HONORS CHEMISTRY SUMMER ASSIGNMENT Video A 1 Honors


















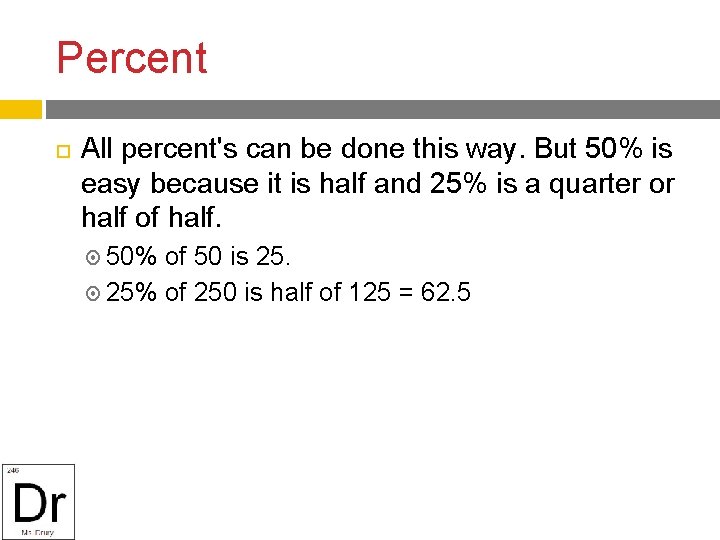











































































- Slides: 119

HONORS CHEMISTRY SUMMER ASSIGNMENT Video A 1: Honors Chemistry Website

HONORS CHEMISTRY SUMMER ASSIGNMENT Video A 2: Laboratory Preparation

Objectives By the end of this video you should be able to… • Identify and explain the use of laboratory equipment.

Beaker Glass used to hold or heat solutions. Although it has graduations, it is NOT USED FOR MEASUREMENT!

Erlenmeyer Flask Cone like shaped glass used for heating and filtering solutions. Although it has graduations, it is NOT USED FOR MEASUREMENT!

Graduated Cylinder Measures volumes of liquids to nearest 0. 01 m. L The plastic rim is used to protect the glass from breaking if the cylinder is accidentally tipped over.

Burette/Buret Used to dispense small amount of liquids. Read to nearest 0. 01 m. L. Read upside down! More accurate than a cylinder.

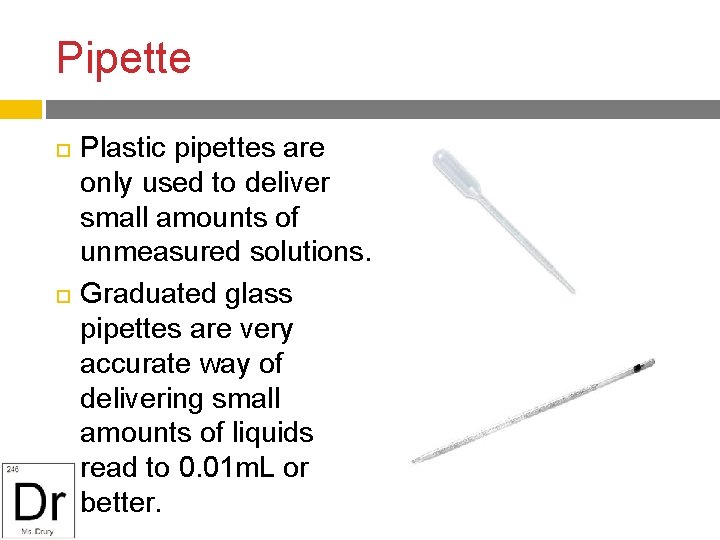
Pipette Plastic pipettes are only used to deliver small amounts of unmeasured solutions. Graduated glass pipettes are very accurate way of delivering small amounts of liquids read to 0. 01 m. L or better.

Volumetric Flask The most accurate piece of glassware. Measures liquids to a very specific volume.

Balance Used to measure masses of substances to nearest 0. 01 g. Can be “tared”.

Mortar and Pestle Used to grind solids into smaller pieces.

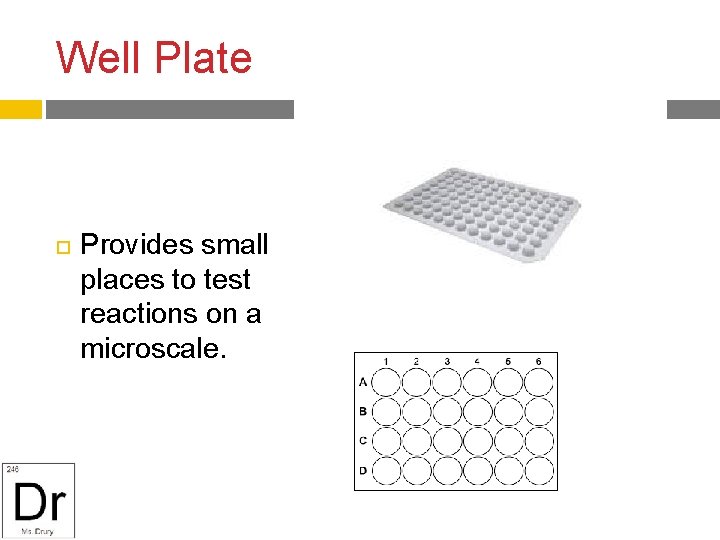
Well Plate Provides small places to test reactions on a microscale.

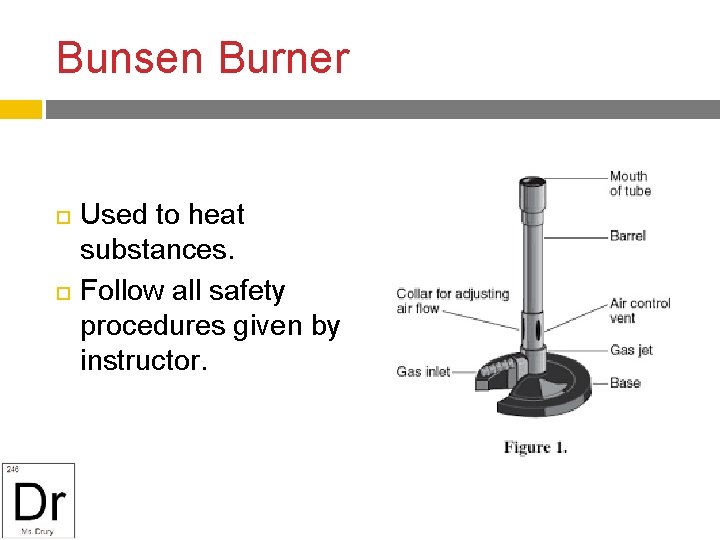
Bunsen Burner Used to heat substances. Follow all safety procedures given by instructor.

