15 Chemical Kinetics Chemical kinetics the study of



![Variation of Reaction rates and Order 2 nd order, rate = k [A]2 rate Variation of Reaction rates and Order 2 nd order, rate = k [A]2 rate](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-7.jpg)


![Differential Rate Law determination – continue The reaction rate –d[H 2 O 2]/dt = Differential Rate Law determination – continue The reaction rate –d[H 2 O 2]/dt =](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-11.jpg)


![A 2 nd Order Example 1 1 —— – —— = k t [B]o A 2 nd Order Example 1 1 —— – —— = k t [B]o](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-21.jpg)
![Half life of 2 nd Order Chemical Kinetics 1/[B] t = 1 5 10 Half life of 2 nd Order Chemical Kinetics 1/[B] t = 1 5 10](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-22.jpg)
![Plot of [B] vs. t & 1/[B] vs. t for 2 nd Order Reactions Plot of [B] vs. t & 1/[B] vs. t for 2 nd Order Reactions](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-23.jpg)

![The Effect of Temperature on Reaction Rates Reaction rate = k [A}x[B]y[C]z constant T) The Effect of Temperature on Reaction Rates Reaction rate = k [A}x[B]y[C]z constant T)](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-29.jpg)

![Steady-state approximation - 3 From the previous result: k 1 k 2 [H 2] Steady-state approximation - 3 From the previous result: k 1 k 2 [H 2]](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-42.jpg)



- Slides: 51

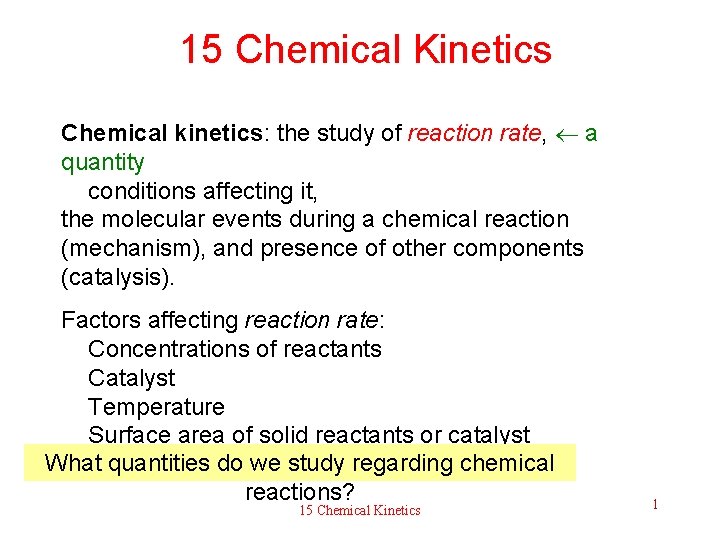
15 Chemical Kinetics Chemical kinetics: the study of reaction rate, a quantity conditions affecting it, the molecular events during a chemical reaction (mechanism), and presence of other components (catalysis). Factors affecting reaction rate: Concentrations of reactants Catalyst Temperature Surface area of solid reactants or catalyst What quantities do we study regarding chemical reactions? 15 Chemical Kinetics 1

Reaction Rate Defined Reaction rate: changes in a concentration of a product or a reactant per unit time. [ ] concentration Reaction rate = —— t chang e [ ] t Define reaction rate and explain Average reaction rate Instantaneous reaction rate (2 tangents shown) 15 Chemical Kinetics Initial reaction rate t 2

Expressing reaction rates For a chemical reaction, there are many ways to express the reaction rate. The relationships among expressions depend on the equation. Note the expression and reasons for their relations for the reaction 2 NO + O 2 (g) = 2 NO 2 (g) [O 2] 1 [NO] 1 [NO 2] Reaction rate = – ——— = – — ———— = — ——— t 2 t Make sure you can write expressions for any reaction and figure out the relationships. For example, give the 3 How can the rate expression be unique and reaction rate expressions for 15 Chemical Kinetics

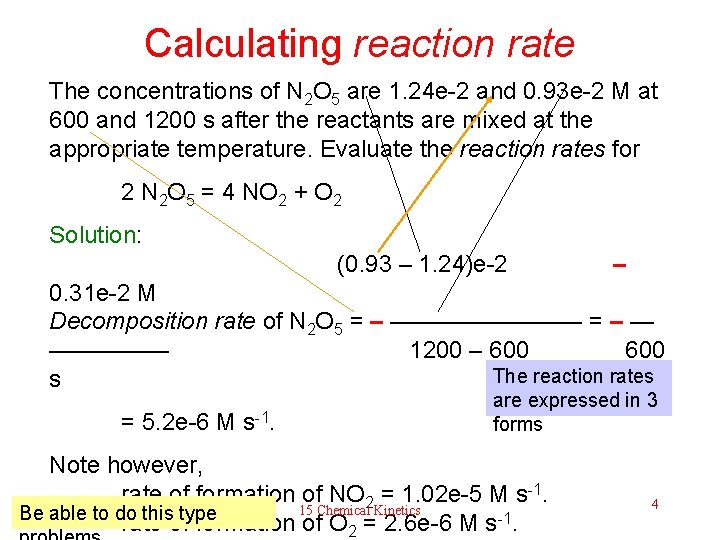
Calculating reaction rate The concentrations of N 2 O 5 are 1. 24 e-2 and 0. 93 e-2 M at 600 and 1200 s after the reactants are mixed at the appropriate temperature. Evaluate the reaction rates for 2 N 2 O 5 = 4 NO 2 + O 2 Solution: (0. 93 – 1. 24)e-2 – 0. 31 e-2 M Decomposition rate of N 2 O 5 = – ———— = – — ————— 1200 – 600 The reaction rates s = 5. 2 e-6 M s-1. are expressed in 3 forms Note however, rate of formation of NO 2 = 1. 02 e-5 M s-1. 15 Chemical Kinetics Be able to do this type rate of formation of O 2 = 2. 6 e-6 M s-1. 4

Determine Reaction Rates To measure reaction rate, we measure the concentration of either a reactant or product at several time intervals. The concentrations are measured using spectroscopic method or pressure (for a gas). For example, the total pressure increases for the reaction: baromet er 2 N 2 O 5 (g) 4 NO 2 (g) + O 2(g) Because 5 moles of gas products are produced from 2 moles of gas reactants. For the reaction Ca. CO 3 (s) Ca. O(s) + CO 2 (g) The increase in gas pressure is entirely due to 15 Chemical Kinetics CO 2 formed. 5

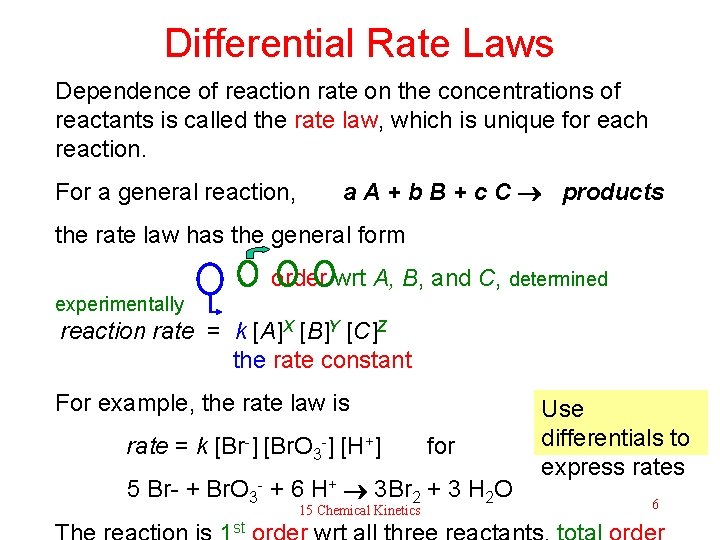
Differential Rate Laws Dependence of reaction rate on the concentrations of reactants is called the rate law, which is unique for each reaction. For a general reaction, a A + b B + c C products the rate law has the general form order wrt A, B, and C, determined experimentally reaction rate = k [A]X [B]Y [C]Z the rate constant For example, the rate law is rate = k [Br-] [Br. O 3 -] [H+] for 5 Br- + Br. O 3 - + 6 H+ 3 Br 2 + 3 H 2 O 15 Chemical Kinetics st Use differentials to express rates 6
![Variation of Reaction rates and Order 2 nd order rate k A2 rate Variation of Reaction rates and Order 2 nd order, rate = k [A]2 rate](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-7.jpg)
Variation of Reaction rates and Order 2 nd order, rate = k [A]2 rate First order, rate = k [A] k = rate, 0 th order [A] = ___? [A] The variation of reaction rates as functions of concentration for various order is interesting. 15 Chemical Kinetics Mathematical analysis is an important scientific tool, 7

