Honors Chemistry Unit 7 Chapter 6 p 175


















- Slides: 56

Honors Chemistry Unit 7 – Chapter 6 (p. 175 -217) Bonding and Lewis Structures

Bond force that holds groups of 2 or more atoms together and makes them function as a unit Bond energy required to break a bond Chemical Bonding 2

Types of Bonds • Ionic- transfer of electrons; held together by the attraction of opposite charges; usually between a metal and nonmetal • Covalent- electrons are shared by two nuclei; held by the attraction of positive nuclei to negative shared electrons – Non-Polar Covalent - electrons are shared equally – Polar Covalent - electrons are shared unequally Chemical Bonding 3


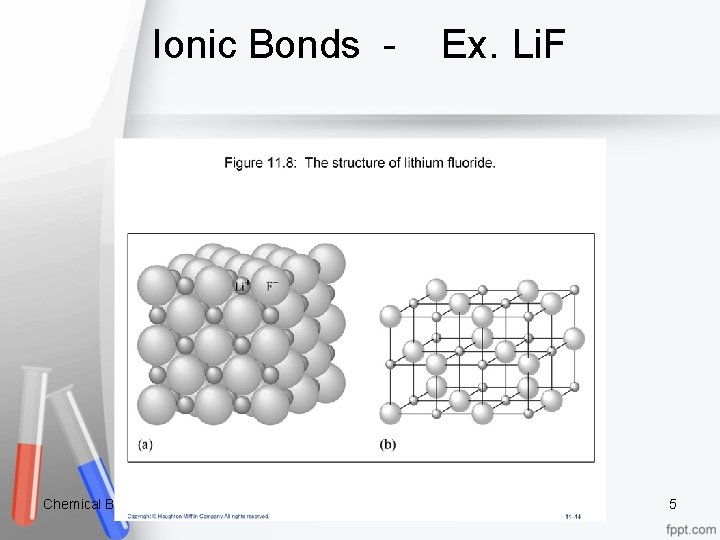
Ionic Bonds - Ex. Li. F Chemical Bonding 5

Ex. Li. F continued When soluble ionic compounds are placed in water, the aqueous ions separate from one another. Ex. Li. F If placed in water, this compound will separate into aqueous Li+1 and F-1 ions. Sketch: Chemical Bonding 6

Polyatomic Salts Compounds with polyatomic ions are held together by the attraction of opposite charges. However, the individual polyatomic ion is held together by covalent bonds and does not break apart. Chemical Bonding 7

Polyatomic Salts – continued Ex. Na. NO 3 if placed in water will separate into aqueous Na+1 and NO 3 -1 ions Note: the ionic bond is broken, but NOT the covalent bond holding the nitrate ion together. Sketch: Chemical Bonding 8

Isoelectronicwhen two atoms have the same number of electrons in the same energy levels, sublevels and orbitals Ex. Cl (7 valence electrons) = [Ne] 3 s 23 p 5 Cl-1 is isoelectronic to [Ne] 3 s 23 p 6 – the same as [Ar] Chemical Bonding 9

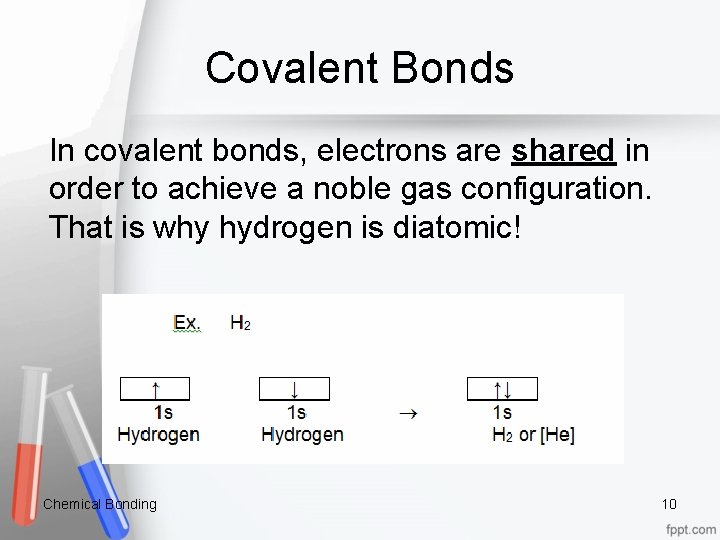
Covalent Bonds In covalent bonds, electrons are shared in order to achieve a noble gas configuration. That is why hydrogen is diatomic! Chemical Bonding 10

Chemical Bonding 11

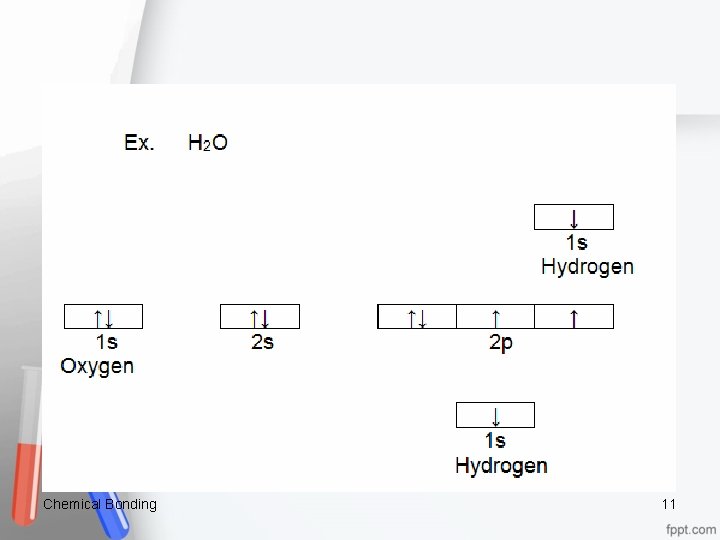
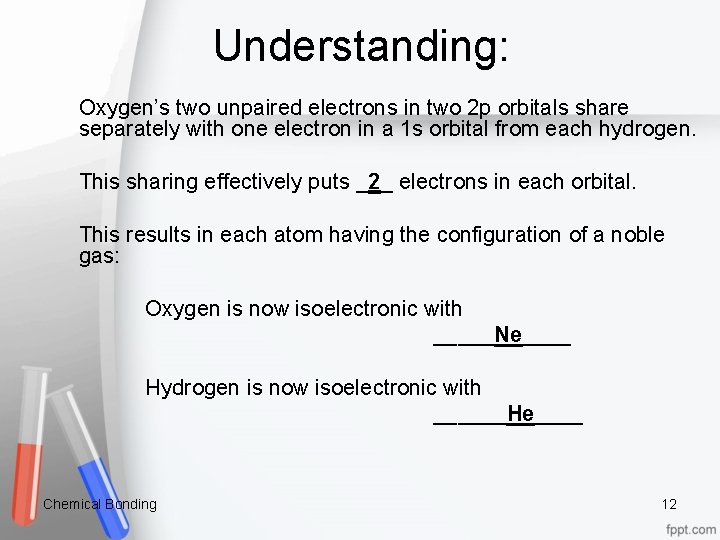
Understanding: Oxygen’s two unpaired electrons in two 2 p orbitals share separately with one electron in a 1 s orbital from each hydrogen. This sharing effectively puts _2_ electrons in each orbital. This results in each atom having the configuration of a noble gas: Oxygen is now isoelectronic with _____Ne____ Hydrogen is now isoelectronic with ______He____ Chemical Bonding 12