Clamp and Ring Stand Used to hold beakers, thermometers, funnels, and other material in place when heating.

Crucible Porcelain cup used to heat substances very hot. Caution: porcelain gets very hot!

Evaporating Dish Used to hold solutions while heating to evaporate them.

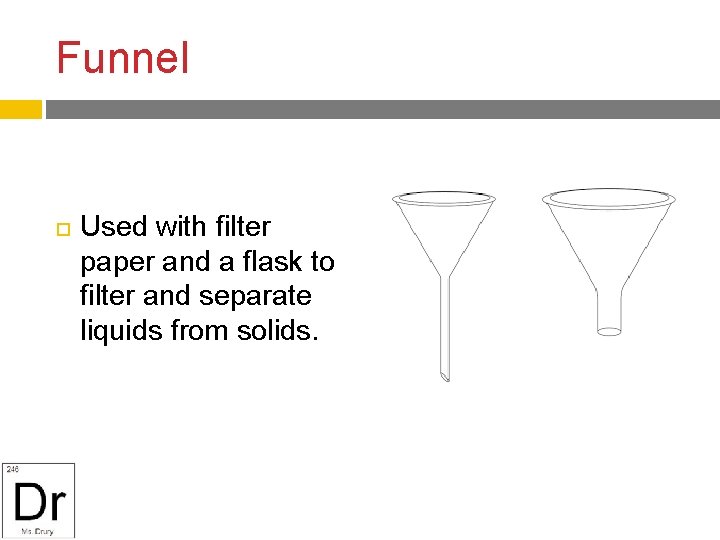
Funnel Used with filter paper and a flask to filter and separate liquids from solids.

Graphs The independent variable is the factor that you have control over and change yourself. It belongs on the x-axis. The dependent variable is the factor being measured belongs on the y-axis.

Objectives Now you should be able to… • Identify and explain the use of laboratory equipment.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video A 3: Percent, Decimals, Fractions, and Estimating

Objectives By the end of this video you should be able to… • Calculate simple decimal, percent, and fraction examples relating to chemistry calculations.

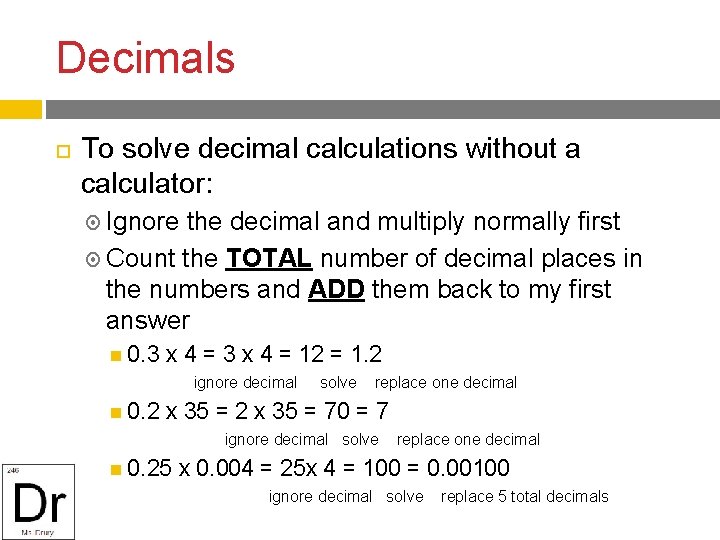
Decimals To solve decimal calculations without a calculator: Ignore the decimal and multiply normally first Count the TOTAL number of decimal places in the numbers and ADD them back to my first answer 0. 3 x 4 = 12 = 1. 2 ignore decimal solve replace one decimal 0. 2 x 35 = 70 = 7 ignore decimal solve replace one decimal 0. 25 x 0. 004 = 25 x 4 = 100 = 0. 00100 ignore decimal solve replace 5 total decimals

Decimals This also works with division but you add places BEFORE the decimal based on the two number’s decimal DIFFERENCE: 24 / 0. 4 = 24 / 4 = 60 ignore decimal solve replace one extra place before decimal 120 / 0. 03 = 120 / 3 = 4000 ignore decimal solve replace two extra places before decimal 0. 3 / 0. 003 = 3/3 = 100 ignore decimal solve replace 2 extra places before decimal

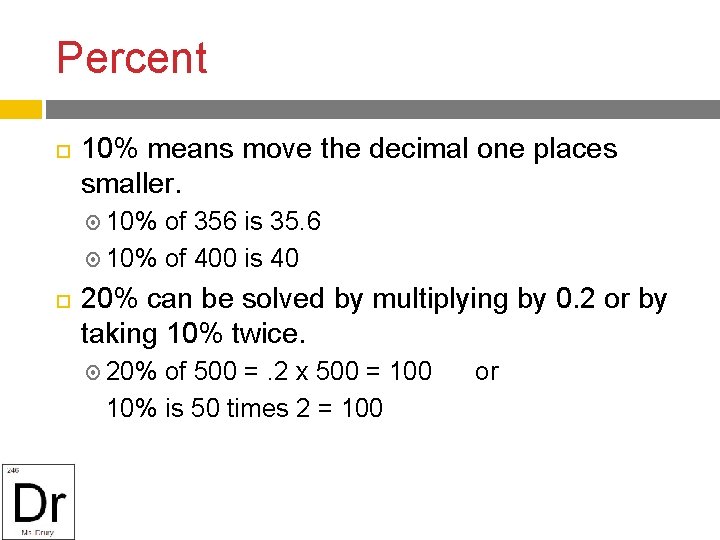
Percent 10% means move the decimal one places smaller. 10% of 356 is 35. 6 10% of 400 is 40 20% can be solved by multiplying by 0. 2 or by taking 10% twice. 20% of 500 =. 2 x 500 = 100 or 10% is 50 times 2 = 100
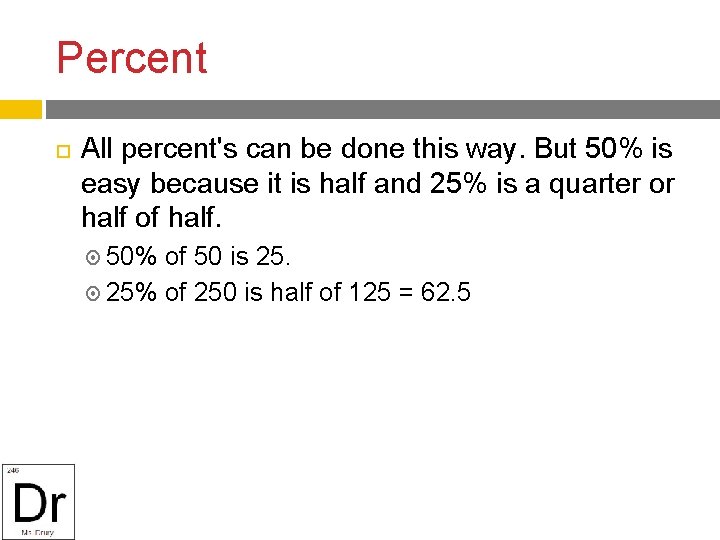
Percent All percent's can be done this way. But 50% is easy because it is half and 25% is a quarter or half of half. 50% of 50 is 25. 25% of 250 is half of 125 = 62. 5