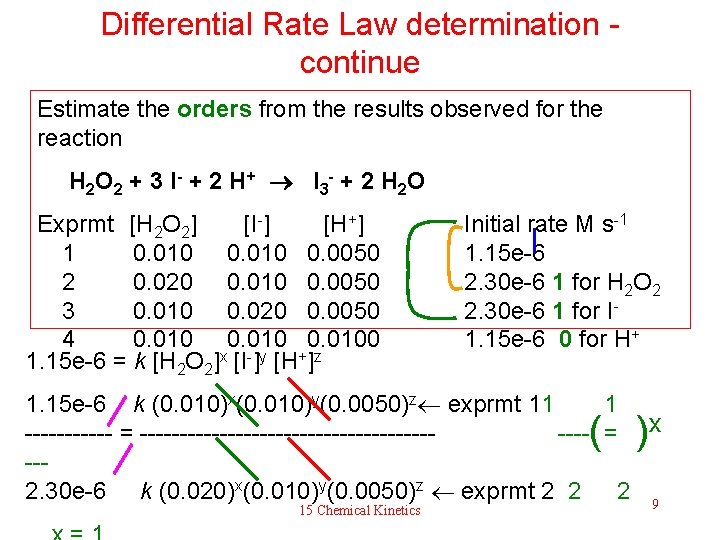
Differential Rate Law determination Estimate the orders and rate constant k from the results observed for the reaction? What is the rate when [H 2 O 2] = [I-] = [H+] = 1. 0 M? H 2 O 2 + 3 I - + 2 H + I 3 - + 2 H 2 O Exprmt [H 2 O 2] [I-] [H+] Initial rate M s-1 1 0. 010 0. 0050 1. 15 e-6 2 0. 020 0. 010 0. 0050 2. 30 e-6 3 0. 010 0. 020 0. 0050 2. 30 e-6 4 0. 0100 1. 15 e-6 Learn the strategy to determine the rate law from this example. Figure out the answer without writing down anything. Solution 15 Chemical Kinetics 8

Differential Rate Law determination - continue Estimate the orders from the results observed for the reaction H 2 O 2 + 3 I - + 2 H + I 3 - + 2 H 2 O Exprmt [H 2 O 2] [I-] [H+] 1 0. 010 0. 0050 2 0. 020 0. 010 0. 0050 3 0. 010 0. 020 0. 0050 4 0. 0100 1. 15 e-6 = k [H 2 O 2]x [I-]y [H+]z Initial rate M s-1 1. 15 e-6 2. 30 e-6 1 for H 2 O 2 2. 30 e-6 1 for I 1. 15 e-6 0 for H+ 1. 15 e-6 k (0. 010)x(0. 010)y(0. 0050)z exprmt 11 1 ------ = ------------------- = x --- 2. 30 e-6 k (0. 020)x(0. 010)y(0. 0050)z exprmt 2 2 2 9 ( ) 15 Chemical Kinetics

Differential Rate Law determination - continue Other orders are determined in a similar way as shown before. Now, lets find k and the rate Thurs, rate = 1. 15 e-6 = k (0. 010) from exprmt 1 k = 1. 15 e-6 M s-1 / (0. 010) M 3 = 0. 0115 M-1 s-1 And the rate law is therefore, – d [H 2 O 2] k rate =————— [I-]a differential rate law dt = 0. 0115 [H 2 O 2] total order 2 The rate when [H 2 O 2] = [I-] = [H+] = 1. 0 M: The rate is the same as the rate constant k, when concentrations of reactants are all unity (exactly 1), 15 Chemical Kinetics doesn’t matter what the orders are. 10
![Differential Rate Law determination continue The reaction rate dH 2 O 2dt Differential Rate Law determination – continue The reaction rate –d[H 2 O 2]/dt =](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-11.jpg)
Differential Rate Law determination – continue The reaction rate –d[H 2 O 2]/dt = 0. 0115 [H 2 O 2] [I–], for H 2 O 2 + 3 I- + 2 H+ I 3 - + 2 H 2 O What is – d[I–]/dt when [H 2 O 2] = [I–] = 0. 5? Solution: Please note the stoichiometry of equation and how the rate changes. – d[I–]/dt = – 3 d[H 2 O 2]/dt = 3* 0. 0115 [H 2 O 2] [I–] = 0. 0345 * 0. 5 = 0. 0086 M s-1 In order to get a unique rate constant k, we evaluate k for the reaction a A + b B product this way rate = -1/a d[A]/dt = -1/b d[B]/dt = k [A]x [B]y 15 Chemical Kinetics Note the reaction rate expression and the stoichiometry 11

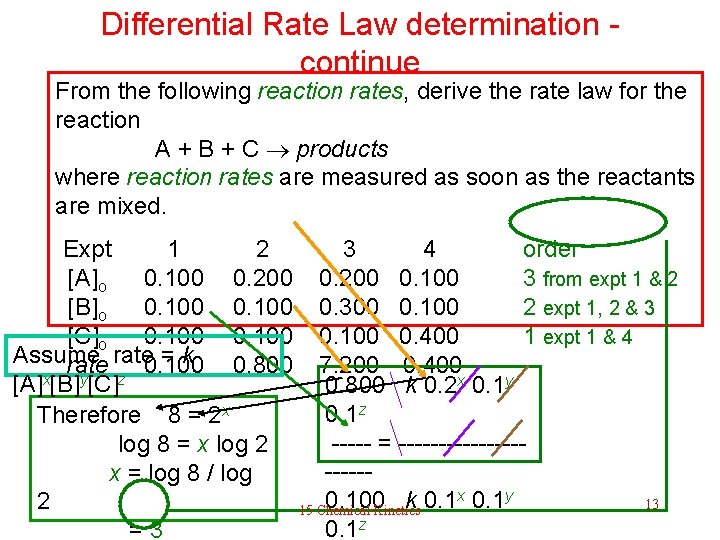
Differential Rate Law determination - continue From the following reaction rates observed in 4 experiments, derive the rate law for the reaction A + B + C products where reaction rates are measured as soon as the reactants are mixed. Expt [A]o [B]o [C]o rate 1 0. 100 2 0. 200 0. 100 0. 800 3 0. 200 0. 300 0. 100 7. 200 4 0. 100 0. 400 This example illustrates the strategy to determine, and a reliable method to solve ratelaw experimentally. 15 Chemical Kinetics Solution next 12

Differential Rate Law determination - continue From the following reaction rates, derive the rate law for the reaction A + B + C products where reaction rates are measured as soon as the reactants are mixed. Expt 1 2 3 4 order [A]o 0. 100 0. 200 0. 100 3 from expt 1 & 2 [B]o 0. 100 0. 300 0. 100 2 expt 1, 2 & 3 [C]o 0. 100 0. 400 1 expt 1 & 4 Assume rate = k rate 0. 100 0. 800 7. 200 0. 400 x y z [A] [B] [C] 0. 800 k 0. 2 x 0. 1 y 0. 1 z Therefore 8 = 2 x log 8 = x log 2 ----- = ----------x = log 8 / log x 0. 1 y 0. 100 k 0. 1 13 2 15 Chemical Kinetics 0. 1 z = 3

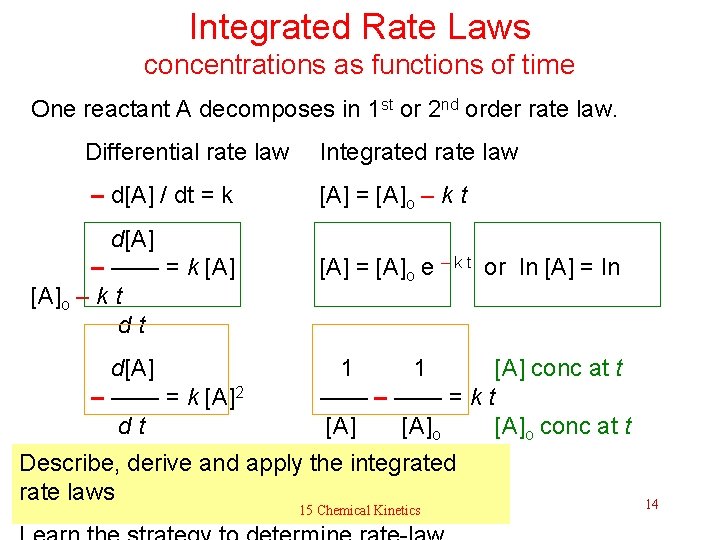
Integrated Rate Laws concentrations as functions of time One reactant A decomposes in 1 st or 2 nd order rate law. Differential rate law Integrated rate law – d[A] / dt = k [A] = [A]o – k t d[A] – —— = k [A]o – k t d[A] – —— = k [A]2 d t [A] = [A]o e – k t or ln [A] = ln 1 1 [A] conc at t —— – —— = k t [A]o conc at t = 0 Describe, derive and apply the integrated rate laws 15 Chemical Kinetics 14

