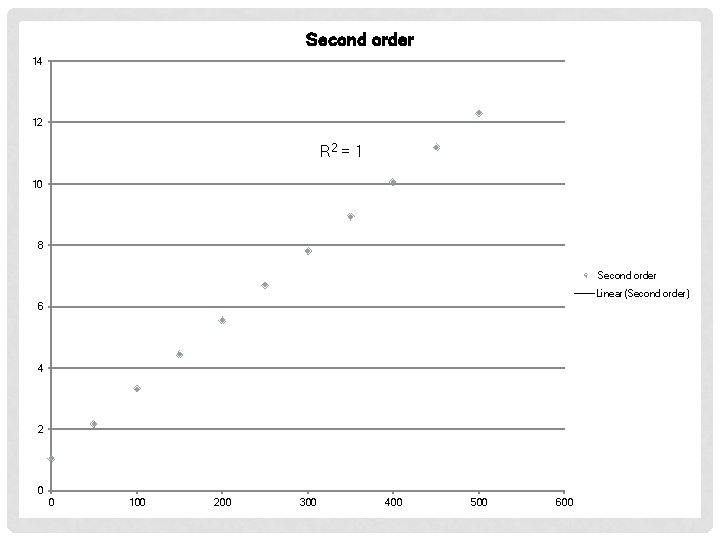
Second order 14 12 R 2 = 1 10 8 Second order Linear(Second order) 6 4 2 0 0 100 200 300 400 500 600

HALF LIFE • Half-life (t 1/2) – the time required for the concentration of a reactant to decrease by one-half of its initial value • EX: If a reaction has a half-life of 100 seconds, and if the initial concentration of the reactant is 1. 0 M, the concentration will fall to 0. 50 M in 100 seconds. • The half-life expression is different for each reaction order!

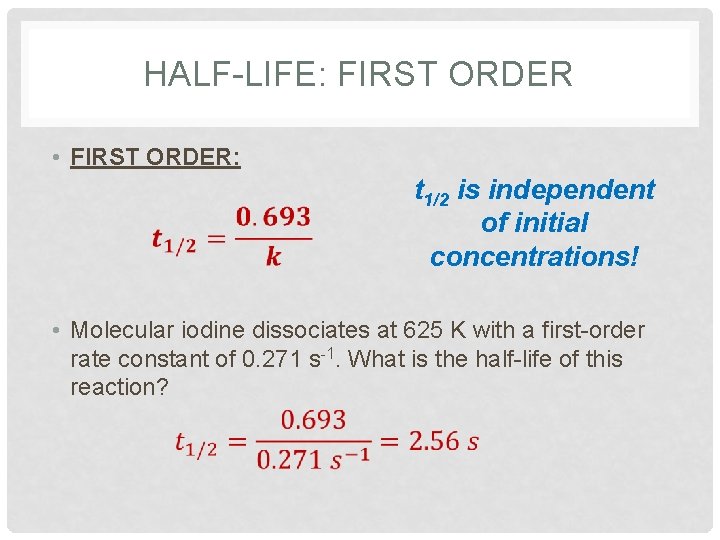
HALF-LIFE: FIRST ORDER • FIRST ORDER: t 1/2 is independent of initial concentrations! • Molecular iodine dissociates at 625 K with a first-order rate constant of 0. 271 s-1. What is the half-life of this reaction?

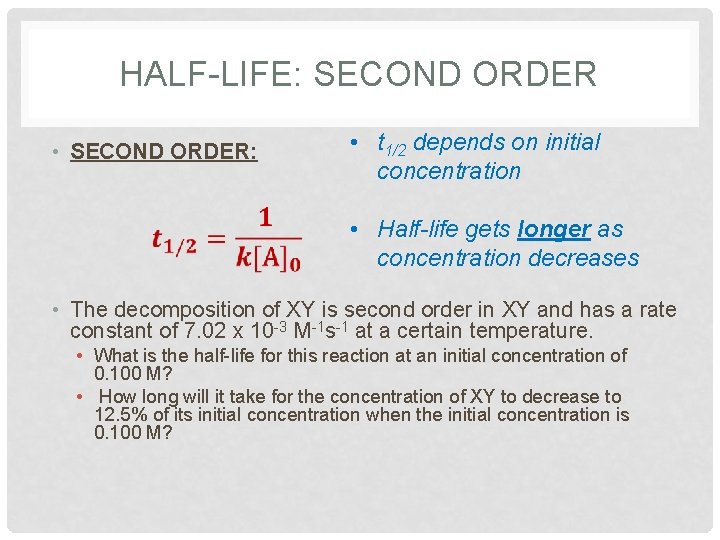
HALF-LIFE: SECOND ORDER • SECOND ORDER: • t 1/2 depends on initial concentration • Half-life gets longer as concentration decreases • The decomposition of XY is second order in XY and has a rate constant of 7. 02 x 10 -3 M-1 s-1 at a certain temperature. • What is the half-life for this reaction at an initial concentration of 0. 100 M? • How long will it take for the concentration of XY to decrease to 12. 5% of its initial concentration when the initial concentration is 0. 100 M?