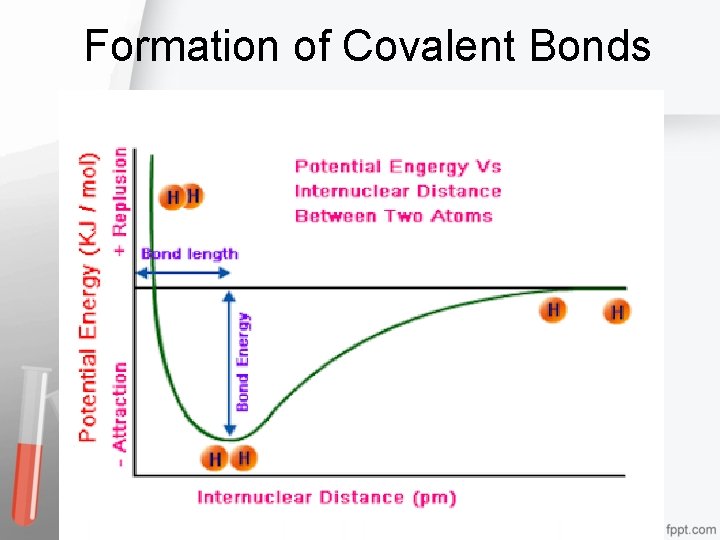
Formation of Covalent Bonds

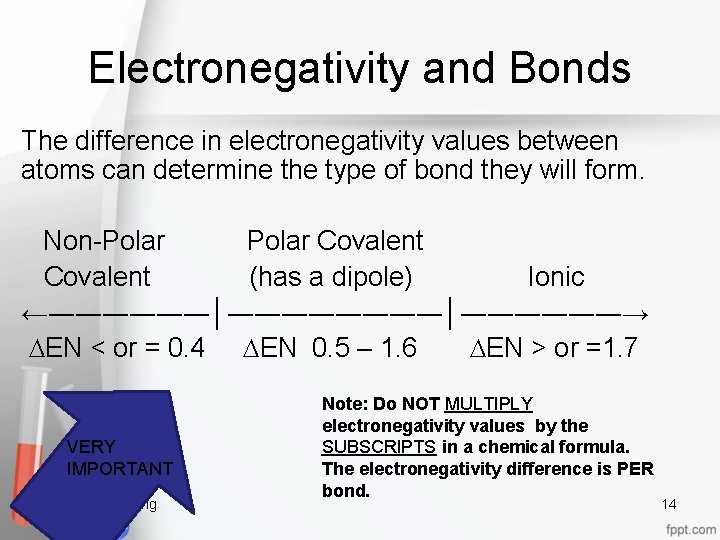
Electronegativity and Bonds The difference in electronegativity values between atoms can determine the type of bond they will form. Non-Polar Covalent (has a dipole) Ionic ←――――――│――――――→ DEN < or = 0. 4 DEN 0. 5 – 1. 6 DEN > or =1. 7 VERY IMPORTANT Chemical Bonding Note: Do NOT MULTIPLY electronegativity values by the SUBSCRIPTS in a chemical formula. The electronegativity difference is PER bond. 14

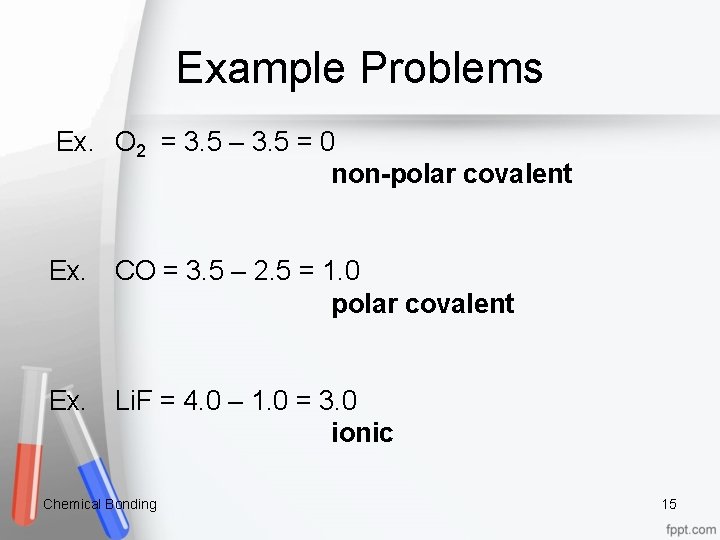
Example Problems Ex. O 2 = 3. 5 – 3. 5 = 0 non polar covalent Ex. CO = 3. 5 – 2. 5 = 1. 0 polar covalent Ex. Li. F = 4. 0 – 1. 0 = 3. 0 ionic Chemical Bonding 15

Polarity / dipole moment- a molecule containing a partial positive and partial negative charge Represented by an arrow pointing toward the partial negative Ex. Since Cl is more electronegative (3. 0) than H (2. 1), electrons spend more time around Cl, giving it a partial negative charge OR Represented by the Greek letter delta d Ex. NEVER use both arrows and deltas! Chemical Bonding 16

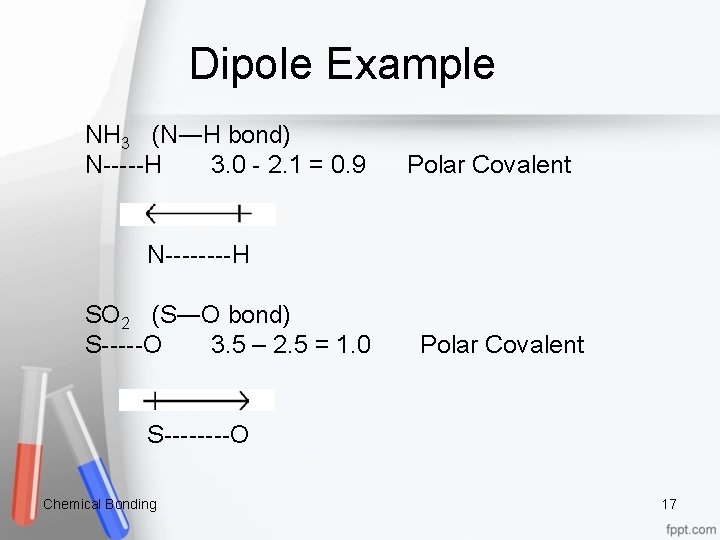
Dipole Example NH 3 (N―H bond) N-----H 3. 0 - 2. 1 = 0. 9 Polar Covalent N----H SO 2 (S―O bond) S-----O 3. 5 – 2. 5 = 1. 0 Polar Covalent S----O Chemical Bonding 17