Estimating 25. 34 x 1. 890 x 0. 00318 x 4. 1689 x 9. 823 Simplify the numbers to 1 -2 numbers each 25 x 2 x 0. 003 x 4 x 10 Find numbers that are easiest to calculate and reduce (25 x 2 = 50 x 4 = 200 x 10 = 2000) x 0. 003 2000 x 0. 003 = 6

Objectives Now you should be able to… • Calculate simple decimal, percent, and fraction examples relating to chemistry calculations.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video A 4: Scientific Method

Objectives By the end of this video you should be able to… • Utilize and explain the steps of the scientific method. • Classify measurements and observations as qualitative or quantitative. • Classify measurements and observations as intensive or extensive.

The Scientific Method 1. 2. 3. 4. 5. 6. 7. Identify the Problem or Purpose Collect Information Form a Hypothesis Including a) Claim: Your intelligent guess b) Evidence: Examples relating to your guess c) Reasoning: Explanation of your guess Create a Procedure Record Observations Analyze data/ Make Inferences Draw Conclusions/Amend Hypothesis

Observation versus Inference Observations use the senses. You observe what you can hear, smell, touch, hear or taste. Most students will have the same observations recorded. Inferences are small conclusions you make based on your observation. Many students will generate different inferences.

Observations and Inferences

Observations are made many ways. They can be either: Qualitative: appearance or behaviors: not measured Quantative: a mathematical description. and either Extensive: dependant on the amount of matter Intensive: dependant on type of matter

Qual or Quant and Intensive or Extensive? Rough or smooth Shiny or dull Large or small Kinetic energy

Objectives Now you should be able to… • Utilize and explain the steps of the scientific method. • Classify measurements and observations as qualitative or quantitative. • Classify measurements and observations as intensive or extensive.

Now What? Please practice problems in the summer assignment handout. Email chemisme@gmail. com or chat me on Remind if you have questions.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video B 1: Scientific Notation

Objectives By the end of this video you should be able to… • Convert numbers into and out of scientific notation.

Scientific Notation What is the purpose for using scientific notation in science?

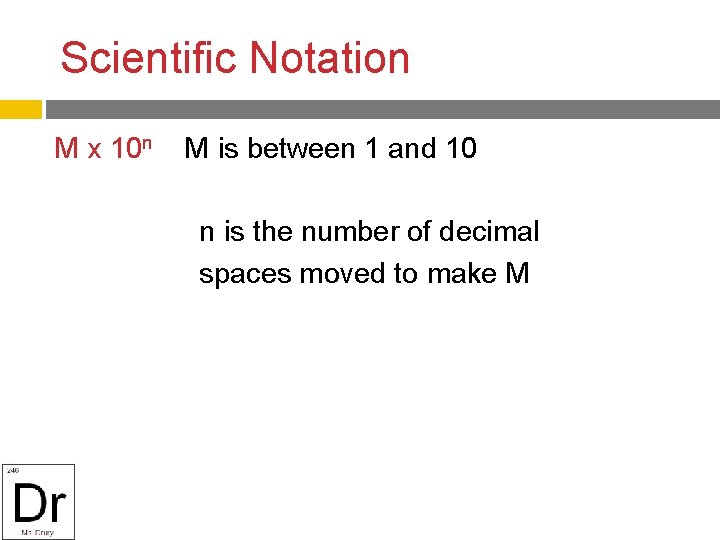
Scientific Notation M x 10 n M is between 1 and 10 n is the number of decimal spaces moved to make M

Rules 1. 2. 3. 4. Find the decimal point. If it is not written, it is at the end of the number. Move the decimal point to make the number between 1 and 10 Place the number of space you moved the decimal in the n spot. If you original number was above 1, the exponent is positive. If the number was smaller that 1, the exponent is negative.

Examples 1020000 is equal to 1. 02 x 106 0. 00789 is equal to 7. 89 x 10 -3 3. 45 x 105 is equal to 345000 1. 23 x 10 -4 is equal to 0. 000123

Objectives Now you should be able to… • Convert numbers into and out of scientific notation.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video B 2: Scientific Notation Multiply and Divide

Objectives By the end of this video you should be able to… • Multiply and divide numbers in scientific notation without a calculator.

Scientific Notation in Mathematics Multiplication and Division: Multiply or divide the base numbers. 2. When multiplying, add exponents. When dividing, subtract exponents. 1. (8 x 105)(2 x 103) = 16 x 108 or 1. 6 x 109 (8 x 105)/(2 x 103) = 4 x 102

Examples (2 x 106) x (4 x 107) = 8 x 1013 (1 x 108) x (5 x 10 -2) = 5 x 106 (8 x 108) / (4 x 104) = 2 x 104 (9 x 106) / (3 x 10 -2) = 3 x 108

Objectives Now you should be able to… • Multiply and divide numbers in scientific notation without a calculator.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video B 3: Scientific Notation Adding and Subtracting

Objectives By the end of this video you should be able to… • Fix numbers that are not in proper scientific notation. • Add and subtract numbers in scientific notation without a calculator.

Proper Scientific Notation The base number can only be between 1 and 10. If it is not in proper scientific notation form, change the base number to a number between 1 and 10 by moving the decimal. Count the movements. If the base number got smaller, increase the exponent the amount of times you moved the decimal. If the base number got larger, decrease the exponent the amount of times you moved the decimal.