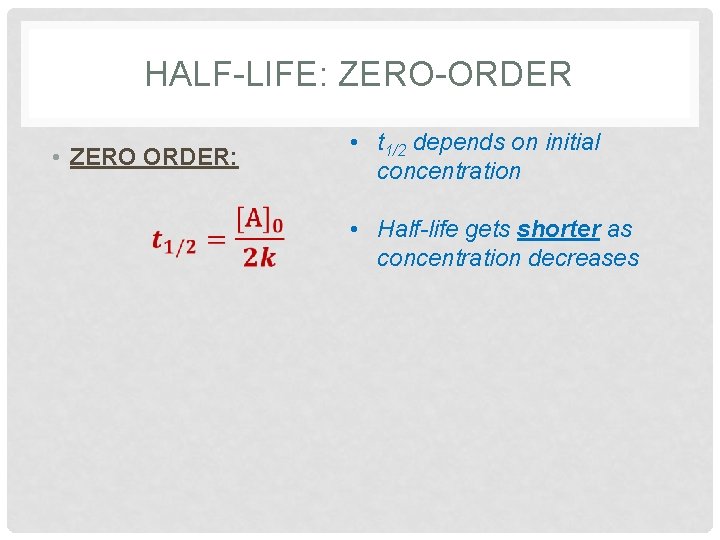
HALF-LIFE: ZERO-ORDER • ZERO ORDER: • t 1/2 depends on initial concentration • Half-life gets shorter as concentration decreases

WARM-UP • How many half lives have passed
![WARMUP 1 A reaction follows the rate law Rate kA2 Which of the WARM-UP #1 A reaction follows the rate law: Rate = k[A]2. Which of the](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-119.jpg)
WARM-UP #1 A reaction follows the rate law: Rate = k[A]2. Which of the following plots will give a straight line? A. 1/[A] versus 1/time B. [A]2 versus time C. 1/[A] versus time D. ln[A] versus time E. [A] versus time

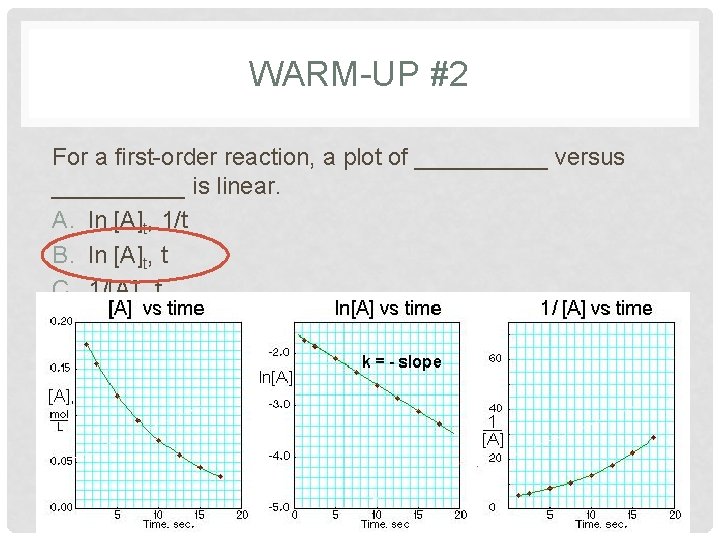
WARM-UP #2 For a first-order reaction, a plot of _____ versus _____ is linear. A. ln [A]t, 1/t B. ln [A]t, t C. 1/[A]t, t D. [A]t, t E. t, 1/[A]t