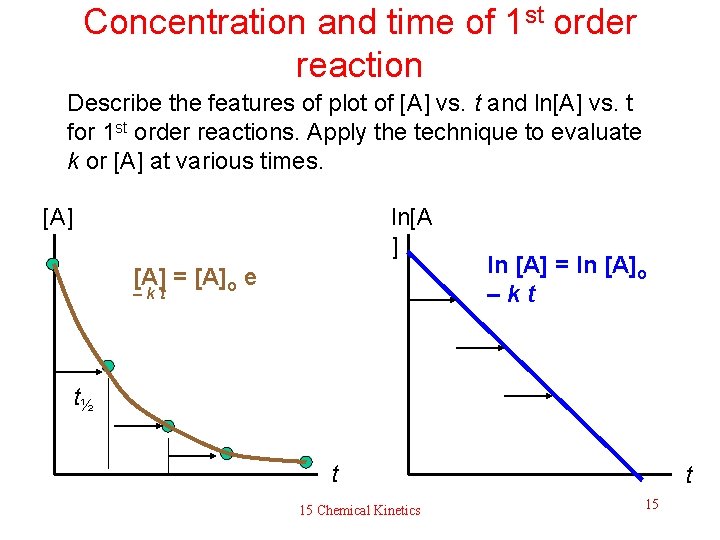
Concentration and time of 1 st order reaction Describe the features of plot of [A] vs. t and ln[A] vs. t for 1 st order reactions. Apply the technique to evaluate k or [A] at various times. [A] ln[A ] [A] = [A]o e –kt ln [A] = ln [A]o –kt t½ t 15 Chemical Kinetics t 15

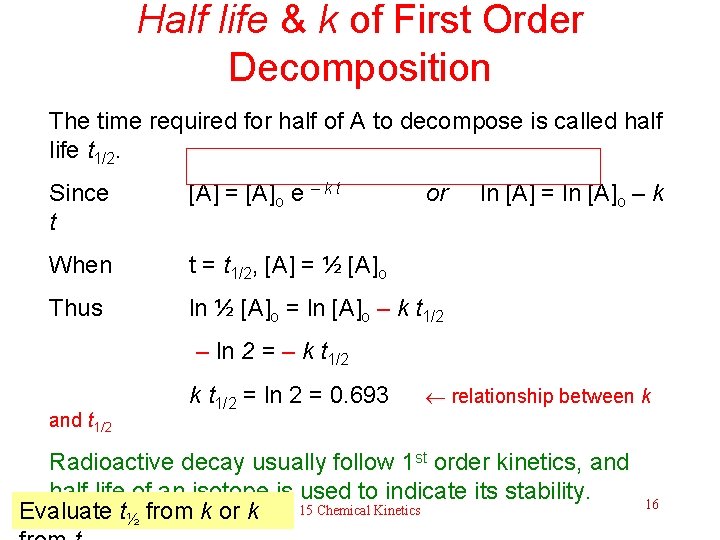
Half life & k of First Order Decomposition The time required for half of A to decompose is called half life t 1/2. Since t [A] = [A]o e – k t or When t = t 1/2, [A] = ½ [A]o Thus ln ½ [A]o = ln [A]o – k t 1/2 ln [A] = ln [A]o – k – ln 2 = – k t 1/2 and t 1/2 k t 1/2 = ln 2 = 0. 693 relationship between k Radioactive decay usually follow 1 st order kinetics, and half life of an isotope is used to indicate its stability. Evaluate t½ from k or k 15 Chemical Kinetics 16

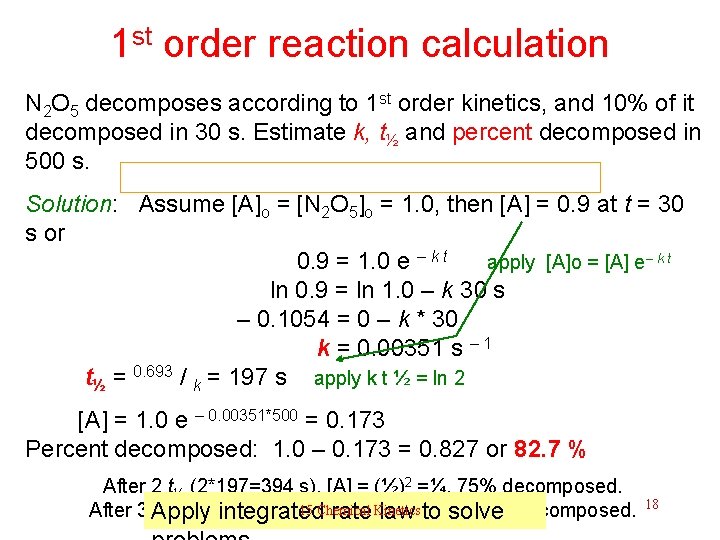
1 st order reaction calculation N 2 O 5 decomposes according to 1 st order kinetics, and 10% of it decomposed in 30 s. Estimate k, t½ and percent decomposed in 500 s. If the rate-law is known, what are the key parameters? 15 Chemical Kinetics Solution next 17

1 st order reaction calculation N 2 O 5 decomposes according to 1 st order kinetics, and 10% of it decomposed in 30 s. Estimate k, t½ and percent decomposed in 500 s. Solution: Assume [A]o = [N 2 O 5]o = 1. 0, then [A] = 0. 9 at t = 30 s or 0. 9 = 1. 0 e – k t apply [A]o = [A] e– k t ln 0. 9 = ln 1. 0 – k 30 s – 0. 1054 = 0 – k * 30 k = 0. 00351 s – 1 t = 0. 693 / = 197 s apply k t ½ = ln 2 ½ k [A] = 1. 0 e – 0. 00351*500 = 0. 173 Percent decomposed: 1. 0 – 0. 173 = 0. 827 or 82. 7 % After 2 t½ (2*197=394 s), [A] = (½)2 =¼, 75% decomposed. 3 15 Chemical Kinetics After 3 t. Apply integrated rate law to solve ½ (3*197=591 s), [A] = (½) =1/8, 87. 5% decomposed. 18

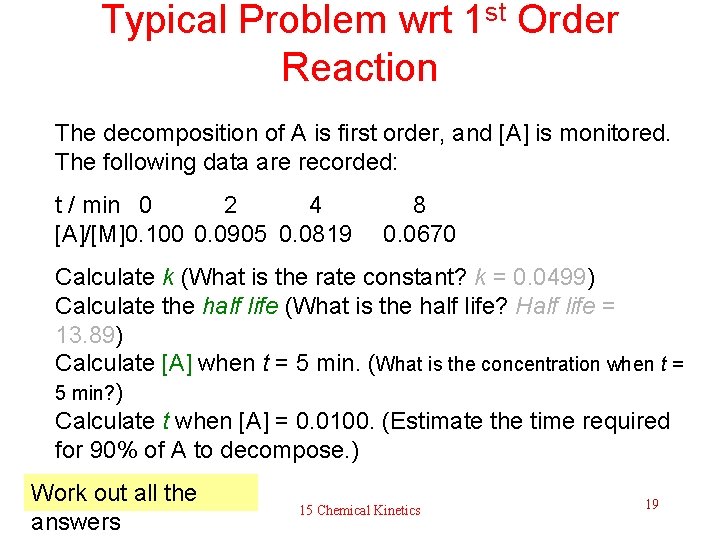
Typical Problem wrt 1 st Order Reaction The decomposition of A is first order, and [A] is monitored. The following data are recorded: t / min 0 2 4 [A]/[M]0. 100 0. 0905 0. 0819 8 0. 0670 Calculate k (What is the rate constant? k = 0. 0499) Calculate the half life (What is the half life? Half life = 13. 89) Calculate [A] when t = 5 min. (What is the concentration when t = 5 min? ) Calculate t when [A] = 0. 0100. (Estimate the time required for 90% of A to decompose. ) Work out all the answers 15 Chemical Kinetics 19