Fix the following numbers 12 x 106 = 1. 2 x 107 250 x 10 -3 = 2. 50 x 10 -1 0. 569 x 106 = 5. 69 x 105 0. 008 x 10 -2 = 8 x 10 -5

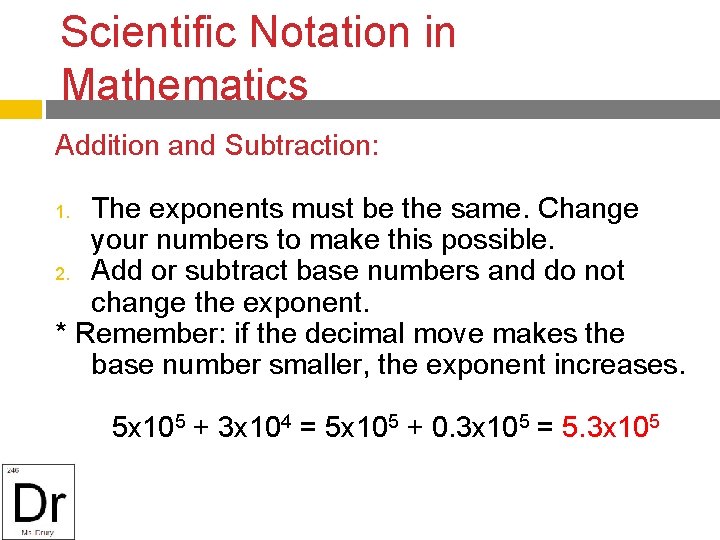
Scientific Notation in Mathematics Addition and Subtraction: The exponents must be the same. Change your numbers to make this possible. 2. Add or subtract base numbers and do not change the exponent. * Remember: if the decimal move makes the base number smaller, the exponent increases. 1. 5 x 105 + 3 x 104 = 5 x 105 + 0. 3 x 105 = 5. 3 x 105

Examples in Calculations (3 x 106) + (4 x 107) = (. 3 x 107) + (4 x 107) = 4. 3 x 107 (5 x 108) + (5 x 109) = (. 5 x 109) + (5 x 109) = 5. 5 x 109 (2 x 105) - (4 x 104) = (2 x 105) - (. 4 x 105) = 1. 6 x 105 (2 x 106) - (4 x 104) = (2 x 106) - (0. 04 x 106) = 1. 96 x 106 When in doubt, you could take it out of scientific notation and then put it back in but that takes a lot of time.

Objectives Now you should be able to… • Fix numbers that are not in proper scientific notation. • Add and subtract numbers in scientific notation without a calculator.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video B 4: Scientific Notation 2

Objectives By the end of this video you should be able to… • Square, square root, and log numbers in scientific notation with out a calculator.

The Inverse of a Number in Scientific Notation If you need to take the inverse of a number in scientific notation, inverse the base number and multiply the exponent by -1. (2 x 105)-1 = 0. 5 x 10 -5 = 5 x 10 -6 1/(4 x 105) = 0. 25 x 10 -5 = 2. 5 x 10 -6

Scientific Notation Number Raised to a Power If a number in scientific notation is raised to a power, raise the base number to that power and multiply the exponents. (2 x 10 -8)2 = 4 x 10 -16 (4 x 103)2 = 16 x 106 = 1. 6 x 107 (2 x 10 -8)3 = 8 x 10 -24

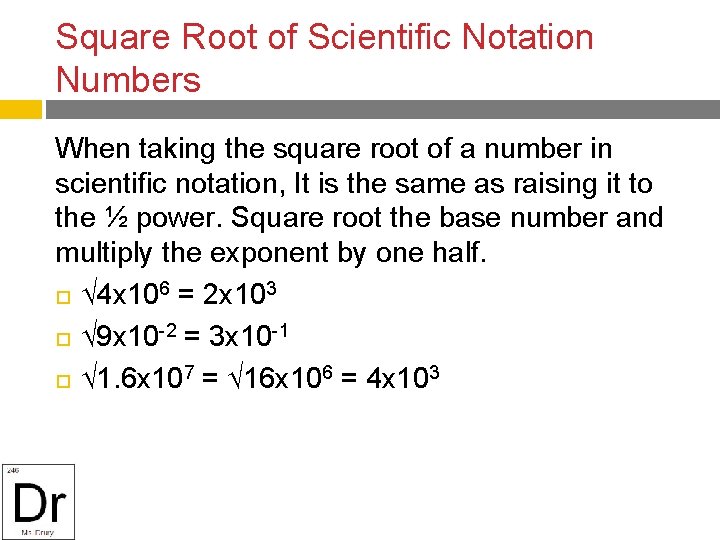
Square Root of Scientific Notation Numbers When taking the square root of a number in scientific notation, It is the same as raising it to the ½ power. Square root the base number and multiply the exponent by one half. 4 x 106 = 2 x 103 9 x 10 -2 = 3 x 10 -1 1. 6 x 107 = 16 x 106 = 4 x 103

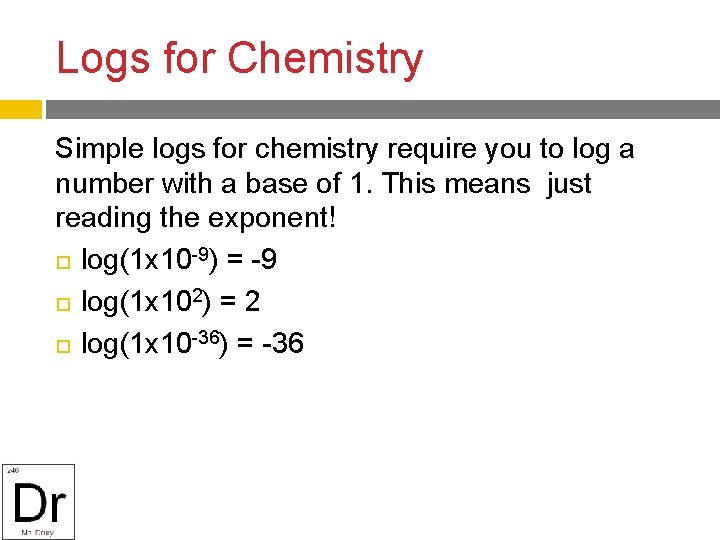
Logs for Chemistry Simple logs for chemistry require you to log a number with a base of 1. This means just reading the exponent! log(1 x 10 -9) = -9 log(1 x 102) = 2 log(1 x 10 -36) = -36

Objectives Now you should be able to… • Square, square root, and log numbers in scientific notation with out a calculator.

Now What? Please practice problems in the summer assignment handout. Email chemisme@gmail. com or chat me on Remind if you have questions.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video C 1: Intro to Metric

Objectives By the end of this video you should be able to… • Identify metric units of measurement. • Convert simple metric measurements.

Our country still uses an old system with non uniform measurements such as: fractions of an inch. . . 12 inches to a foot…. 3 feet to a yard…. 5. 5 yards to a rod. . . 320 rods to a mile. . . 43, 560 sq ft to an acre. . . But almost all other countries use the metric system, which is disadvantageous for us.

But we do use the metric for a few things: We buy cola in liters. . . We buy memory cards in bites… We run 10 km races. . . We swim in 25 meter pools. . . Why haven’t we switched entirely to metric?

Measuring Length in meters When measuring a person we would use meters. If we are measuring an ant, would meters still be feasible? What should we use? If we are measuring the distance from your house to the school, what should we use? Always pick a prefix with a value close to what you are measuring.