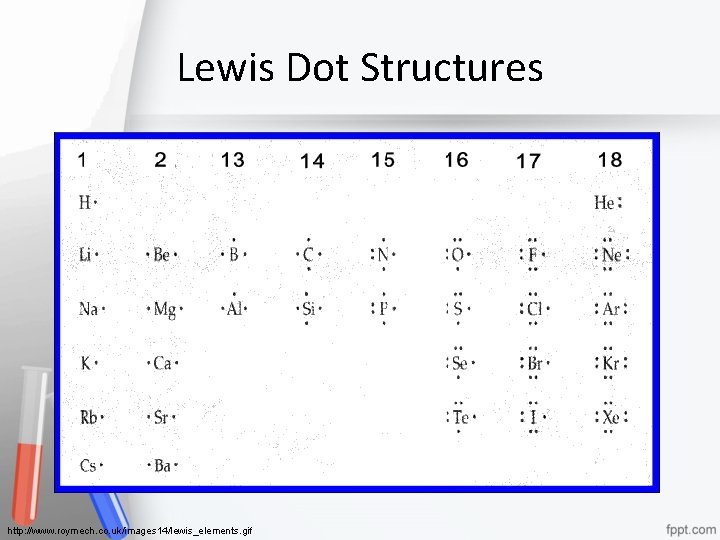
Lewis Dot Diagram Lewis dot diagram- shows how the valence electrons (those involved in bonding) are arranged among the atoms in a molecule • Uses valence electrons • One dot = one valence electron • One dash = a covalent bond = two electrons Chemical Bonding 18

Lewis Dot Structures http: //www. roymech. co. uk/images 14/lewis_elements. gif

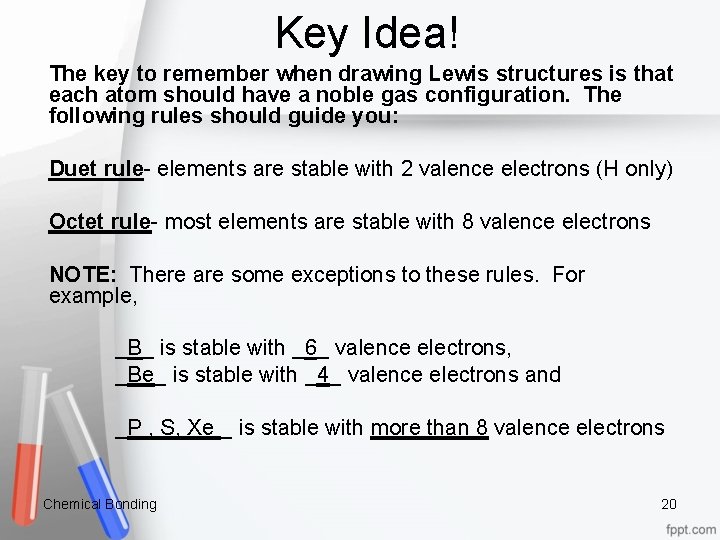
Key Idea! The key to remember when drawing Lewis structures is that each atom should have a noble gas configuration. The following rules should guide you: Duet rule- elements are stable with 2 valence electrons (H only) Octet rule- most elements are stable with 8 valence electrons NOTE: There are some exceptions to these rules. For example, _B_ is stable with _6_ valence electrons, _Be_ is stable with _4_ valence electrons and _P , S, Xe _ is stable with more than 8 valence electrons Chemical Bonding 20

Lewis Structures of Molecules • Determine the number of valance electrons it wants • Determine the number of valance electrons it has • Subtract them to find how many are shared and then divide that by two because there are two electrons per bond. • Subtract the number of it has by how many it is sharing to find how many are left over. • Draw the Lewis Dot Diagram for each element and find the one with the most unpaired electrons and make that the center. Chemical Bonding 21

Multiple Bonds Single, Double, Triple, etc bonds exist. The bond length decreases and the bond strength increases with more bonds in the same bonding area. HINTS: nitrogen will often have 1 unshared pair of electrons oxygen will often have 2 unshared pair of electrons carbon almost never has an unshared pair of electrons Chemical Bonding 22

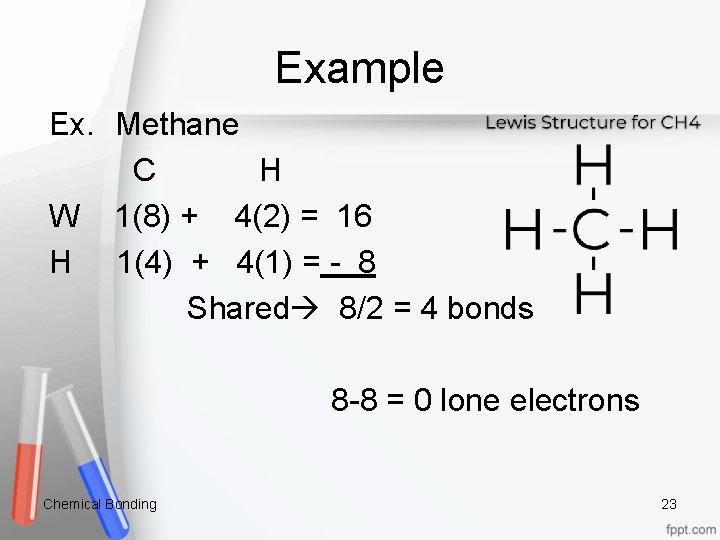
Example Ex. Methane C H W 1(8) + 4(2) = 16 H 1(4) + 4(1) = - 8 Shared 8/2 = 4 bonds 8 -8 = 0 lone electrons Chemical Bonding 23

Practice Examples Ex. ammonia Chemical Bonding 24

Practice Examples Ex. Carbon Dioxide Chemical Bonding 25

Try This One Ex. Nitrogen Chemical Bonding 26

Try This One Nitrate ion Chemical Bonding 27

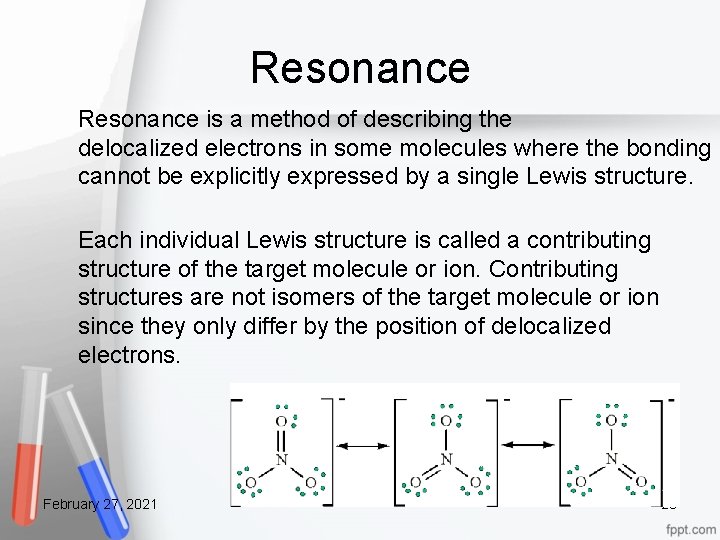
Resonance is a method of describing the delocalized electrons in some molecules where the bonding cannot be explicitly expressed by a single Lewis structure. Each individual Lewis structure is called a contributing structure of the target molecule or ion. Contributing structures are not isomers of the target molecule or ion since they only differ by the position of delocalized electrons. February 27, 2021 28