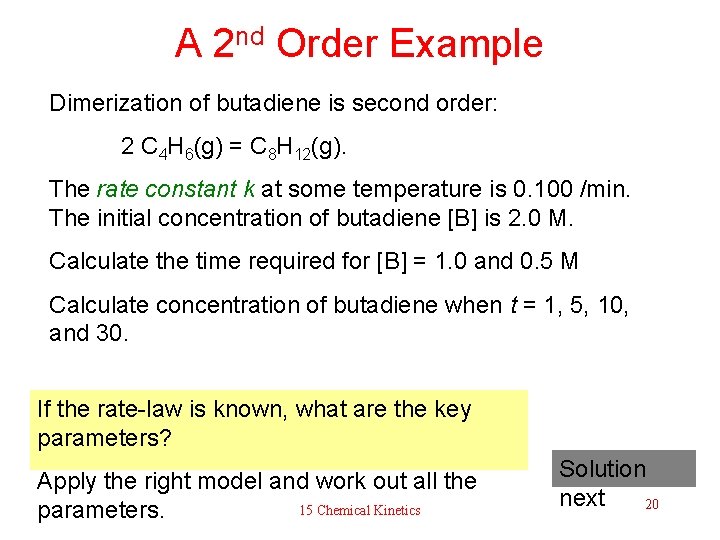
A 2 nd Order Example Dimerization of butadiene is second order: 2 C 4 H 6(g) = C 8 H 12(g). The rate constant k at some temperature is 0. 100 /min. The initial concentration of butadiene [B] is 2. 0 M. Calculate the time required for [B] = 1. 0 and 0. 5 M Calculate concentration of butadiene when t = 1, 5, 10, and 30. If the rate-law is known, what are the key parameters? Apply the right model and work out all the 15 Chemical Kinetics parameters. Solution next 20
![A 2 nd Order Example 1 1 k t Bo A 2 nd Order Example 1 1 —— – —— = k t [B]o](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-21.jpg)
A 2 nd Order Example 1 1 —— – —— = k t [B]o Dimerization of butadiene is second order: 1 1 —— – —— [B]o t = ———— k 2 C 4 H 6(g) = C 8 H 12(g). The rate constant k at some temperature is 0. 100 /min. The initial concentration of butadiene [B] is 2. 0 M. Calculate the time t required for [B] = 1. 0 and 0. 5 M Calculate concentration of t = 1 5 10 15 30 butadiene when t = 1, 5, 10, and 30. 35 [B]o [B] = —————— [B]o k t + 1 [B] = 1. 671. 00. 67 0. 50 0. 29 0. 25 Work out the formulas and then 15 Chemical Kinetics 21
![Half life of 2 nd Order Chemical Kinetics 1B t 1 5 10 Half life of 2 nd Order Chemical Kinetics 1/[B] t = 1 5 10](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-22.jpg)
Half life of 2 nd Order Chemical Kinetics 1/[B] t = 1 5 10 15 30 35 [B] = 1. 671. 00. 67 0. 50 0. 29 0. 25 Kinetics How does half life vary in 215 nd. Chemical order 22
![Plot of B vs t 1B vs t for 2 nd Order Reactions Plot of [B] vs. t & 1/[B] vs. t for 2 nd Order Reactions](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-23.jpg)
Plot of [B] vs. t & 1/[B] vs. t for 2 nd Order Reactions t = 1 5 10 15 30 35 [B] = 1. 671. 00. 67 0. 50 0. 29 0. 25 [B] 1 — [B]o [B] = ————— — [B]o k t + 1 t 15 Chemical Kineticsnd st What kind of plot is linear for 1 and 2 1 1 —— – —— = k t [B]o t 23

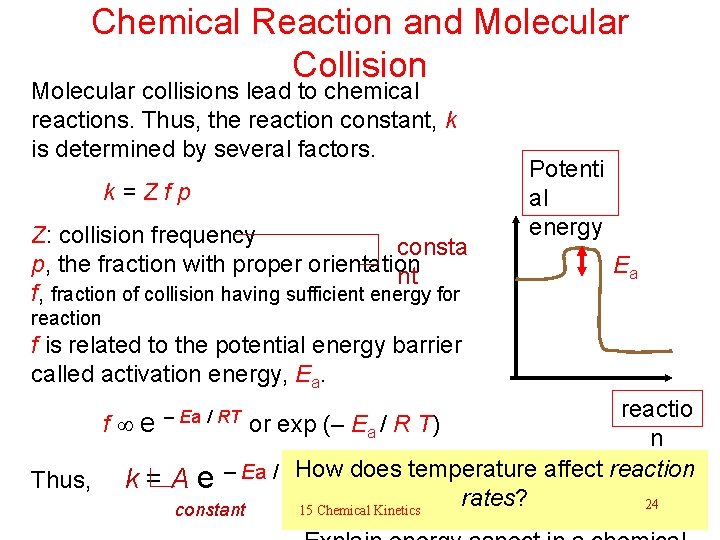
Chemical Reaction and Molecular Collision Molecular collisions lead to chemical reactions. Thus, the reaction constant, k is determined by several factors. k=Zfp Z: collision frequency consta p, the fraction with proper orientation nt f, fraction of collision having sufficient energy for Potenti al energy Ea reaction f is related to the potential energy barrier called activation energy, Ea. reactio n How does temperature affect reaction Thus, k = A e – Ea / RT rates? 24 15 Chemical Kinetics constant f e – Ea / RT or exp (– Ea / R T)

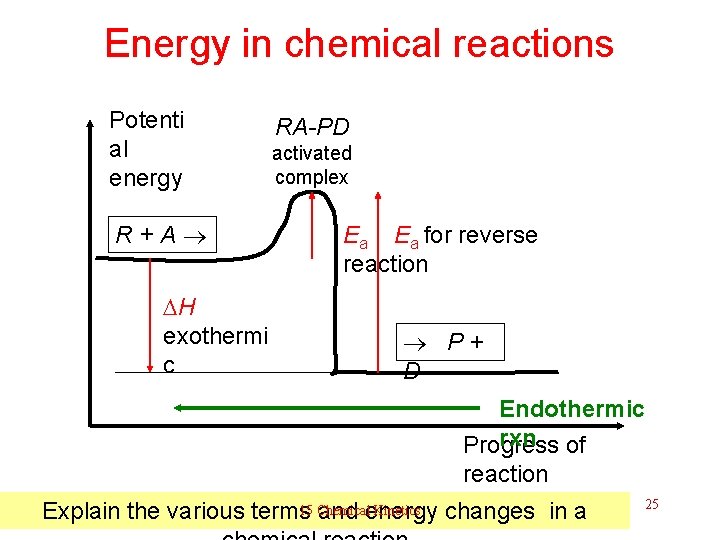
Energy in chemical reactions Potenti al energy R + A H exothermi c RA-PD activated complex Ea Ea for reverse reaction P + D Endothermic rxn Progress of reaction 15 Chemical Kinetics Explain the various terms and energy changes in a 25

The Arrhenius Equation The temperature dependence of the rate constant k is best described by the Arrhenius equation: or k = A e – Ea / R T ln k = ln A – Ea / R T If k 1 and k 2 are the rate constants at T 1 and T 2 respectively, then k 1 Ea 1 1 ln —— = – — — – — k 2 R T 1 T 2 1903 Nobel Prize citation” …in recognition of the extraordinary services he has rendered to the advancement of chemistry by his electrolytic theory of dissociation” How does temperature affect reaction rates? Derive and apply these relationship to solve 15 Chemical Kinetics problems, and recall the Clausius-Clapeyron 26