Simple Conversions Convert 3, 000 g to kg Convert 0. 0007 g to mg Convert 250 cm to m Convert 9000 mm to m Convert 95 L to m. L Convert 2500 m. L to L Convert 2. 0 pm to m Convert 350 nm to m 3 Kg 0. 7 mg 2. 50 m 95000 m. L 2. 5 L 2. 0 x 10 -12 m 3. 5 x 10 -7 m

Objectives Now you should be able to… • Identify metric units of measurement. • Convert simple metric measurements.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video C 2: Metric Conversions

Objectives By the end of this video you should be able to… • Convert numbers in metric measurements.

The Metric System If a unit is getting larger (m km) the number must get smaller. [If the unit gets smaller (m cm) the number gets larger. ] Examples: 1. 23. 5 cm 2. 3567 m. L 3. 0. 0984 mg 0. 000235 = km 0. 003567 = k. L 98. 4 = ug

More Examples Convert 3, 500 mg to kg 0. 035 kg Convert 0. 0057 kg to mg 570 mg 0. 0025 km Convert 250 cm to km Convert 9000 mm to cm 900 cm 950 m. L Convert 95 c. L to m. L 2. 5 x 1012 pg Convert 2500 mg to pg Convert 2. 0 pm to mm 2 x 10 -9 mm Convert 350 nm to mm 0. 000350 or 3. 50 x 10 -4 mm

Objectives Now you should be able to… • Convert numbers in metric measurements.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video C 3: Temperature Conversions

Objectives By the end of this video you should be able to… • Convert temperature measurements between Celsius and Kelvin.

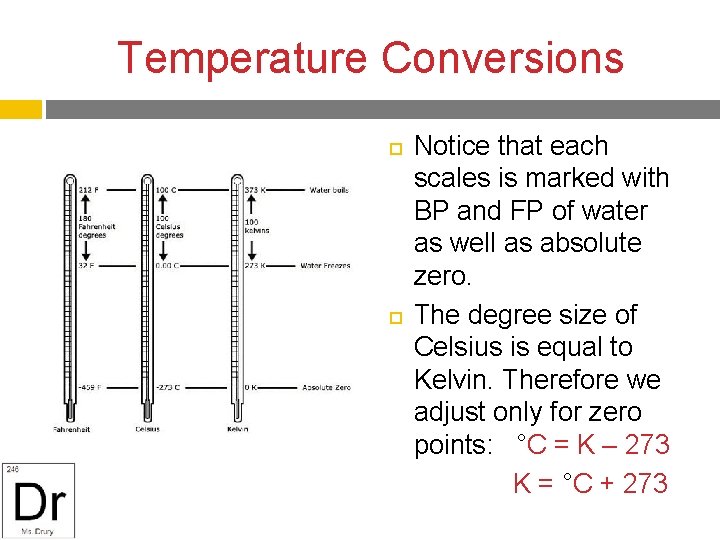
Temperature Conversions Notice that each scales is marked with BP and FP of water as well as absolute zero. The degree size of Celsius is equal to Kelvin. Therefore we adjust only for zero points: °C = K – 273 K = °C + 273

More Examples Convert 0°C to K Convert 25°C to K Convert 100°C to K Convert 0 K to°C Convert 298 K to°C Convert 273 K to°C 273 K 298 K 373 K -273 C 25 C 0 C

Thinking in Celsius -10° Celsius = frigid (14° F) 0° Celsius = cold (32° F) 10° Celsius = cool (50° F) 20° Celsius = comfortably warm (68° F) 30° Celsius = hot (86° F) 40° Celsius = very hot (104° F) 50° Celsius = Phoenix Hot (120°F)

Objectives Now you should be able to… • Convert temperature measurements between Celsius and Kelvin.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video C 4: Density

Objectives By the end of this video you should be able to… • Calculate density

Density depends on: Mass: the amount of matter an object contains. (This is different than weight, which is mass plus gravity) Volume: The amount of space a substance occupies

How do we measure mass in the lab? Electronic Balance

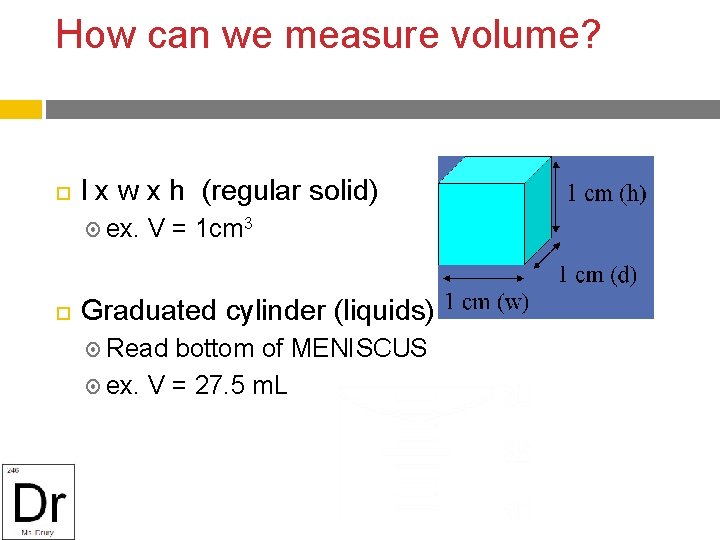
How can we measure volume? l x w x h (regular solid) ex. V = 1 cm 3 Graduated cylinder (liquids) Read bottom of MENISCUS ex. V = 27. 5 m. L

Measuring Volume: Irregular Solid Water displacement method: 1. 2. 3. Measure initial volume Measure final volume with object The Difference is the volume of the object

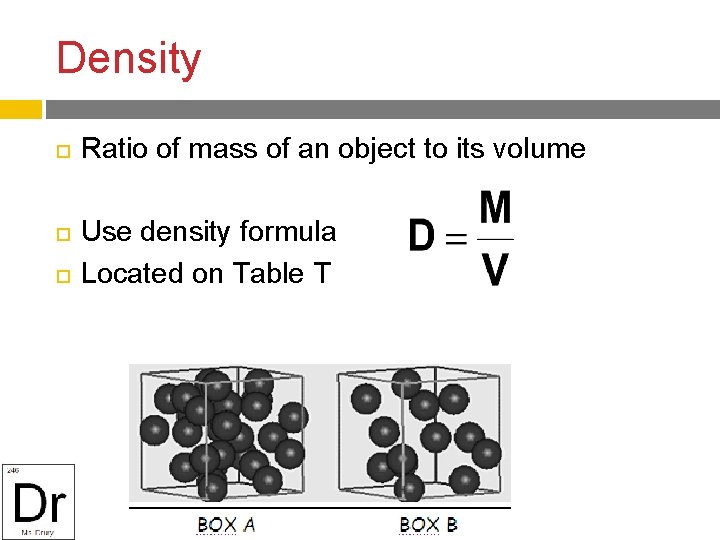
Density Ratio of mass of an object to its volume Use density formula Located on Table T