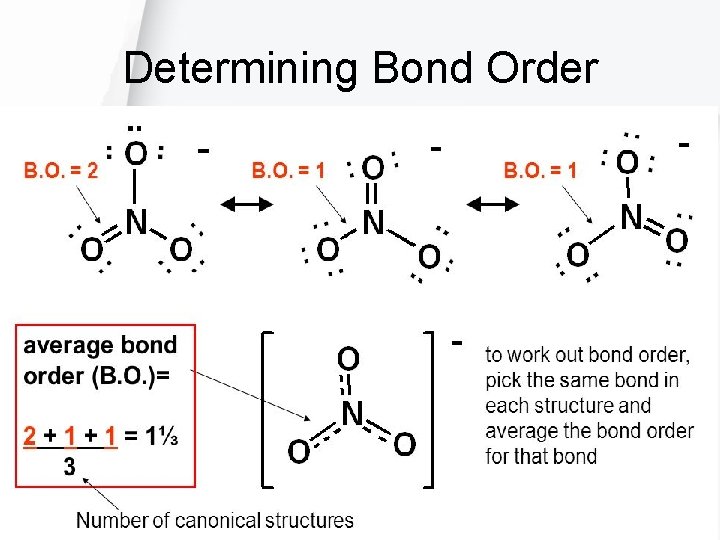
Determining Bond Order

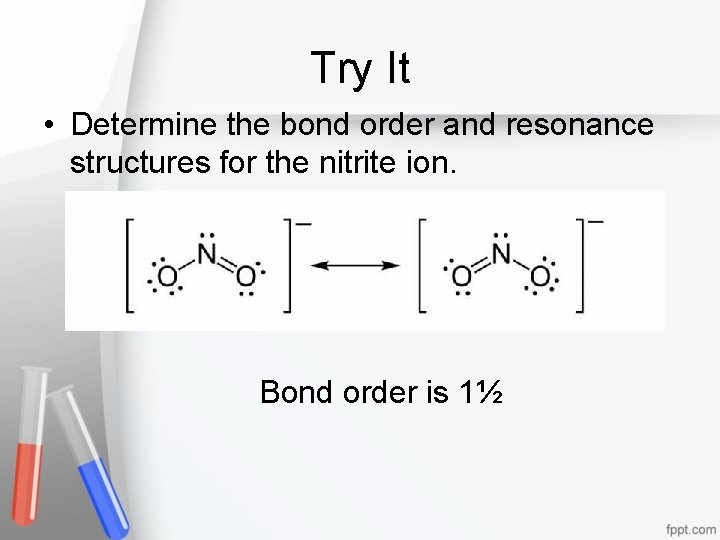
Try It • Determine the bond order and resonance structures for the nitrite ion. Bond order is 1½

Molecular Structure Molecular structure / geometric shape- the 3 -D arrangement of atoms in a molecule VSEPR theory- Valence Shell Electron Pair Repulsion theory; • Useful for predicting molecular shapes • Bonding and nonbonding electron pairs around the central atom are positioned as far apart as possible (because they will repel each other) • Lone pairs can spread out a little more since not held in place by two nuclei Chemical Bonding 31

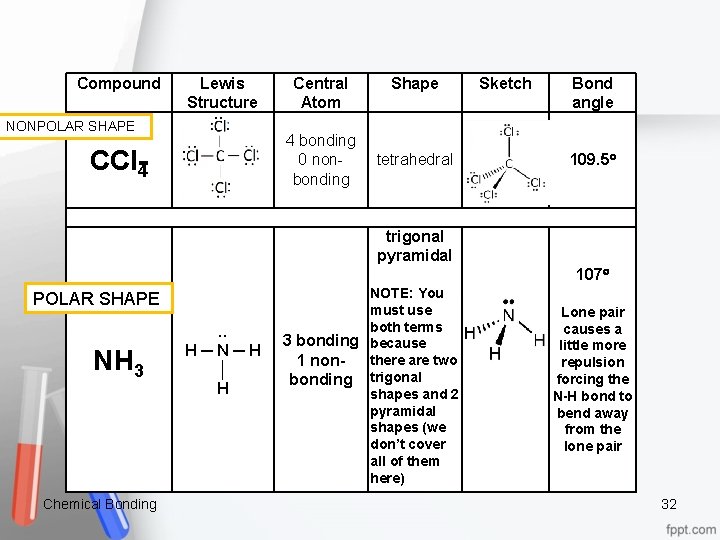
Compound Lewis Structure Central Atom Shape Sketch Bond angle NONPOLAR SHAPE 4 bonding 0 nonbonding CCl 4 tetrahedral 109. 5 trigonal pyramidal POLAR SHAPE NH 3 Chemical Bonding . . H ─ N ─ H │ H NOTE: You must use both terms 3 bonding because there are two 1 non bonding trigonal shapes and 2 pyramidal shapes (we don’t cover all of them here) 107 Lone pair causes a little more repulsion forcing the N H bond to bend away from the lone pair 32

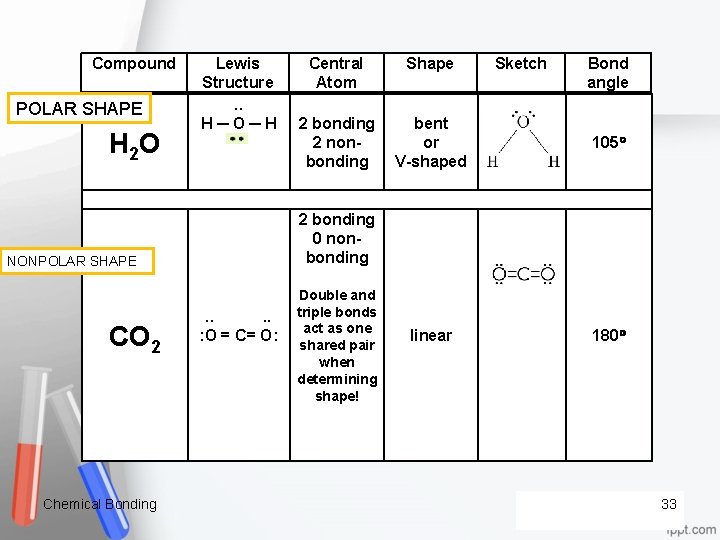
Compound POLAR SHAPE H 2 O Lewis Structure. . H ─ O ─ H Shape . . : O = C= O: Sketch Bond angle 2 bonding 2 non bonding bent or V shaped 2 bonding 0 non bonding NONPOLAR SHAPE CO 2 Central Atom Double and triple bonds act as one shared pair when determining shape! 105 linear 180 Chemical Bonding 33