WARM-UP #3 The reaction 2 NOBr(g) → 2 NO(g) + Br 2(g) is a second-order reaction with a rate constant of 0. 50 M-1 s-1 at 11ᵒC. If the initial concentration of NOBr is 1. 0 M, the concentration of NOBr after 10. 0 seconds is _____. A. 0. 0500 M B. 0. 0250 M C. 0. 0312 M D. 0. 5000 M E. 0. 0625 M
![WARMUP 4 The following reaction is second order in A and the rate constant WARM-UP #4 The following reaction is second order in [A] and the rate constant](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-122.jpg)
WARM-UP #4 The following reaction is second order in [A] and the rate constant is 0. 039 M-1 s-1 A → B The concentration of A was 0. 30 M at 23 s. The initial concentration of A was _____ M. A. 2. 4 B. 0. 27 C. 0. 41 D. 3. 7 E. 1. 2 x 10 -2
![WARMUP 5 The reaction A B is first order in A Consider the WARM-UP #5 The reaction A → B is first order in [A]. Consider the](https://slidetodoc.com/presentation_image_h/c5b15c192c532746ba54469d3ee0485e/image-123.jpg)
WARM-UP #5 The reaction A → B is first order in [A]. Consider the following data. The half-life of this reaction is _____ s. A. 0. 97 B. 7. 1 C. 5. 0 D. 3. 0 E. 0. 14

INTEGRATED RATE LAWS

MAXWELL BOLTZMAN DIAGRAM

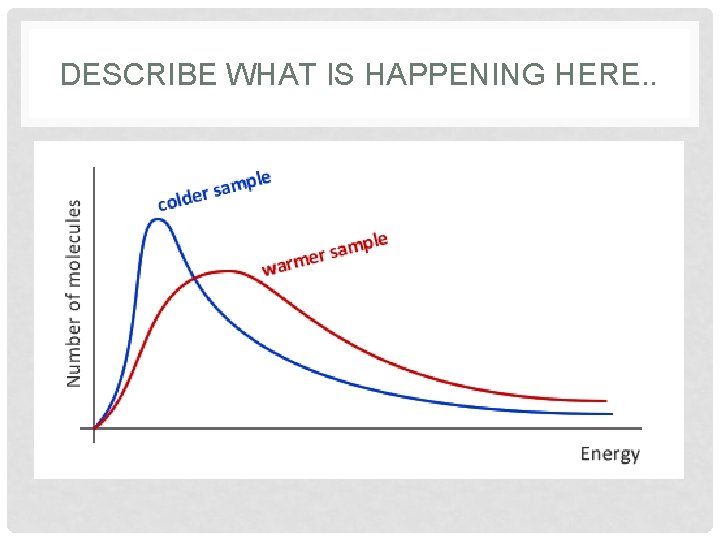
DESCRIBE WHAT IS HAPPENING HERE. .

MC QUESTIONS 2. The specific rate constant, k, for radioactive beryllium– 11 is 0. 049 s– 1. What mass of a 0. 500 mg sample of beryllium– 11 remains after 28 seconds? a) 0. 250 mg b) 0. 125 mg c) 0. 0625 mg d) 0. 375 mg e) 0. 500 mg

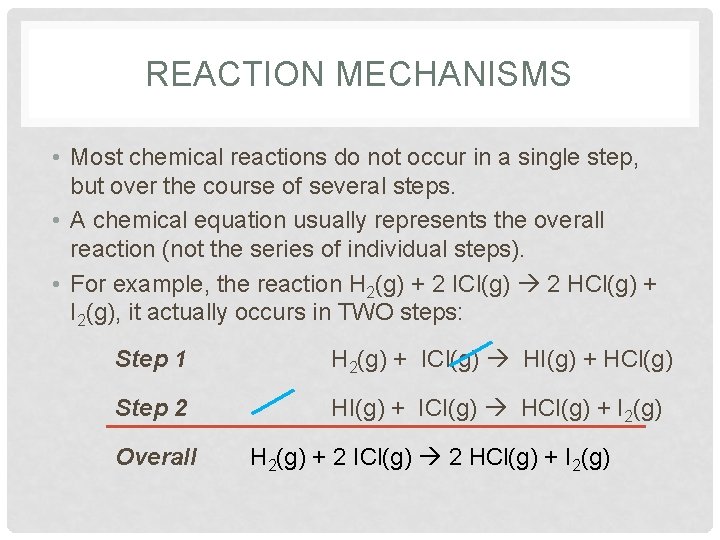
REACTION MECHANISMS • Most chemical reactions do not occur in a single step, but over the course of several steps. • A chemical equation usually represents the overall reaction (not the series of individual steps). • For example, the reaction H 2(g) + 2 ICl(g) 2 HCl(g) + I 2(g), it actually occurs in TWO steps: Step 1 H 2(g) + ICl(g) HI(g) + HCl(g) Step 2 HI(g) + ICl(g) HCl(g) + I 2(g) Overall H 2(g) + 2 ICl(g) 2 HCl(g) + I 2(g)