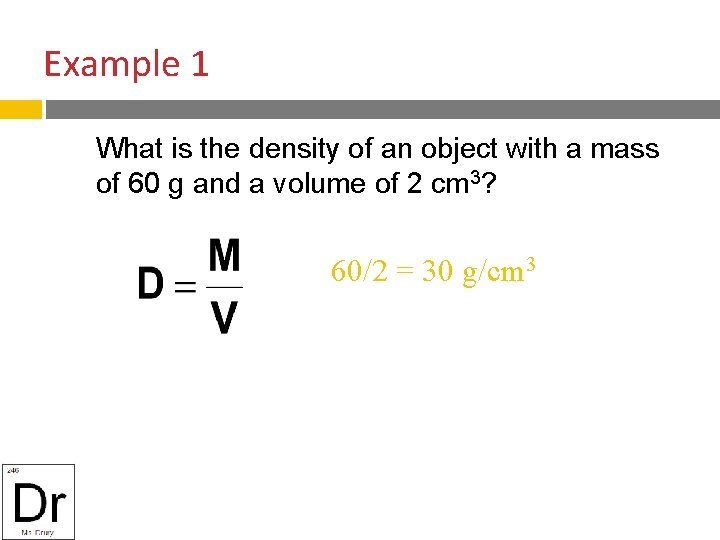
Example 1 What is the density of an object with a mass of 60 g and a volume of 2 cm 3? 60/2 = 30 g/cm 3

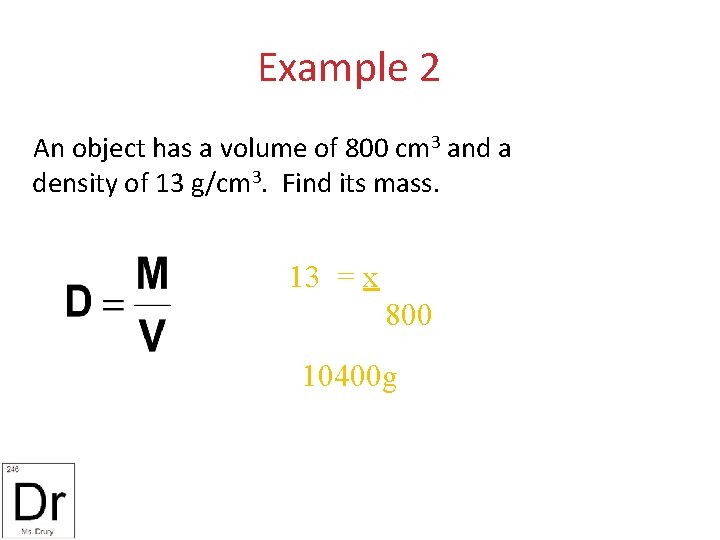
Example 2 An object has a volume of 800 cm 3 and a density of 13 g/cm 3. Find its mass. 13 = x 800 10400 g

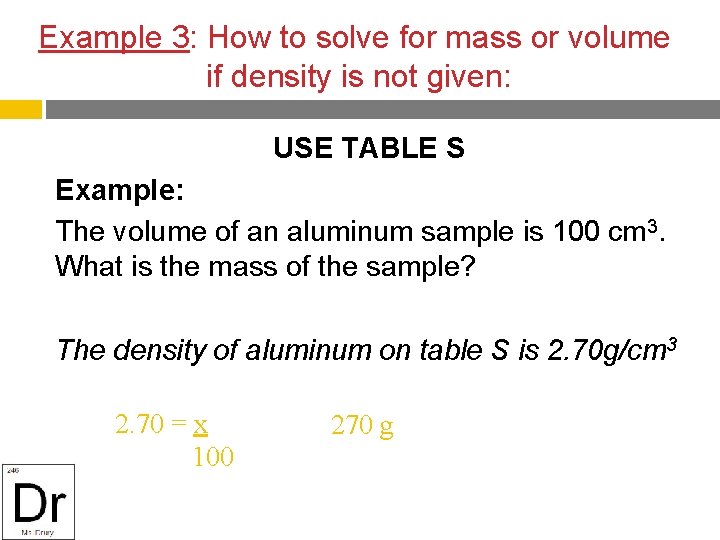
Example 3: How to solve for mass or volume if density is not given: USE TABLE S Example: The volume of an aluminum sample is 100 cm 3. What is the mass of the sample? The density of aluminum on table S is 2. 70 g/cm 3 2. 70 = x 100 270 g

Objectives Now you should be able to… • Calculate density.

Now What? Please practice problems in the summer assignment handout. Email chemisme@gmail. com or chat me on Remind if you have questions.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video D 1: Precision and Accuracy

Objectives By the end of this video you should be able to… • Compare accuracy and precision.

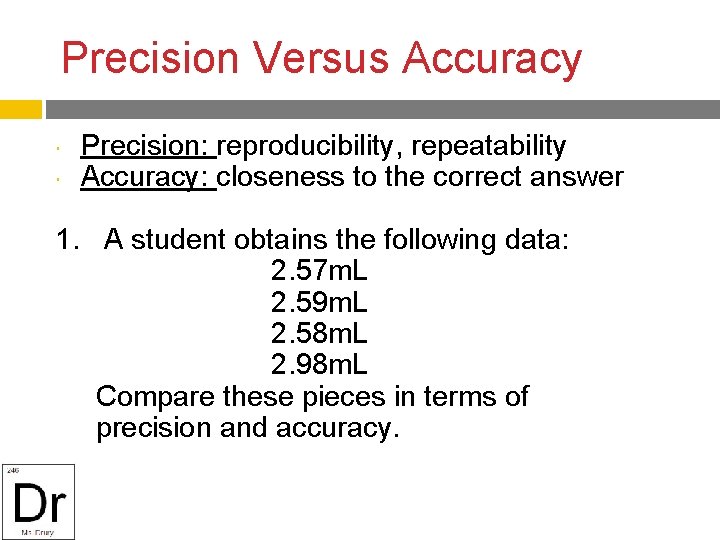
Precision Versus Accuracy Precision: reproducibility, repeatability Accuracy: closeness to the correct answer 1. A student obtains the following data: 2. 57 m. L 2. 59 m. L 2. 58 m. L 2. 98 m. L Compare these pieces in terms of precision and accuracy.

