Solids CHEM HONORS Solids Crystalline vs Amorphous a






























- Slides: 35

Solids CHEM HONORS

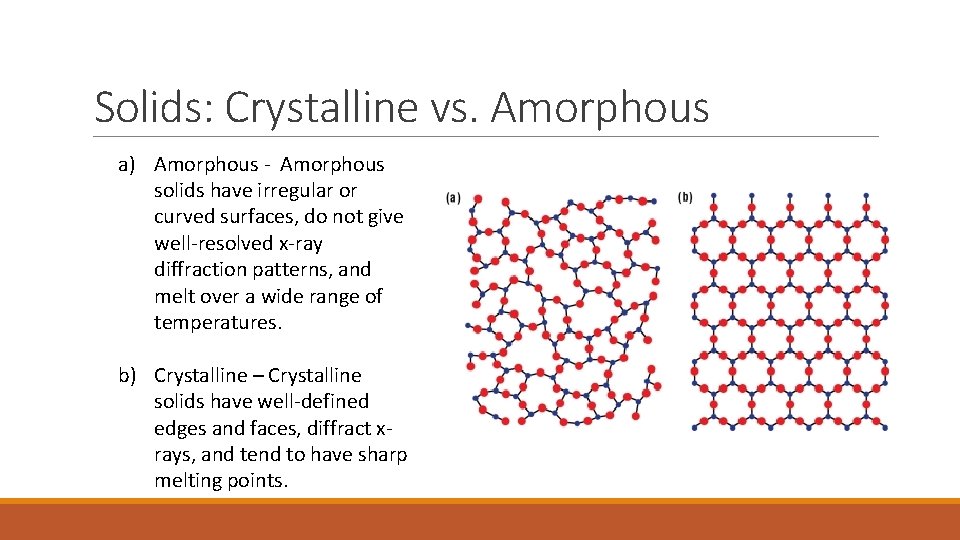
Solids: Crystalline vs. Amorphous a) Amorphous - Amorphous solids have irregular or curved surfaces, do not give well-resolved x-ray diffraction patterns, and melt over a wide range of temperatures. b) Crystalline – Crystalline solids have well-defined edges and faces, diffract xrays, and tend to have sharp melting points.

Types of Solids Ionic Solids (Ionic Bonds) • Molecular Solids (Covalent Bonds) • Atomic Solids • Metallic Solids (Metallic Bonds) •

Ionic Bonds • Ionic bonds commonly contain a metal and a non-metal • The metal is oxidized and loses an electron – becomes a CATION (positive charge) • The non-metal is reduced and gains an electron – becomes an ANION (negative charge) • An electrostatic attraction holds the two ions together

Ionic Bonds – Types of Ions Charge of ion predicted using the electronegativity and electron configuration of the atom - Atoms with loosely held electrons form positive ions (Cations) - Atoms able to hold additional electrons form negative ions (Anions) Ex) Group I atoms loose electrons, become positive (by losing an electron they fulfill the octet rule)

Ionic Solids • Contains ions at the point of the lattice that describes the structure of the solid (ex Na. Cl) • VERY high MP’s (500 -3000 C⁰) • Very Hard • Ion-ion Coulombic forces are the strongest of all attractive forces • IMF usually implies covalently bonded substances, but can apply to both types • Very STABLE because they are held together by strong electrostatic forces. Na. Cl crystal lattice: Na – purple, Cl - green

Ionic Solids – Recap Strong attraction between the 2 ions due to the Coulombic force Solids at room temp High melting points Huge, 3 D networks of ions of opposite charge held together by ionic bonds

Questions • Which has a stronger ionic bond, Na. Cl or Na. F? ◦ Na. F because F- is smaller; the particles/ions will pack tighter in the crystal lattice • Melting point measures what? ◦ How strongly ions are held together in a bond; the more strongly the ions are held together, the higher the MP • Which has a higher MP? Na. Cl vs. Na. Br; Mg. O vs. Ca. O • Na. Cl and Mg. O because they’re smaller • Smaller ions = greater bond, because the in Coulombic force equation distance and force are inversely proportional:

Lattice Energy • Lattice Energy: amount of energy needed to completely break apart the ions in one mole of an ionic compound • Which has a larger lattice energy: Na. Cl v. Na. Br; Na. Cl v. Mg. S: • Na. Cl because it has a higher melting point, and thus stronger ionic bonds, so it will take more energy to break the bonds • Mg. S because it has a higher charge (Mg 2+ and S 2 - v. Na+ and Cl-), and thus stronger bonds (because in Coulombic force equation force and charge are directly proportional), so it will take more energy to break the bonds

Summary Ionic Bonds + Ionic Solids • Ionic Bonds • NM (gains electrons)+ Metal (loses electrons) • Smaller ion = greater force = higher MP • Greater charge = greater force = higher MP • Ionic Solids • Solids at room temp • High melting points • Huge, 3 D networks of ions of opposite charge held together by ionic bonds