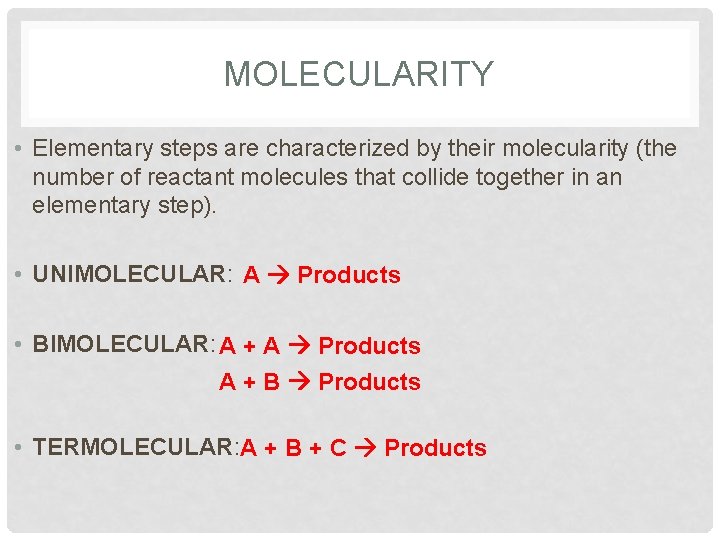
MOLECULARITY • Elementary steps are characterized by their molecularity (the number of reactant molecules that collide together in an elementary step). • UNIMOLECULAR: A Products • BIMOLECULAR: A + A Products A + B Products • TERMOLECULAR: A + B + C Products

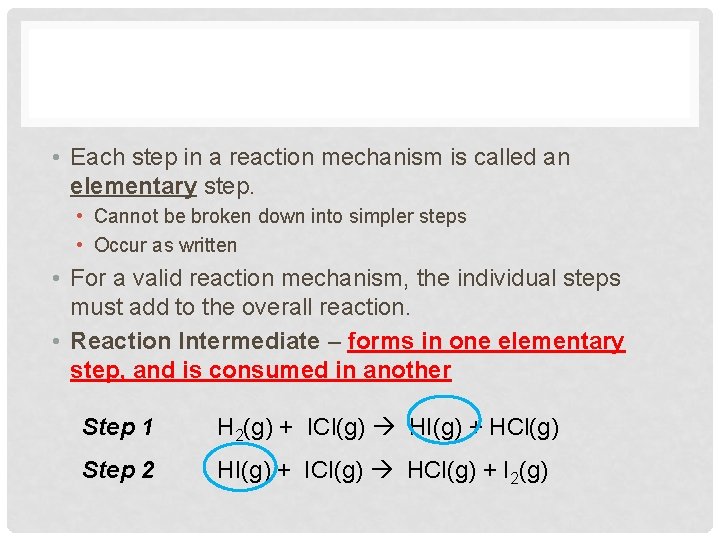
• Each step in a reaction mechanism is called an elementary step. • Cannot be broken down into simpler steps • Occur as written • For a valid reaction mechanism, the individual steps must add to the overall reaction. • Reaction Intermediate – forms in one elementary step, and is consumed in another Step 1 H 2(g) + ICl(g) HI(g) + HCl(g) Step 2 HI(g) + ICl(g) HCl(g) + I 2(g)