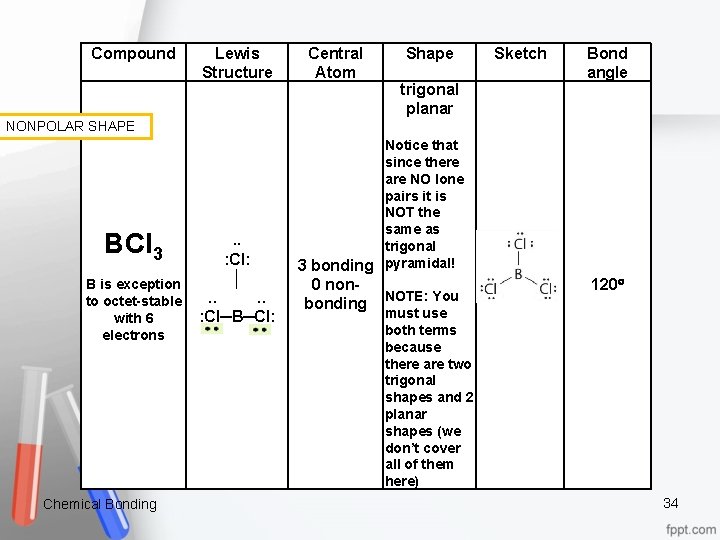
Compound Lewis Structure NONPOLAR SHAPE BCl 3 . . : Cl: │ B is exception . . to octet stable : Cl─B─Cl: with 6 electrons Chemical Bonding . . Central Atom Shape trigonal planar Notice that since there are NO lone pairs it is NOT the same as trigonal 3 bonding pyramidal! 0 non bonding NOTE: You must use both terms because there are two trigonal shapes and 2 planar shapes (we don’t cover all of them here) Sketch Bond angle 120 34

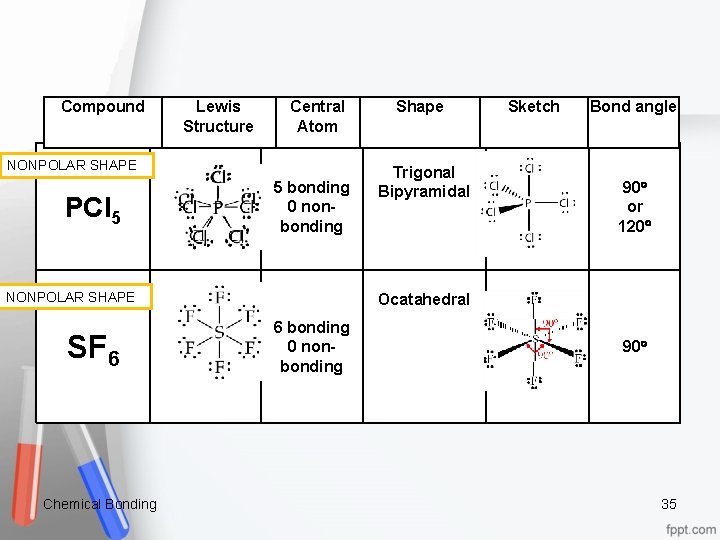
Compound Lewis Structure Central Atom NONPOLAR SHAPE PCl 5 5 bonding 0 non bonding Chemical Bonding Trigonal Bipyramidal Sketch Bond angle 90 or 120 Ocatahedral NONPOLAR SHAPE SF 6 Shape 6 bonding 0 non bonding 90 35

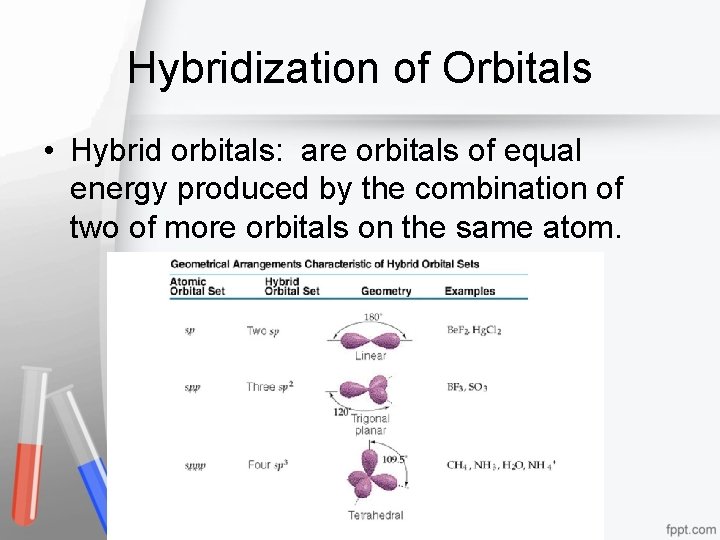
Hybridization of Orbitals • Hybrid orbitals: are orbitals of equal energy produced by the combination of two of more orbitals on the same atom.

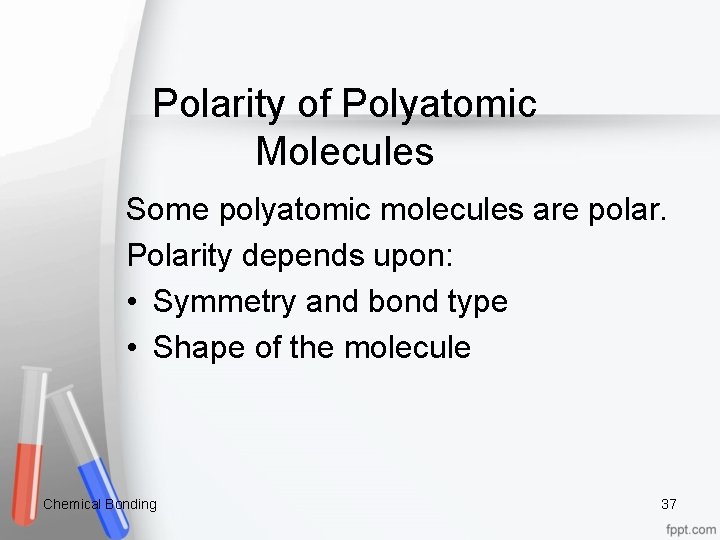
Polarity of Polyatomic Molecules Some polyatomic molecules are polar. Polarity depends upon: • Symmetry and bond type • Shape of the molecule Chemical Bonding 37