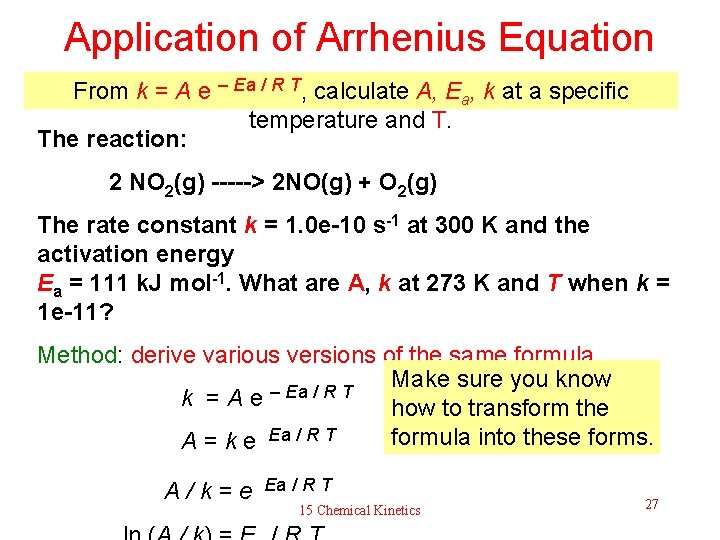
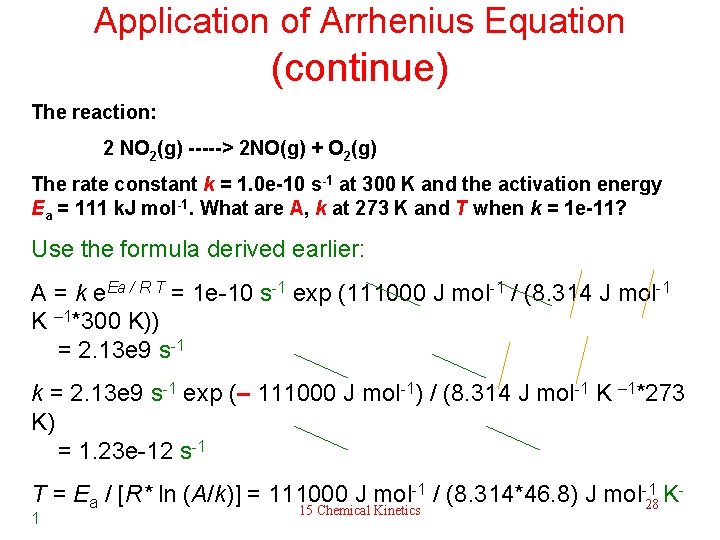
Application of Arrhenius Equation From k = A e – Ea / R T, calculate A, Ea, k at a specific temperature and T. The reaction: 2 NO 2(g) -----> 2 NO(g) + O 2(g) The rate constant k = 1. 0 e-10 s-1 at 300 K and the activation energy Ea = 111 k. J mol-1. What are A, k at 273 K and T when k = 1 e-11? Method: derive various versions of the same formula Make sure you know – Ea / R T k = A e how to transform the formula into these forms. A = k e Ea / R T A / k = e Ea / R T 15 Chemical Kinetics 27

Application of Arrhenius Equation (continue) The reaction: 2 NO 2(g) -----> 2 NO(g) + O 2(g) The rate constant k = 1. 0 e-10 s-1 at 300 K and the activation energy Ea = 111 k. J mol-1. What are A, k at 273 K and T when k = 1 e-11? Use the formula derived earlier: A = k e. Ea / R T = 1 e-10 s-1 exp (111000 J mol-1 / (8. 314 J mol-1 K – 1*300 K)) = 2. 13 e 9 s-1 k = 2. 13 e 9 s-1 exp (– 111000 J mol-1) / (8. 314 J mol-1 K – 1*273 K) = 1. 23 e-12 s-1 T = Ea / [R* ln (A/k)] = 111000 J mol-1 / (8. 314*46. 8) J mol-128 K 15 Chemical Kinetics 1
![The Effect of Temperature on Reaction Rates Reaction rate k AxByCz constant T The Effect of Temperature on Reaction Rates Reaction rate = k [A}x[B]y[C]z constant T)](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-29.jpg)
The Effect of Temperature on Reaction Rates Reaction rate = k [A}x[B]y[C]z constant T) (Concentration effect at k = A exp ( – Ea / RT) (Temperature effect) Use graphic method to discuss the variation of k vs. T variation of k vs 1 / T variation of ln(k) vs 1 / T See a potential multiple choice question in an exam? 15 Chemical Kinetics 29

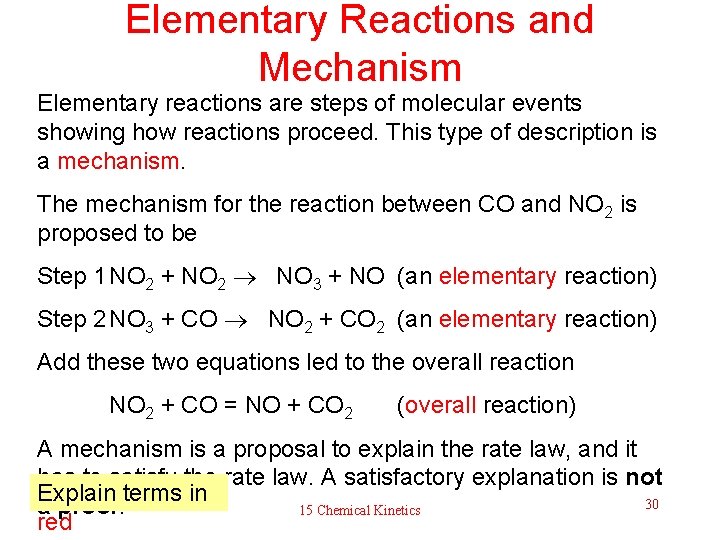
Elementary Reactions and Mechanism Elementary reactions are steps of molecular events showing how reactions proceed. This type of description is a mechanism. The mechanism for the reaction between CO and NO 2 is proposed to be Step 1 NO 2 + NO 2 NO 3 + NO (an elementary reaction) Step 2 NO 3 + CO NO 2 + CO 2 (an elementary reaction) Add these two equations led to the overall reaction NO 2 + CO = NO + CO 2 (overall reaction) A mechanism is a proposal to explain the rate law, and it has to satisfy the rate law. A satisfactory explanation is not Explain terms in 30 a proof. 15 Chemical Kinetics red

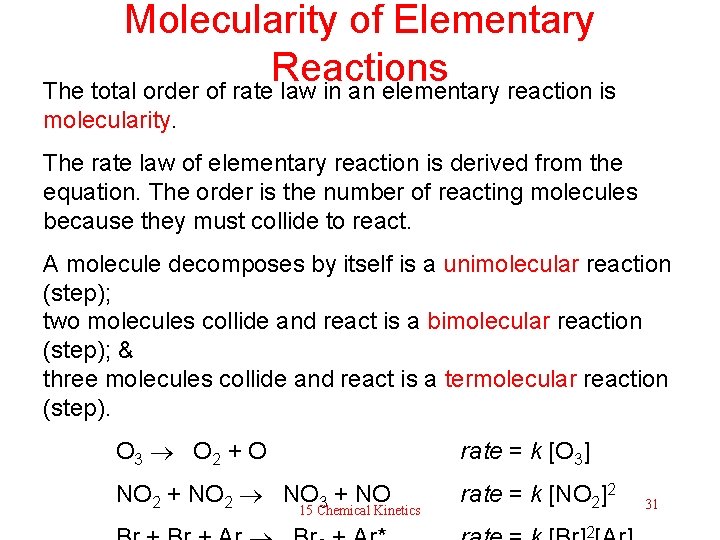
Molecularity of Elementary Reactions The total order of rate law in an elementary reaction is molecularity. The rate law of elementary reaction is derived from the equation. The order is the number of reacting molecules because they must collide to react. A molecule decomposes by itself is a unimolecular reaction (step); two molecules collide and react is a bimolecular reaction (step); & three molecules collide and react is a termolecular reaction (step). O 3 O 2 + O rate = k [O 3] NO 2 + NO 2 NO 3 + NO rate = k [NO 2]2 15 Chemical Kinetics 2 31

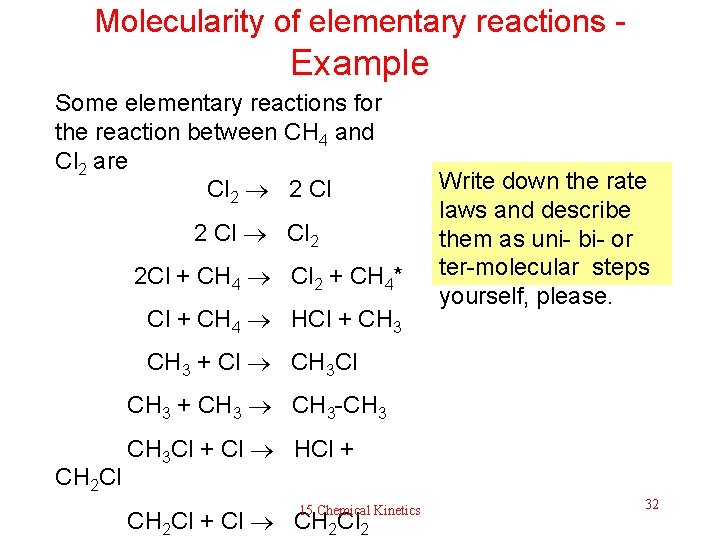
Molecularity of elementary reactions - Example Some elementary reactions for the reaction between CH 4 and Cl 2 are Cl 2 2 Cl Cl 2 2 Cl + CH 4 Cl 2 + CH 4* Cl + CH 4 HCl + CH 3 Write down the rate laws and describe them as uni- bi- or ter-molecular steps yourself, please. CH 3 + Cl CH 3 + CH 3 -CH 3 CH 2 Cl CH 3 Cl + Cl HCl + 15 Chemical Kinetics CH 2 Cl + Cl CH 2 Cl 2 32