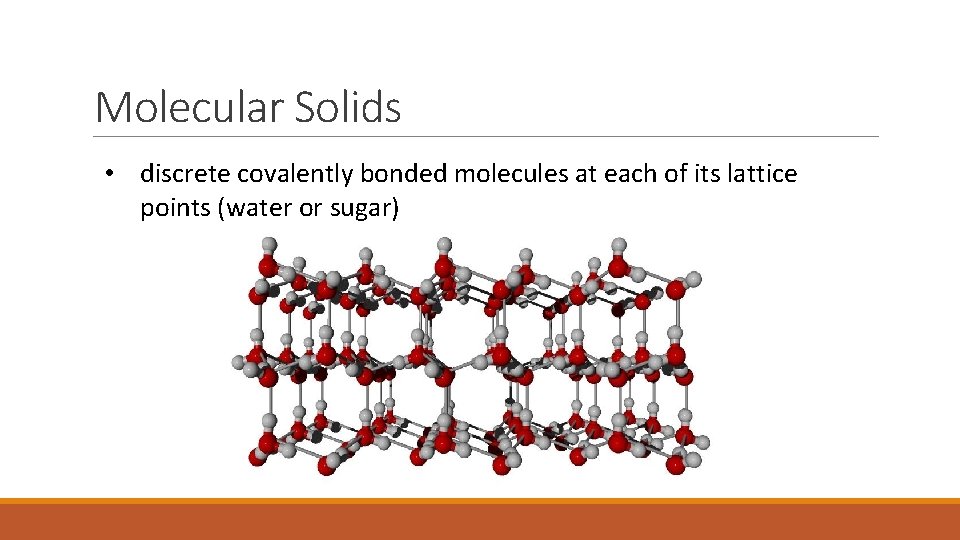
Molecular Solids • discrete covalently bonded molecules at each of its lattice points (water or sugar)

Molecular Solids • Characterized by strong COVALENT bonding within the molecule yet weak forces between the molecule • It takes 6. 0 k. J of energy to melt one mole of solid water since you have to overcome H-bonding while it takes 470 k. J of energy to break one mole of O-H bonds • Molecules such as CO 2, I 2, P 4, and S 8 have no dipole moment (London Dispersion Forces) • As the size of the molecule increases the London Dispersion Forces increase because the larger the molecule the more electrons, the more polarizable its electron cloud. • If it is polarizable, temporary dipoles can easily form. This causes the melting point and boiling point to increase due to the molecules becoming more attracted to one another

Molecular Solids Carbon Dioxide Iodine (I 2) Sulfur (S 8)

Network Atomic Solids • AKA Network Covalent • Composed of strong directional covalent bonds that are best viewed as a “giant molecule”. • Examples include diamond and graphite (both are composed of strictly carbon atoms) • Allotropes are different structural modifications of an element; the atoms of the element are bonded together in a different manner. Diamond • Diamond - bonds with neighbors in a tetrahedral 3 -D fashion • Graphite – only has WEAK bonding in the 3 rd dimension Graphite

Network Atomic Solids Network Solids are often: • Brittle – diamond is the hardest substance on the planet, but when a diamond is “cut” it is actually fractured to make the facets • They DO NOT conduct heat or electricity • They are carbon or silicon based

Diamond Network atomic solid • Diamond is hard, colorless and an insulator. • It consists of carbon atoms ALL bonded tetrahedrally. • Therefore they are sp 3 hybridization and 109. 5 o bond angles

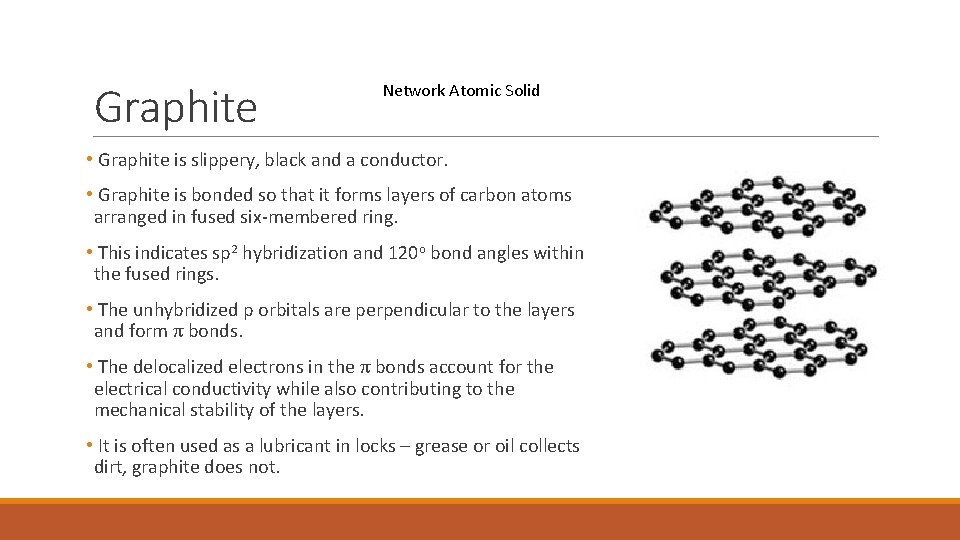
Graphite Network Atomic Solid • Graphite is slippery, black and a conductor. • Graphite is bonded so that it forms layers of carbon atoms arranged in fused six-membered ring. • This indicates sp 2 hybridization and 120 o bond angles within the fused rings. • The unhybridized p orbitals are perpendicular to the layers and form π bonds. • The delocalized electrons in the π bonds account for the electrical conductivity while also contributing to the mechanical stability of the layers. • It is often used as a lubricant in locks – grease or oil collects dirt, graphite does not.