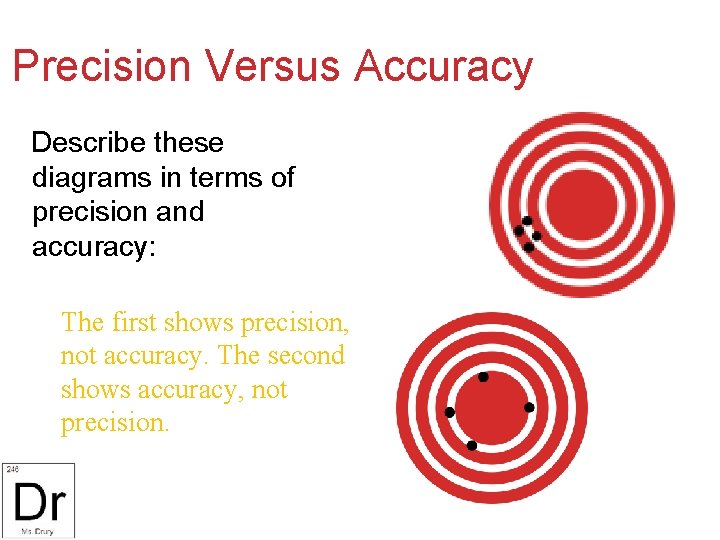
Precision Versus Accuracy Describe these diagrams in terms of precision and accuracy: The first shows precision, not accuracy. The second shows accuracy, not precision.

In this classroom, what is more important: Precision or

Precision! Due to lack of precise equipment and variable climates we will most likely not end up with accurate results. Therefore, we will focus on refining our lab skills and strive for precise results.

Objectives Now you should be able to… • Compare accuracy and precision.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video D 2: Significant Figures

Objectives By the end of this video you should be able to… • Count the number of significant figures in a number.

Significant Figures When scientists take measurements their equipment can measure with varying degrees of precision. A scientists final calculation can only be as precise as their least precise measurement. Count digits in your measured number to determine their level of precision.

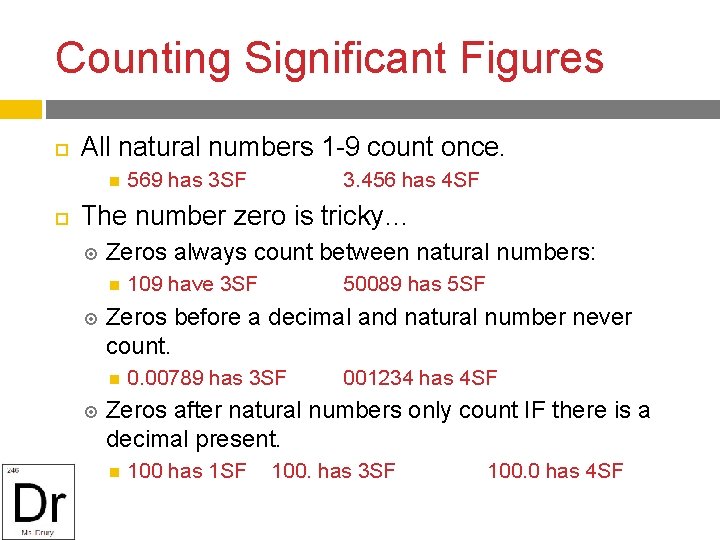
Counting Significant Figures All natural numbers 1 -9 count once. 569 has 3 SF 3. 456 has 4 SF The number zero is tricky… Zeros always count between natural numbers: 50089 has 5 SF Zeros before a decimal and natural number never count. 109 have 3 SF 0. 00789 has 3 SF 001234 has 4 SF Zeros after natural numbers only count IF there is a decimal present. 100 has 1 SF 100. has 3 SF 100. 0 has 4 SF

Objectives Now you should be able to… • Count the number of significant figures in a number.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video D 3: Rounding with Sig Figs

Objectives By the end of this video you should be able to… • Round calculated numbers to the appropriate number of significant figures.

Adding/Subtracting Significant Figures Remember: you can only be as precise as your least precise measurement. Therefore, when adding and subtracting, round your answer to the least number of DECIMAL PLACES. 2. 0 + 5. 61 = 7. 6 5. 67 + 102. 111 = 107. 78 23 + 11. 10 = 34

Multiplying/Dividing Significant Figures Remember: you can only be as precise as your least precise measurement. Therefore, when multiplying or dividing, round your answer to the least number of significant figures. 2. 0 x 35. 1 = 70. 2 = 70. 5. 11 x 98. 654 = 504. 12194 = 504 72. 1 / 3. 123 = 23. 0867755 = 23. 1

More Examples Record the following with appropriate sig figs. 110. 3 45. 2 + 65. 12 = 746 780 - 34. 2 = 43. 9 89. 52/45. 6 = 100 5 * 23 = 5. 6 (32. 4 -2. 3)/5. 4 = 140 3. 5(523. 6 -123. 12) =

Objectives Now you should be able to… • Round calculated numbers to the appropriate number of significant figures.

HONORS CHEMISTRY SUMMER ASSIGNMENT Video D 4: Errors in the Lab

Objectives By the end of this video you should be able to… • Calculate percent error.

Percent Error The measured value is a number from the lab data. The accepted value is a number published or given to you by a teacher. It doesn’t really matter which order you subtract in. You should take the absolute value. Therefore I usually subtract the big-small number. But ALWAYS DIVIDE BY ACCEPTED VALUE!

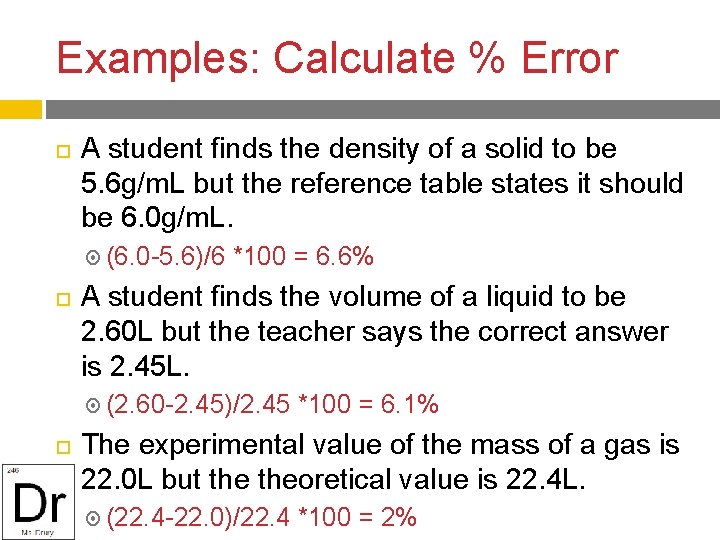
Examples: Calculate % Error A student finds the density of a solid to be 5. 6 g/m. L but the reference table states it should be 6. 0 g/m. L. (6. 0 -5. 6)/6 *100 = 6. 6% A student finds the volume of a liquid to be 2. 60 L but the teacher says the correct answer is 2. 45 L. (2. 60 -2. 45)/2. 45 *100 = 6. 1% The experimental value of the mass of a gas is 22. 0 L but theoretical value is 22. 4 L. (22. 4 -22. 0)/22. 4 *100 = 2%

Objectives Now you should be able to… • Calculate percent error.