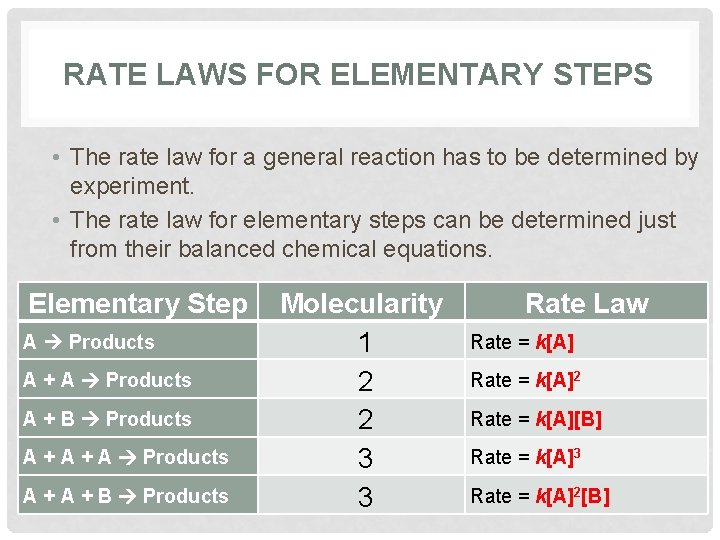
RATE LAWS FOR ELEMENTARY STEPS • The rate law for a general reaction has to be determined by experiment. • The rate law for elementary steps can be determined just from their balanced chemical equations. Elementary Step A Products A + B Products A + A Products A + B Products Molecularity 1 2 2 3 3 Rate Law Rate = k[A]2 Rate = k[A][B] Rate = k[A]3 Rate = k[A]2[B]

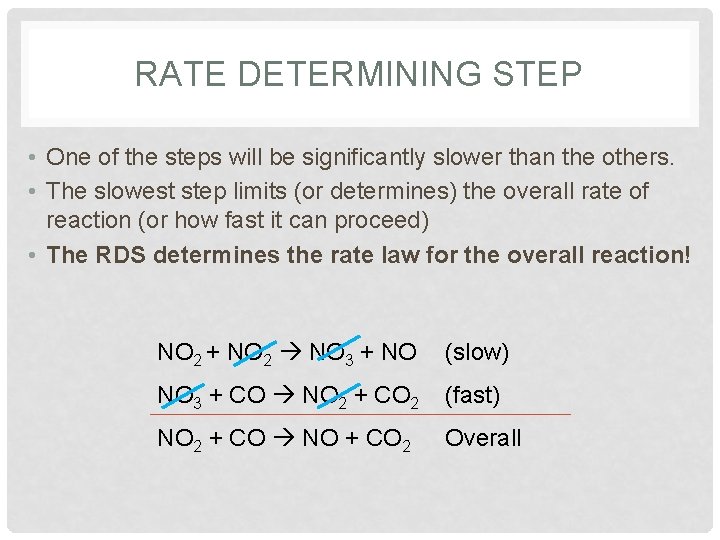
RATE DETERMINING STEP • One of the steps will be significantly slower than the others. • The slowest step limits (or determines) the overall rate of reaction (or how fast it can proceed) • The RDS determines the rate law for the overall reaction! NO 2 + NO 2 NO 3 + NO (slow) NO 3 + CO NO 2 + CO 2 (fast) NO 2 + CO NO + CO 2 Overall

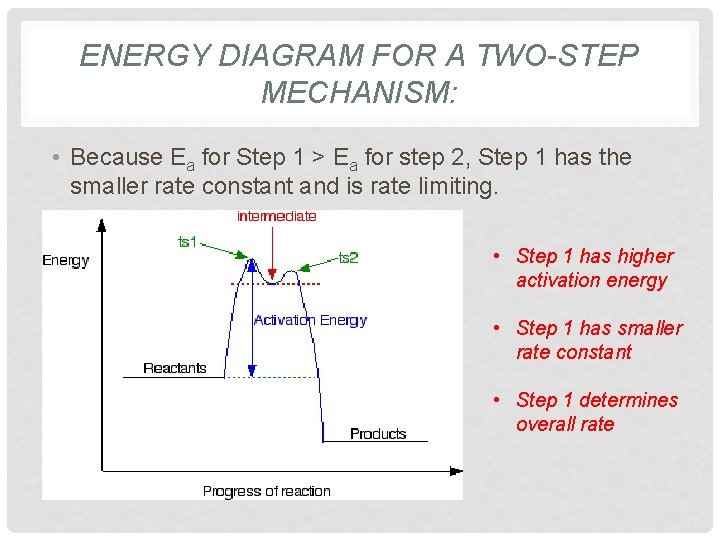
ENERGY DIAGRAM FOR A TWO-STEP MECHANISM: • Because Ea for Step 1 > Ea for step 2, Step 1 has the smaller rate constant and is rate limiting. • Step 1 has higher activation energy • Step 1 has smaller rate constant • Step 1 determines overall rate