Elementary Reactions are Molecular Events N 2 O 5 NO 2 + NO 3 NO + O 2 + NO 3 15 Chemical Kinetics Explain differences between elementary and over 33

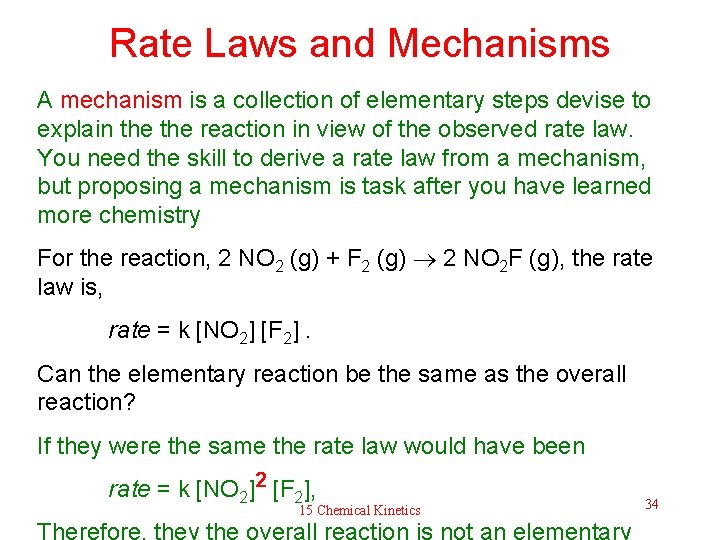
Rate Laws and Mechanisms A mechanism is a collection of elementary steps devise to explain the reaction in view of the observed rate law. You need the skill to derive a rate law from a mechanism, but proposing a mechanism is task after you have learned more chemistry For the reaction, 2 NO 2 (g) + F 2 (g) 2 NO 2 F (g), the rate law is, rate = k [NO 2] [F 2]. Can the elementary reaction be the same as the overall reaction? If they were the same the rate law would have been rate = k [NO 2]2 [F 2], 15 Chemical Kinetics Therefore, they the overall reaction is not an elementary 34

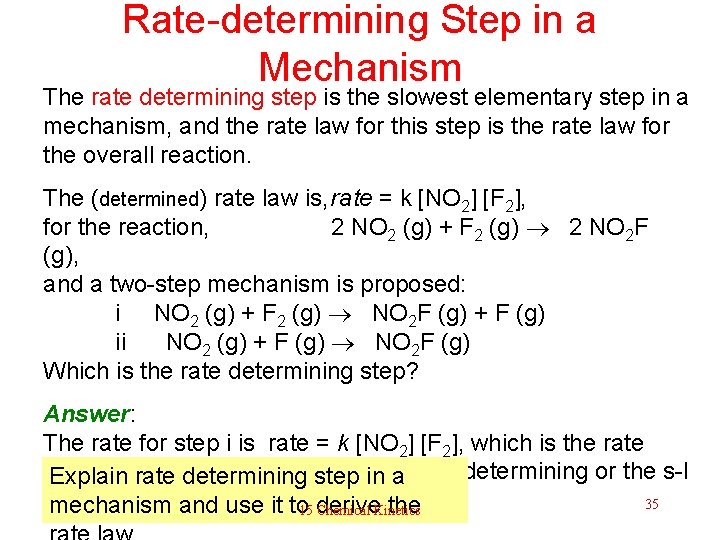
Rate-determining Step in a Mechanism The rate determining step is the slowest elementary step in a mechanism, and the rate law for this step is the rate law for the overall reaction. The (determined) rate law is, rate = k [NO 2] [F 2], for the reaction, 2 NO 2 (g) + F 2 (g) 2 NO 2 F (g), and a two-step mechanism is proposed: i NO 2 (g) + F 2 (g) NO 2 F (g) + F (g) ii NO 2 (g) + F (g) NO 2 F (g) Which is the rate determining step? Answer: The rate for step i is rate = k [NO 2] [F 2], which is the rate law, this suggests that step i is the rate-determining or the s-l Explain rate determining step in a -o-w step. 35 mechanism and use it to derive the 15 Chemical Kinetics

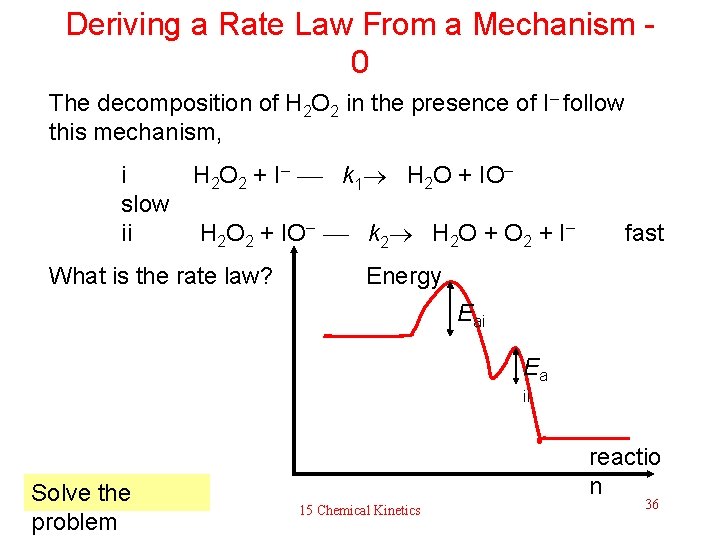
Deriving a Rate Law From a Mechanism - 0 The decomposition of H 2 O 2 in the presence of I– follow this mechanism, i H 2 O 2 + I– k 1 H 2 O + IO– slow ii H 2 O 2 + IO– k 2 H 2 O + O 2 + I– fast What is the rate law? Energy Eai Ea ii Solve the problem reactio n 15 Chemical Kinetics 36

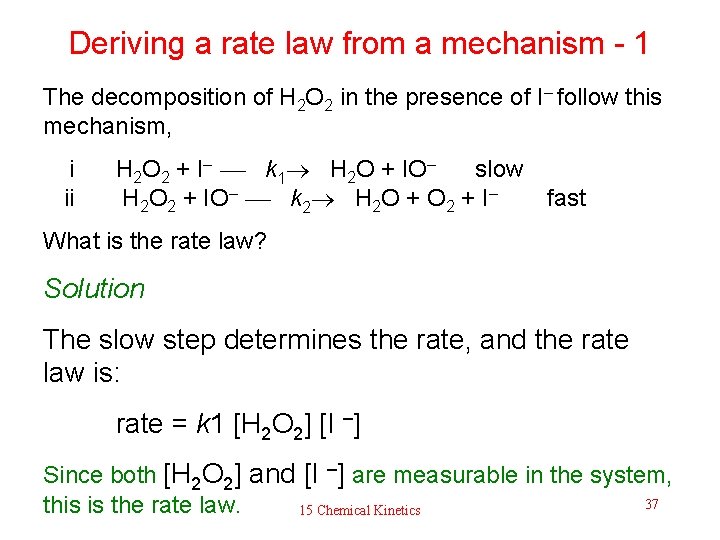
Deriving a rate law from a mechanism - 1 The decomposition of H 2 O 2 in the presence of I– follow this mechanism, i ii H 2 O 2 + I– k 1 H 2 O + IO– slow H 2 O 2 + IO– k 2 H 2 O + O 2 + I– fast What is the rate law? Solution The slow step determines the rate, and the rate law is: rate = k 1 [H 2 O 2] [I –] Since both [H 2 O 2] and [I –] are measurable in the system, 37 this is the rate law. 15 Chemical Kinetics