Now What? Please practice problems in the summer assignment handout. I will be checking for evidence of your attempt in September. Email chemisme@gmail. com or chat me on Remind if you have questions. See you soon!
 Honors chemistry summer assignment
Honors chemistry summer assignment Kuei honors chemistry
Kuei honors chemistry Honors chemistry unit 4 review answers
Honors chemistry unit 4 review answers Bond order formula
Bond order formula Video yandex ru search
Video yandex ru search Yahoo gravity
Yahoo gravity Video.search.yahoo
Video.search.yahoo Digital media primer
Digital media primer Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Creighton university honors program
Creighton university honors program James scholar uiuc aces
James scholar uiuc aces Policy guidelines on awards and recognition
Policy guidelines on awards and recognition Ucsb honors seminars
Ucsb honors seminars Honors physics semester 1 review
Honors physics semester 1 review Honors biology ecology test
Honors biology ecology test 04.05 uncle sams toolbox-honors
04.05 uncle sams toolbox-honors Quadrilateral test review
Quadrilateral test review Honors earth science
Honors earth science Honors math 3
Honors math 3 Hilton honors military program
Hilton honors military program Honors project
Honors project Honors biology properties of water lab
Honors biology properties of water lab Honors geometry parallel lines and transversals worksheet
Honors geometry parallel lines and transversals worksheet Tulane honors program
Tulane honors program Honors geometry chapter 3
Honors geometry chapter 3 Pre calculus chapter 1
Pre calculus chapter 1 Vyi physics
Vyi physics Honors biology unit 4 test
Honors biology unit 4 test Smc scholars classes
Smc scholars classes English 2 honors vocabulary unit 1
English 2 honors vocabulary unit 1 Math 3 honors
Math 3 honors Chem
Chem Stlucieschools skyward
Stlucieschools skyward Mahurin honors college
Mahurin honors college Honors algebra 2 final exam
Honors algebra 2 final exam Umes honors program
Umes honors program Latin honors smith college
Latin honors smith college What is the earth's nearest celestial neighbor
What is the earth's nearest celestial neighbor Physics honors notes
Physics honors notes Foldable assignment
Foldable assignment Assignmentpoint.com bangladesh
Assignmentpoint.com bangladesh Encyclopedia entry assignment
Encyclopedia entry assignment Create your own parable
Create your own parable They do their homework last night
They do their homework last night Slidetodoc.com
Slidetodoc.com My 10 year plan answers
My 10 year plan answers Volume of cylinders cones and spheres assignment
Volume of cylinders cones and spheres assignment Vertex form of a quadratic
Vertex form of a quadratic Unix c sys wipro
Unix c sys wipro Special consideration uoa
Special consideration uoa All real numbers graph
All real numbers graph Unit 5 international business assignment 2
Unit 5 international business assignment 2 Unit 22 assignment 2
Unit 22 assignment 2 Unit 14 physiological disorders p1
Unit 14 physiological disorders p1 Cost accumulation and cost assignment
Cost accumulation and cost assignment Lirik lagu selembar kertas putih
Lirik lagu selembar kertas putih Travel brochure assignment
Travel brochure assignment Transportation and assignment problems and solutions
Transportation and assignment problems and solutions Transparent assignment template
Transparent assignment template Scarlet ibis writing prompt
Scarlet ibis writing prompt Napoleon's rise and fall assignment
Napoleon's rise and fall assignment The great gatsby song project
The great gatsby song project Economic naturalist essay
Economic naturalist essay Joshua is doing his assignment
Joshua is doing his assignment Tilt assignment template
Tilt assignment template Fetac levels chart
Fetac levels chart Static single assignment form
Static single assignment form Social 30-2 writing assignment 3
Social 30-2 writing assignment 3 Shake
Shake Random assignment vs random sampling
Random assignment vs random sampling 5 ways to prove lines are parallel
5 ways to prove lines are parallel Uno proofs
Uno proofs Product photography ads
Product photography ads Cost accumulation and cost assignment
Cost accumulation and cost assignment Systematic sampling example situation
Systematic sampling example situation Print ad assignment
Print ad assignment Ad deconstruction
Ad deconstruction Dream school
Dream school Deconstruction examples
Deconstruction examples Random assignment vs random selection
Random assignment vs random selection Slopes of parallel and perpendicular lines assignment
Slopes of parallel and perpendicular lines assignment Cwv origins assignment
Cwv origins assignment One hot state machine
One hot state machine Njnin
Njnin Curriculum-led budget template npqh
Curriculum-led budget template npqh Dedicated processor assignment
Dedicated processor assignment Dissection alternative assignment
Dissection alternative assignment Mgt 340 report
Mgt 340 report Maximo asset management scheduler
Maximo asset management scheduler Maps and scales maths lit grade 12
Maps and scales maths lit grade 12 Contoh soal dan penyelesaian mengenai metode penugasan
Contoh soal dan penyelesaian mengenai metode penugasan Masalah penugasan (assignment problem)
Masalah penugasan (assignment problem) Hadoop assignments with solutions
Hadoop assignments with solutions Hypothesis testing assignment
Hypothesis testing assignment Macbeth twitter project
Macbeth twitter project Close reading assignment
Close reading assignment Letter to self assignment
Letter to self assignment 12-6 surface areas and volumes of spheres answers
12-6 surface areas and volumes of spheres answers Geometry section 11-4 areas of regular polygons answers
Geometry section 11-4 areas of regular polygons answers Business research methods assignment
Business research methods assignment Unit 14 physiological disorders assignment 1
Unit 14 physiological disorders assignment 1 Eth306w assignment 2 answers 2020
Eth306w assignment 2 answers 2020 Trapeze bar comfort device
Trapeze bar comfort device Good luck with your assignment
Good luck with your assignment Joint footed biology
Joint footed biology Higher media assignment
Higher media assignment Risk assesment example
Risk assesment example Pub1601
Pub1601 Gravimetric analysis notes
Gravimetric analysis notes General responsibility assignment software patterns
General responsibility assignment software patterns Transformations of tangent functions
Transformations of tangent functions Structure chart symbols
Structure chart symbols Random assignment vs selection
Random assignment vs selection Static single assignment form
Static single assignment form Student-led discussion assignment
Student-led discussion assignment Binary addition 1+1+1
Binary addition 1+1+1 Dominant impression examples
Dominant impression examples Q
Q Computer networks assignment 1
Computer networks assignment 1