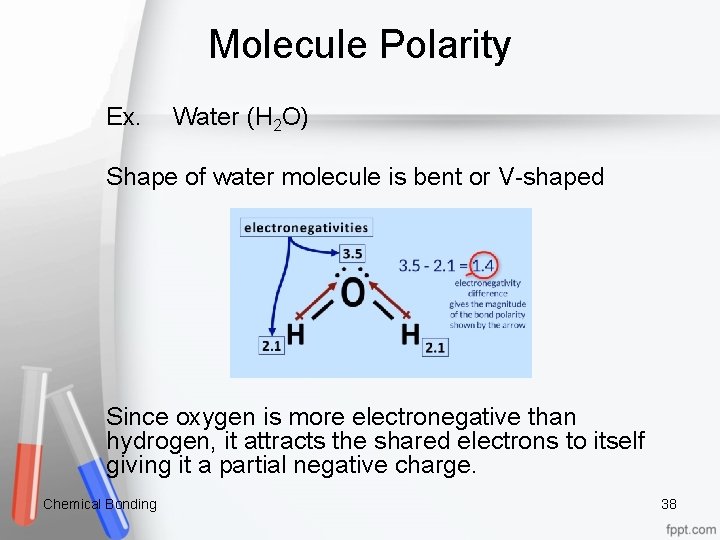
Molecule Polarity Ex. Water (H 2 O) Shape of water molecule is bent or V-shaped Since oxygen is more electronegative than hydrogen, it attracts the shared electrons to itself giving it a partial negative charge. Chemical Bonding 38

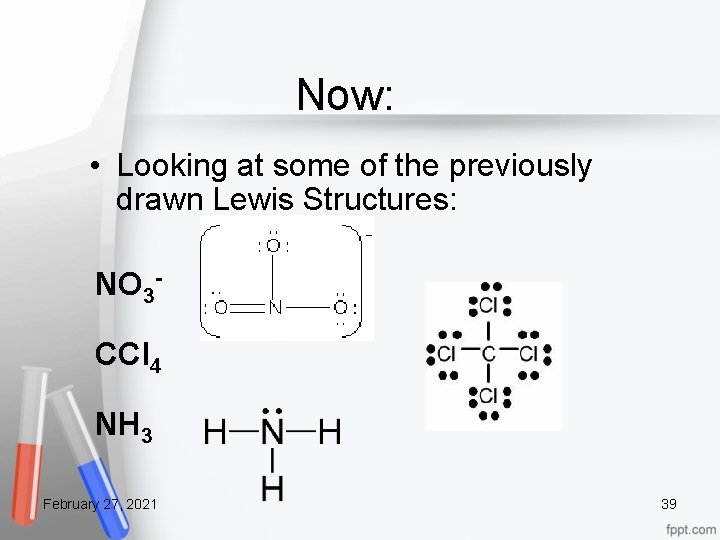
Now: • Looking at some of the previously drawn Lewis Structures: NO 3 CCl 4 NH 3 February 27, 2021 39

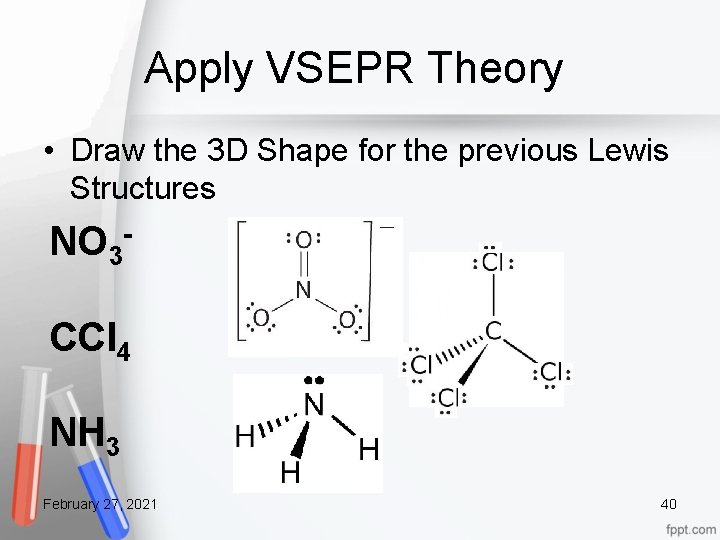
Apply VSEPR Theory • Draw the 3 D Shape for the previous Lewis Structures NO 3 CCl 4 NH 3 February 27, 2021 40

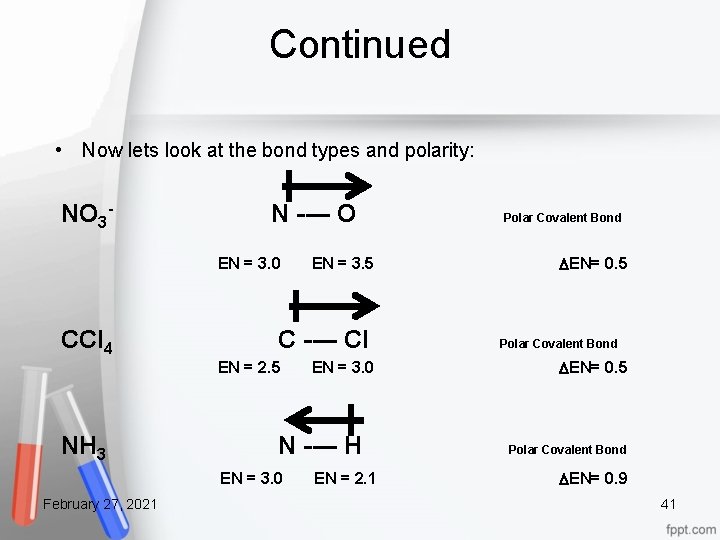
Continued • Now lets look at the bond types and polarity: NO 3 N O EN = 3. 0 EN = 3. 5 CCl 4 NH 3 February 27, 2021 C Cl EN = 2. 5 EN = 3. 0 N H EN = 3. 0 EN = 2. 1 Polar Covalent Bond DEN= 0. 5 Polar Covalent Bond DEN= 0. 9 41