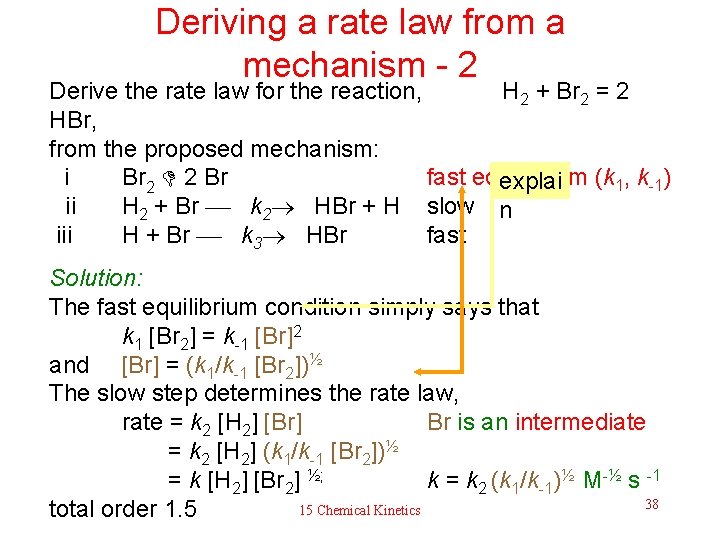
Deriving a rate law from a mechanism - 2 Derive the rate law for the reaction, H 2 + Br 2 = 2 HBr, from the proposed mechanism: i Br 2 2 Br fast equilibrium (k explai 1, k-1) ii H 2 + Br k 2 HBr + H slow n iii H + Br k 3 HBr fast Solution: The fast equilibrium condition simply says that k 1 [Br 2] = k-1 [Br]2 and [Br] = (k 1/k-1 [Br 2])½ The slow step determines the rate law, rate = k 2 [H 2] [Br] Br is an intermediate = k 2 [H 2] (k 1/k-1 [Br 2])½ = k [H 2] [Br 2] ½; k = k 2 (k 1/k-1)½ M-½ s -1 38 15 Chemical Kinetics total order 1. 5

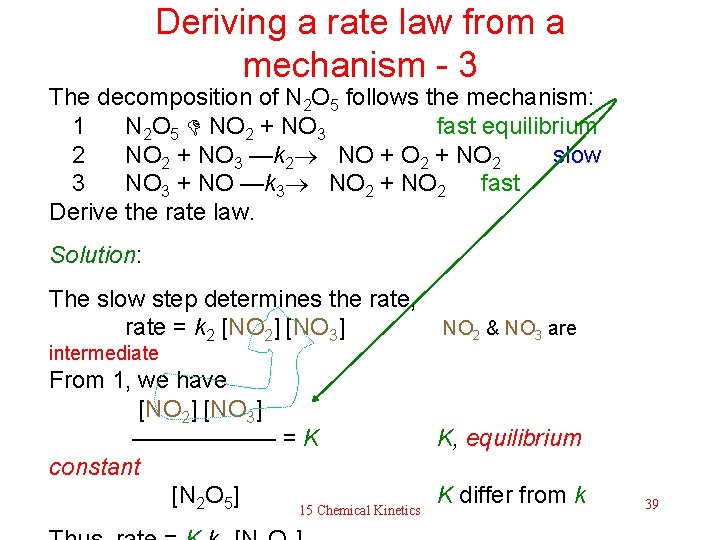
Deriving a rate law from a mechanism - 3 The decomposition of N 2 O 5 follows the mechanism: 1 N 2 O 5 NO 2 + NO 3 fast equilibrium 2 NO 2 + NO 3 —k 2 NO + O 2 + NO 2 slow 3 NO 3 + NO —k 3 NO 2 + NO 2 fast Derive the rate law. Solution: The slow step determines the rate, rate = k 2 [NO 2] [NO 3] NO 2 & NO 3 are intermediate From 1, we have [NO 2] [NO 3] —————— = K K, equilibrium constant [N 2 O 5] K differ from k 15 Chemical Kinetics 39

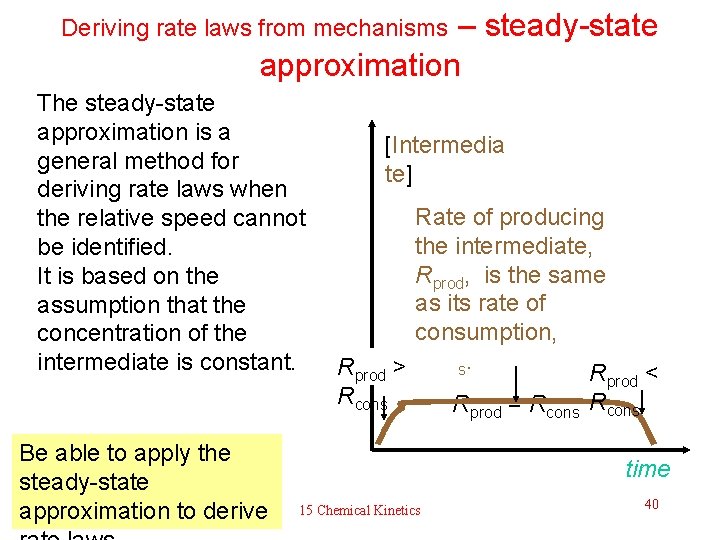
Deriving rate laws from mechanisms – steady-state approximation The steady-state approximation is a general method for deriving rate laws when the relative speed cannot be identified. It is based on the assumption that the concentration of the intermediate is constant. [Intermedia te] Rate of producing the intermediate, Rprod, is the same as its rate of consumption, Rprod > Rcons. Rprod < Rcons R = R prod Be able to apply the steady-state approximation to derive cons time 15 Chemical Kinetics 40

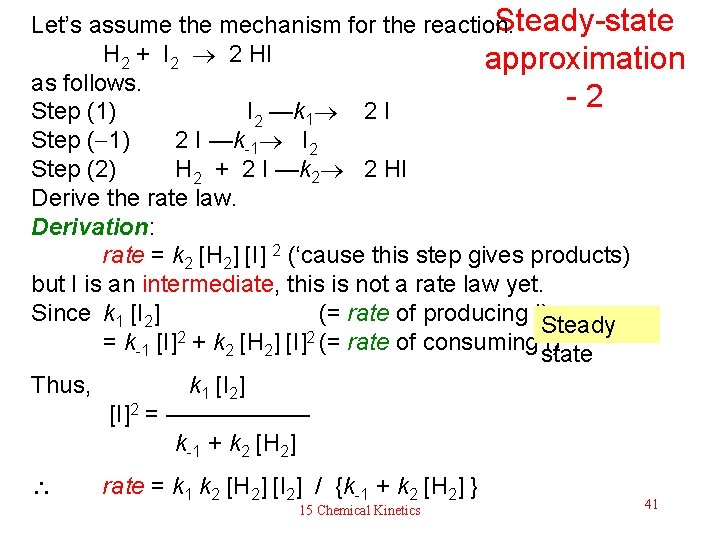
Steady-state Let’s assume the mechanism for the reaction. H 2 + I 2 2 HI approximation as follows. - 2 Step (1) I 2 —k 1 2 I Step ( 1) 2 I —k-1 I 2 Step (2) H 2 + 2 I —k 2 2 HI Derive the rate law. Derivation: rate = k 2 [H 2] [I] 2 (‘cause this step gives products) but I is an intermediate, this is not a rate law yet. Since k 1 [I 2] (= rate of producing I) Steady 2 2 = k-1 [I] + k 2 [H 2] [I] (= rate of consuming I) state Thus, k 1 [I 2] [I]2 = —————— k-1 + k 2 [H 2] rate = k 1 k 2 [H 2] [I 2] / {k-1 + k 2 [H 2] } 15 Chemical Kinetics 41
![Steadystate approximation 3 From the previous result k 1 k 2 H 2 Steady-state approximation - 3 From the previous result: k 1 k 2 [H 2]](https://slidetodoc.com/presentation_image/3cce740703772e2b6fc15b208ca6cc9a/image-42.jpg)
Steady-state approximation - 3 From the previous result: k 1 k 2 [H 2] [I 2] rate = ——————— {k-1 + k 2 [H 2] } Discussion: (i) If k-1 << k 2 [H 2] then {k-1 + k 2 [H 2]} = k 2 [H 2] , then rate = k 1 k 2 [H 2] [I 2] / {k 2 [H 2] } = k 1 [I 2] (pseudo 1 st order wrt I 2) using large concentration of H 2 or step 2 is fast (will meet this condition). (ii) If step (2) is slow, then k 2 << k 1, and if [H 2] is not large, we have {k-1 + k 2 [H 2]} = k-1 and rate = k 1 k 2 [H 2] [I 2] / k 1 = k 2 [H 2] [I 2] 15 Chemical Kinetics 42