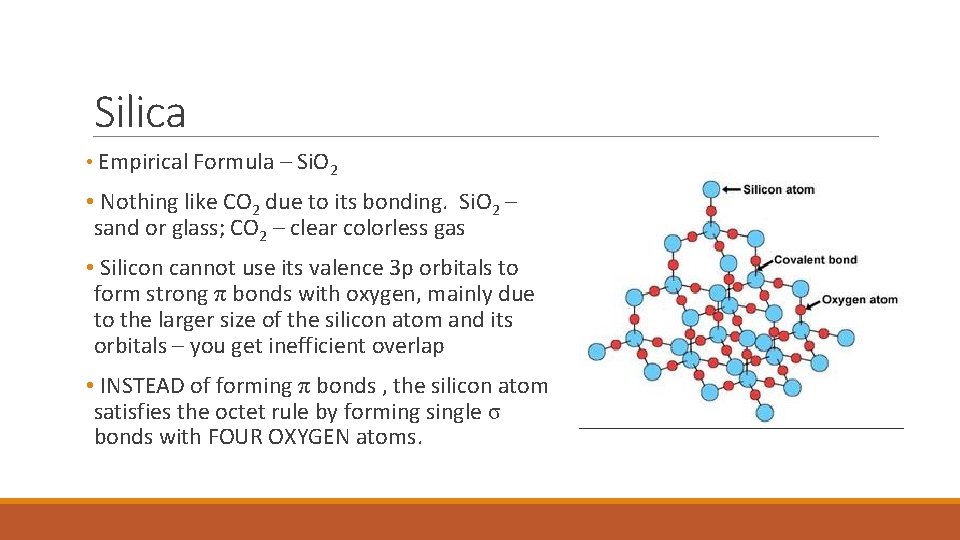
Silica • Empirical Formula – Si. O 2 • Nothing like CO 2 due to its bonding. Si. O 2 – sand or glass; CO 2 – clear colorless gas • Silicon cannot use its valence 3 p orbitals to form strong π bonds with oxygen, mainly due to the larger size of the silicon atom and its orbitals – you get inefficient overlap • INSTEAD of forming π bonds , the silicon atom satisfies the octet rule by forming single σ bonds with FOUR OXYGEN atoms.

Silica Network Atomic Solid • Each silicon is in the center of a tetrahedral arrangement of oxygen atoms. • The structure is based on a network of Si. O 4 tetrahedral with shared oxygen atoms. • When silica is heated above its melting point of about 1600 o. C and cooled rapidly, an amorphous solid forms. We call it glass – it’s really a super-cooled, ultra viscous liquid with a great deal of disorder.

Structure and Bonding in Metals • Metals are characterized by high thermal and electrical conductivity, malleability, and ductility. • Closest Packing – a model that uses hard spheres to represent the atoms of a metal. These atoms are packed together and bonded to each other equally in all directions. • It will be easiest for you to understand if you can imagine taking a cubic box and pouring in golf balls. • The balls will layer, perhaps directly on top of one another, but perhaps one layer slides into the “dimple” made by the first layer so that the two

Metals – Properties • Have shine or luster • Malleable – can be hammered or pressed into different shapes without breaking • Ductile – can be drawn into thin sheets or wires without breaking • Conduct heat and electricity

Nonmetals • NOT malleable • NOT ductile • Poor conductors of heat and electricity • High Electronegativity • Generally, a gas

Bonding Models for Metals: ◦ ◦ ◦ Conduct Heat Conduct Electricity Are Malleable Are Ductile Have High Melting Points Indicates that bonding in metals is both strong and non-directional Difficult to separate atoms, but easy to move them provided they stay in contact with each other

Bonding Models in Metals Electron Sea Model: A regular array of metals in a “sea” of electrons.