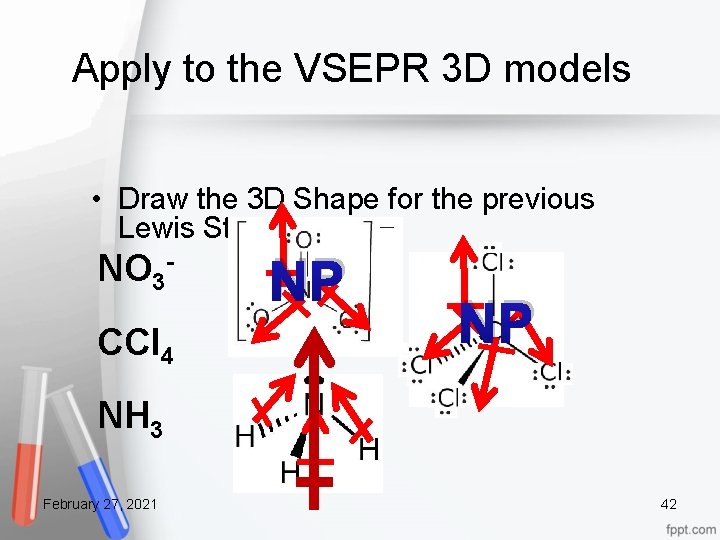
Apply to the VSEPR 3 D models • Draw the 3 D Shape for the previous Lewis Structures NO 3 CCl 4 NP NP NH 3 February 27, 2021 42

Multiple Center Atoms • Remember That Hydrogen Is Never Central • Halogens (Cl, Br, I, F) Are Rarely Ever Central • Carbon is Always Central If Present and Will Make Chains • Otherwise Look For How Many Unpaired Electrons are Present In Each Atom February 27, 2021 43

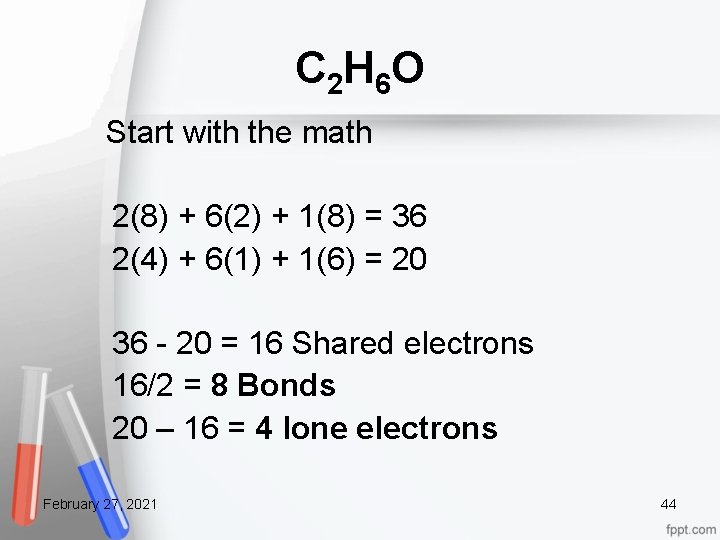
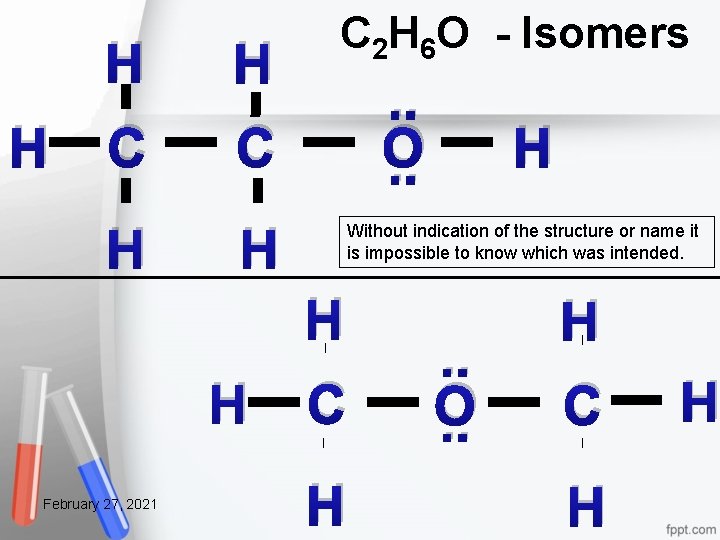
C 2 H 6 O Start with the math 2(8) + 6(2) + 1(8) = 36 2(4) + 6(1) + 1(6) = 20 36 - 20 = 16 Shared electrons 16/2 = 8 Bonds 20 – 16 = 4 lone electrons February 27, 2021 44

H H H C C H H C 2 H 6 O Isomers . . O. . Without indication of the structure or name it is impossible to know which was intended. H H February 27, 2021 H C H . . O. . H C H H

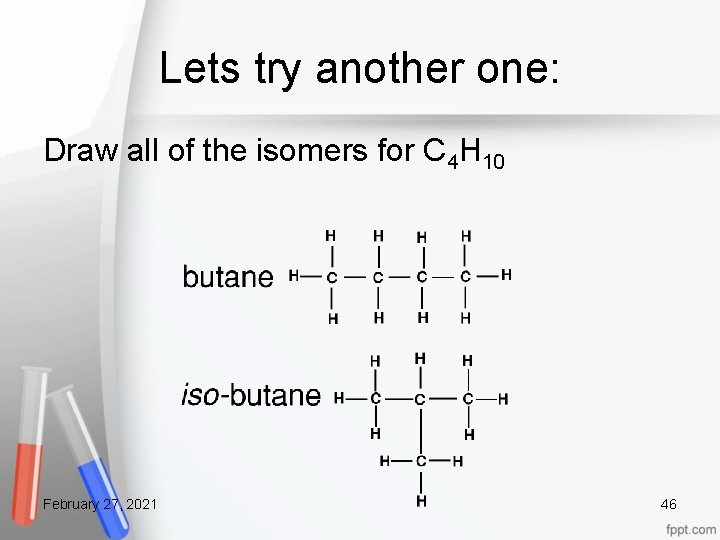
Lets try another one: Draw all of the isomers for C 4 H 10 February 27, 2021 46

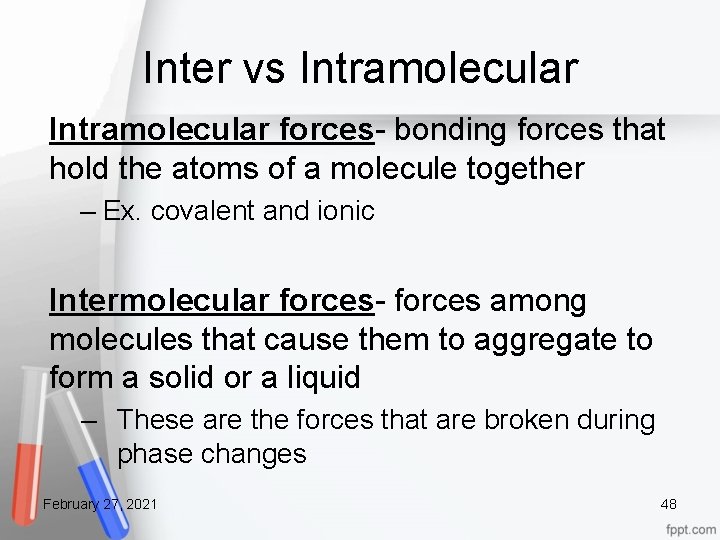
Inter vs Intramolecular forces- bonding forces that hold the atoms of a molecule together – Ex. covalent and ionic Intermolecular forces- forces among molecules that cause them to aggregate to form a solid or a liquid – These are the forces that are broken during phase changes February 27, 2021 48