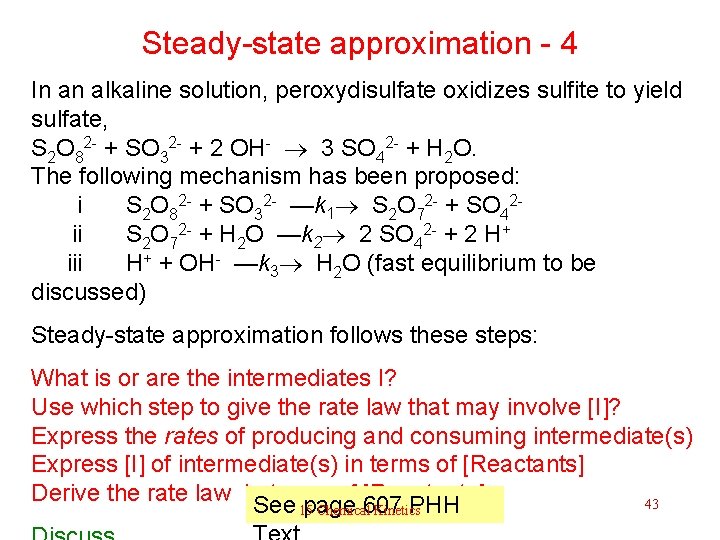
Steady-state approximation - 4 In an alkaline solution, peroxydisulfate oxidizes sulfite to yield sulfate, S 2 O 82 - + SO 32 - + 2 OH- 3 SO 42 - + H 2 O. The following mechanism has been proposed: i S 2 O 82 - + SO 32 - —k 1 S 2 O 72 - + SO 42 - ii S 2 O 72 - + H 2 O —k 2 2 SO 42 - + 2 H+ iii H+ + OH- —k 3 H 2 O (fast equilibrium to be discussed) Steady-state approximation follows these steps: What is or are the intermediates I? Use which step to give the rate law that may involve [I]? Express the rates of producing and consuming intermediate(s) Express [I] of intermediate(s) in terms of [Reactants] Derive the rate law in terms of [Reactants] 43 See page 607 PHH 15 Chemical Kinetics

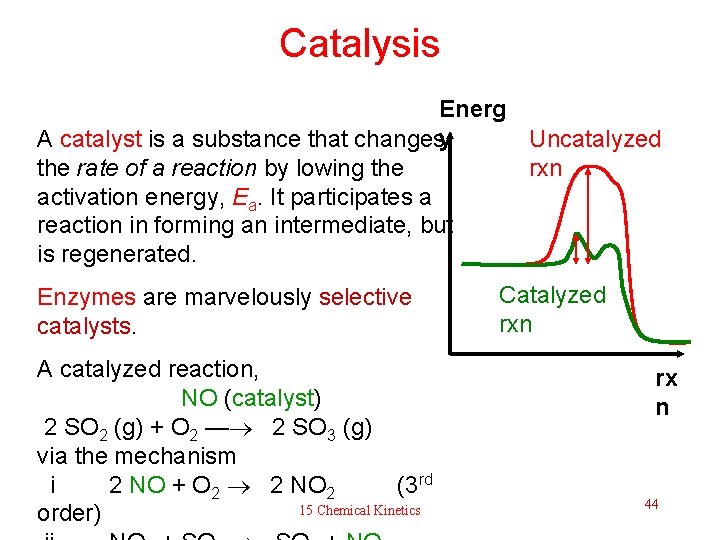
Catalysis Energ y Uncatalyzed A catalyst is a substance that changes rxn the rate of a reaction by lowing the activation energy, Ea. It participates a reaction in forming an intermediate, but is regenerated. Enzymes are marvelously selective catalysts. A catalyzed reaction, NO (catalyst) 2 SO 2 (g) + O 2 — 2 SO 3 (g) via the mechanism i 2 NO + O 2 2 NO 2 (3 rd 15 Chemical Kinetics order) Catalyzed rxn rx n 44

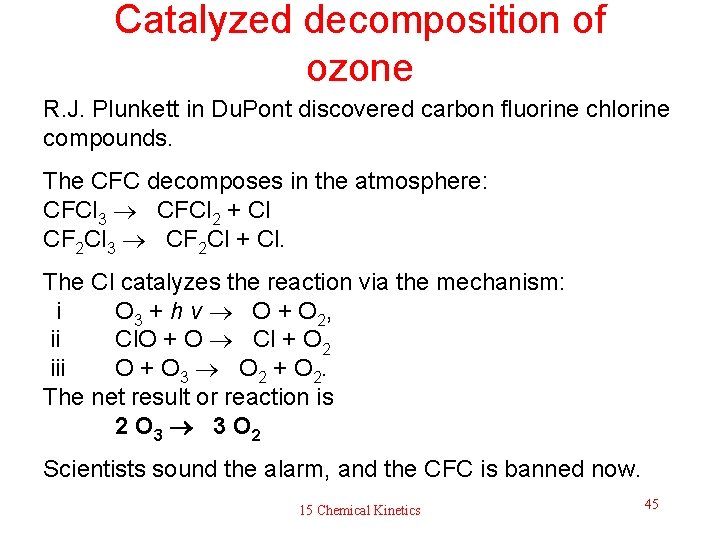
Catalyzed decomposition of ozone R. J. Plunkett in Du. Pont discovered carbon fluorine chlorine compounds. The CFC decomposes in the atmosphere: CFCl 3 CFCl 2 + Cl CF 2 Cl 3 CF 2 Cl + Cl. The Cl catalyzes the reaction via the mechanism: i O 3 + h v O + O 2, ii Cl. O + O Cl + O 2 iii O + O 3 O 2 + O 2. The net result or reaction is 2 O 3 3 O 2 Scientists sound the alarm, and the CFC is banned now. 15 Chemical Kinetics 45

Homogenous vs. heterogeneous catalysts A catalyst in the same phase (gases and solutions) as the reactants is a homogeneous catalyst. It effective, but recovery is difficult. When the catalyst is in a different phase than reactants (and products), the process involve heterogeneous catalysis. Chemisorption, absorption, and adsorption cause reactions to take place via different pathways. Platinum is often used to catalyze hydrogenation Catalytic converters reduce CO and NO emission. 15 Chemical Kinetics 46

Heterogeneous catalysts Ceryx's vision is to design, produce, and commercialize advanced systems that balance Cost, Performance, Emissions Reduction, and Fuel Penalty to make the economics of pollution control viable. We explore new ways to look Catalyzed reactions: at the air quality challenges CO + O 2 CO 2 faced by industry and search 2 NO N 2 + O 2 for potential solutions by combining proven 47 15 Chemical Kinetics technologies with state-of-the-

15 Chemical Kinetics 48

Enzymes – selective catalysts Enzymes are a long protein molecules that fold into balls. They often have a metal coordinated to the O and N sites. Molecules catalyzed by enzymes are called substrates. They are held by various sites (together called the active site) of the enzyme molecules and just before and during the reaction. After having reacted, the products P 1 & P 2 are released. Enzyme + Substrate ES (activated complex) ES P 1 + P 2 + E Enzymes are biological catalysts for biological systems. 15 Chemical Kinetics 49

15 Chemical Kinetics X-ray 3 -D structure of fumarate reductase. It reduces fumerate, an important role 50 in the metabolism

Chemical Kinetics - Summary Explain how the various factors affect reaction rates. Define reaction rates, average rates, initial rates and rate constants. Evaluate rate law from experiments Properly apply 1 st and 2 nd differential rate laws and integrated rate laws. Interpret elementary reactions and mechanisms. Derive rate laws from a given mechanism. Apply the steady-state method to derive the rate law of a given mechanism, and discuss the results. Explain the action of catalysts in terms of chemistry and in terms of energy of activation. 15 Chemical Kinetics 51
 Molekülerite nedir
Molekülerite nedir Grade 11 chemistry unit 4
Grade 11 chemistry unit 4 Kinetics half life
Kinetics half life Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics What is quasi steady state
What is quasi steady state Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Số nguyên tố là gì
Số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Kinetics and equilibrium
Kinetics and equilibrium Zero order elimination drugs
Zero order elimination drugs Plane kinetics of rigid bodies
Plane kinetics of rigid bodies