WARM UP • Know which plots give a straight line for which reaction • Know half life formula for first order • Understand elementary reactions
 Molekülerite nedir
Molekülerite nedir Chemical reactions grade 11
Chemical reactions grade 11 Order of kinetics
Order of kinetics Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Steady state kinetics
Steady state kinetics Paradigm shift from women studies to gender studies
Paradigm shift from women studies to gender studies Empirical formula pogil
Empirical formula pogil Chemical formulas and chemical compounds chapter 7
Chemical formulas and chemical compounds chapter 7 Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Kinetics and equilibrium
Kinetics and equilibrium Zero order elimination drugs
Zero order elimination drugs Fbd and kd
Fbd and kd Antagonistic effect
Antagonistic effect Difference between 1st order and zero order kinetics
Difference between 1st order and zero order kinetics Collision theory of kinetics
Collision theory of kinetics Kinetics flotation reagents
Kinetics flotation reagents Kinetics of rigid bodies
Kinetics of rigid bodies Kinetics of rigid bodies engineering mechanics
Kinetics of rigid bodies engineering mechanics Kinetics of a particle force and acceleration
Kinetics of a particle force and acceleration Ap chem kinetics
Ap chem kinetics Kinetics of crystal violet fading
Kinetics of crystal violet fading Octet kinetics
Octet kinetics Enzyme catalyze
Enzyme catalyze Pseudo first order equation
Pseudo first order equation Kinetics of particles newton's second law
Kinetics of particles newton's second law Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Dynafit kinetics
Dynafit kinetics Enzyme kinetics
Enzyme kinetics Abbas et al
Abbas et al Planar kinematics of a rigid body
Planar kinematics of a rigid body Data kinetics ltd
Data kinetics ltd Kcat
Kcat Planar kinetics of a rigid body work and energy
Planar kinetics of a rigid body work and energy Kinetics reaction
Kinetics reaction Growth kinetics
Growth kinetics Kinetics is the branch of
Kinetics is the branch of Fermenter
Fermenter Vampnets for deep learning of molecular kinetics
Vampnets for deep learning of molecular kinetics Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chemical names and formulas chapter 9
Chemical names and formulas chapter 9 Malaysian studies chapter 1
Malaysian studies chapter 1 Grade 9 social studies chapter 3 test
Grade 9 social studies chapter 3 test Social studies chapter 2
Social studies chapter 2 Chapter 13 experiments and observational studies
Chapter 13 experiments and observational studies Mississippi tornado
Mississippi tornado Mississippi studies chapter 1
Mississippi studies chapter 1 Mississippi studies chapter 1
Mississippi studies chapter 1 What influenced mexico’s political and social structures
What influenced mexico’s political and social structures Matched pairs design statistics
Matched pairs design statistics Ms studies chapter 1
Ms studies chapter 1 Chapter 4 designing studies
Chapter 4 designing studies Social studies chapter 3
Social studies chapter 3 Social studies chapter 3
Social studies chapter 3 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 review chemical equilibrium
Chapter 18 review chemical equilibrium Chapter 6
Chapter 6 Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide Chapter 9 chemical names and formulas
Chapter 9 chemical names and formulas Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 Label an atom
Label an atom Double flat wrap perm
Double flat wrap perm Chapter 18 review chemical equilibrium
Chapter 18 review chemical equilibrium Chemical equilibrium chapter 18 review
Chemical equilibrium chapter 18 review Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 7 chemical quantities answer key
Chapter 7 chemical quantities answer key Chapter 6
Chapter 6 Chemical quantities chapter 10
Chemical quantities chapter 10 Chemical and waste management chapter 23
Chemical and waste management chapter 23 Chapter 6 review chemical bonding
Chapter 6 review chemical bonding Chapter 6 how cells harvest chemical energy
Chapter 6 how cells harvest chemical energy Chapter 6 chemical bonding section 4
Chapter 6 chemical bonding section 4 Chemical bonding chapter 6 review
Chemical bonding chapter 6 review Chapter 2 the chemical context of life
Chapter 2 the chemical context of life Synthesis reaction
Synthesis reaction Examples of double replacement reactions
Examples of double replacement reactions Chapter 10 chemical quantities practice problems answer key
Chapter 10 chemical quantities practice problems answer key Water vaporization equation
Water vaporization equation Chapter 18 chemical equilibrium answer key
Chapter 18 chemical equilibrium answer key Chapter 6 chemical bonding
Chapter 6 chemical bonding