Dipole dipole attraction • Dipole dipole attraction- polar molecules have dipole moments; molecules line up so that the partial positive end of one molecule is next to the partial negative end of another molecule – 1% as strong as a covalent or ionic bond. – They are only effective when molecules are close together, such as in solids and liquids. – They are not a factor in gases because the molecules are too far apart. February 27, 2021 49

Hydrogen bonding • Hydrogen bonding- special dipole attraction that occurs among molecules in which hydrogen is bonded directly to a highly electronegative element (ex. N, O, F) – the great polarity of the bond and the small size of the hydrogen atom makes this an unusually strong force. – Although called Hydrogen “bonding”- they are not a true bond like ionic or covalent! February 27, 2021 50

London dispersion forces- instantaneous induced dipole occurring in all atoms and molecules. – An instantaneous dipole in one atom can induce a similar dipole in a neighboring atom. – The molecules are then attracted to each other. These attractions are very weak and short-lived. February 27, 2021 51

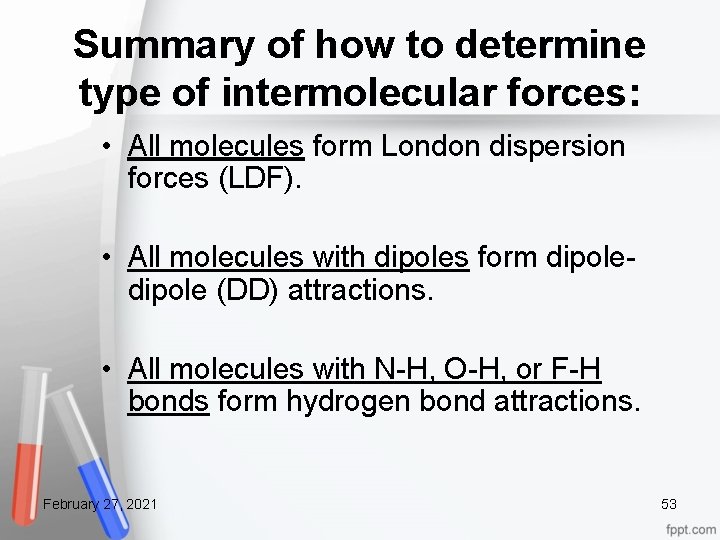
Summary of how to determine type of intermolecular forces: • All molecules form London dispersion forces (LDF). • All molecules with dipoles form dipole (DD) attractions. • All molecules with N-H, O-H, or F-H bonds form hydrogen bond attractions. February 27, 2021 53

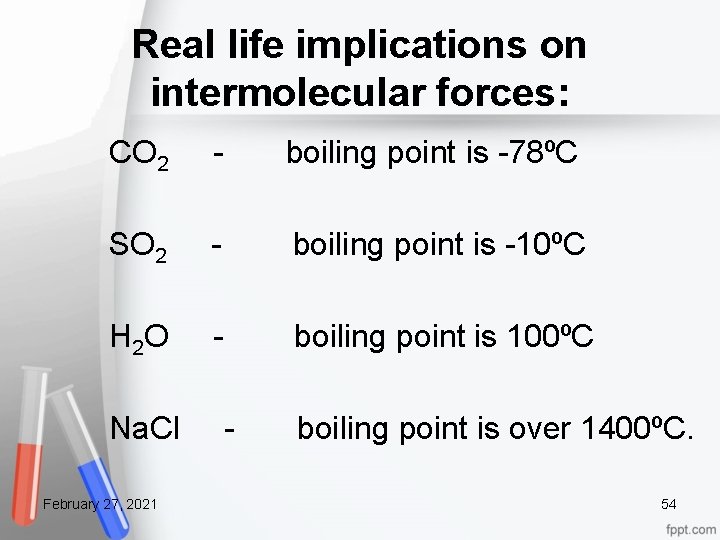
Real life implications on intermolecular forces: CO 2 - boiling point is -78ºC SO 2 - boiling point is -10ºC H 2 O - boiling point is 100ºC Na. Cl - boiling point is over 1400ºC. February 27, 2021 54

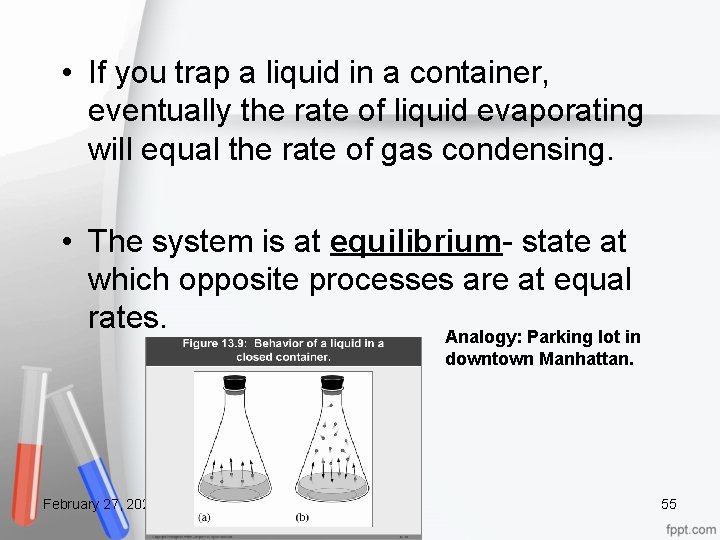
• If you trap a liquid in a container, eventually the rate of liquid evaporating will equal the rate of gas condensing. • The system is at equilibrium- state at which opposite processes are at equal rates. Analogy: Parking lot in downtown Manhattan. February 27, 2021 55

Physical Properties and IMF Vapor pressure- the pressure of the vapor present at equilibrium with its liquid – Varies with temperature – Varies with intermolecular forces (large forces = low vapor pressure) – Varies with molecular size (large molecule = low vapor pressure) Volatile- to evaporate rapidly due to high vapor pressure Ex. Rubbing Alcohol February 27, 2021 56

Ex. Which has the largest vapor pressure: H 2 O or CH 3 OH? • H 2 O contains 2 very polar O-H bonds while CH 3 OH only contains 1 polar O -H bond. As a result, H 2 O is more likely to have strong intermolecular forces (such as hydrogen bonding) and therefore will have the lower vapor pressure (less molecules go into the vapor phase and produce pressure). February 27, 2021 57

Ex. Which will have the lower vapor pressure: CH 3 OH or CH 3 CH 2 CH 2 OH? • Both have 1 very polar O-H bond, but since CH 3 CH 2 CH 2 OH is a larger molecule, it has the lower vapor pressure (less molecules go into the vapor phase and produce pressure). February 27, 2021 58