Electronic Structure of Metals • Valence electrons on a metal atom are shared with many neighboring atoms, not just one • Electrons not tightly bonded to individual atoms, so they move freely throughout the metal • Force of attraction btwn (+) metal ions and the sea of mobile (–)electrons forms a metallic bond that holds these particles together • Metals hold electrons loosely due to low electronegativity • Valence electrons are delocalized over a number of metal atoms • Metals exist as extended arrays of packed spherical atoms so each atom can touch as many neighboring atoms as possible

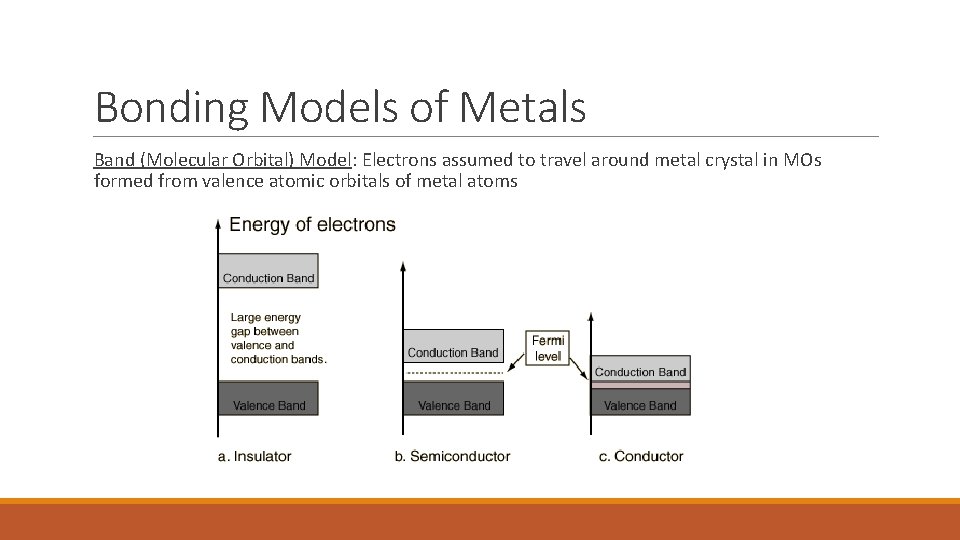
Bonding Models of Metals Band (Molecular Orbital) Model: Electrons assumed to travel around metal crystal in MOs formed from valence atomic orbitals of metal atoms

Metal Alloys – a substance that has a mixture of elements and has metallic properties Substitution Alloys – ◦ ◦ in brass 1/3 of the atoms in the host copper metal have been replaced by zinc atoms. Sterling Silver – 93% silver and 7% copper Pewter – 85% Tin, 7% copper, 6% bismuth, and 2% antimony Plumber’s solder – 95% tin and 5% antimony

Review • Molecular Solids • Crystallizes • Low MP • Metallic Solids • High MP • Insoluble in H 2 O • Used in Structural Material • Ductile • Malleable • Not Crystalline • Conducts electricity and heat • Ionic Solids • High MP • Crystallizes • Hard • Brittle • Soluble in H 2 O • Atomic Network Solids • Insoluble in H 2 O • Crystallizes • Doesn’t conduct electricity or heat • High MP • Brittle

Bond-Type Triangle • Bond-type triangle: chart used to predict properties of a compound based on the electronegativities of the elements that compromise the compound • Can be divided into regions which indicate the predominant type of bonding present in compounds • Semiconductor: compound with properties intermediate btwn metallic and covalent • Ex) Si ΔEN = (absolute) difference in electronegati vity btwn 2 elements E N = avg. electronegati vity of 2 elements

Checkpoint Identify each solid as molecular, ionic, or atomic. A. ) Ar (s) B. ) H 2 O (s) C. ) K 2 O (s) D. ) Fe (s)

Checkpoint Identify each solid as molecular, ionic, or atomic. A. ) Ca. Cl 2 (s) B. ) CO 2 (s) C. ) Ni (s) D. ) I 2 (s)

Checkpoint Rank the solid by increasing melting points. Why? Ar (s), CCl 4 (s), Li. Cl (s), CH 3 OH (s)

Checkpoint Rank the solids by increasing melting points. Why? C (s, diamond), Kr (s), Na. Cl (s), H 2 O (s)

Checkpoint Which solid in each pair has the higher melting point and why? A. ) Ti. O 2 (s) or HOOH (s) B. ) CCl 4 (s) or Si. Cl 4 (s) C. ) Kr (s) or Xe (s) D. ) Na. Cl (s) or Ca. O (s)

Checkpoint Which solid in each pair has the higher melting point and why? A. ) Fe (s) or CCl 4 (s) B. ) KCl (s) or HCl (s) C. ) Ti (s) or Ne (s) D. ) H 2 O (s) or H 2 S (s)