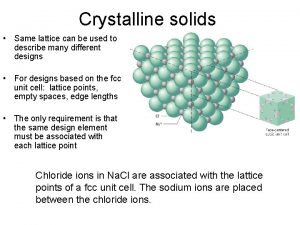
The Structure of Crystalline Solids ISSUES TO ADDRESS






- Slides: 24

The Structure of Crystalline Solids ISSUES TO ADDRESS. . . • How do atoms assemble into solid structures? • How does the density of a material depend on its structure? • When do material properties vary with the sample (i. e. , part) orientation? 1

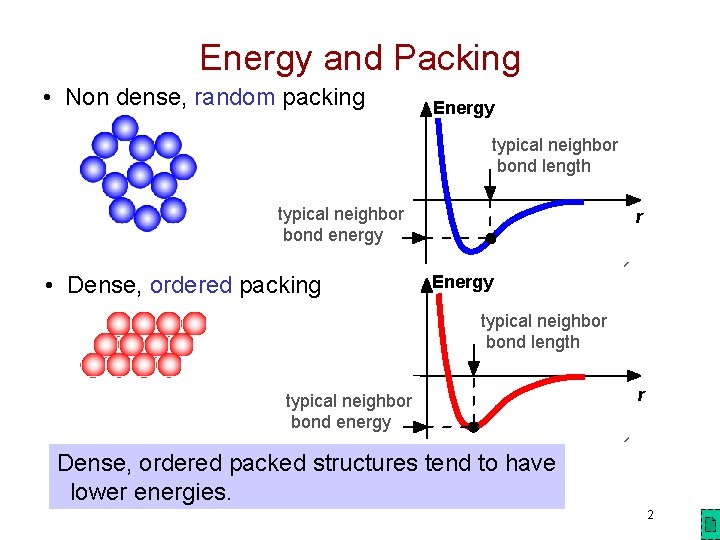
Energy and Packing • Non dense, random packing Energy typical neighbor bond length typical neighbor bond energy • Dense, ordered packing r Energy typical neighbor bond length typical neighbor bond energy r Dense, ordered packed structures tend to have lower energies. 2

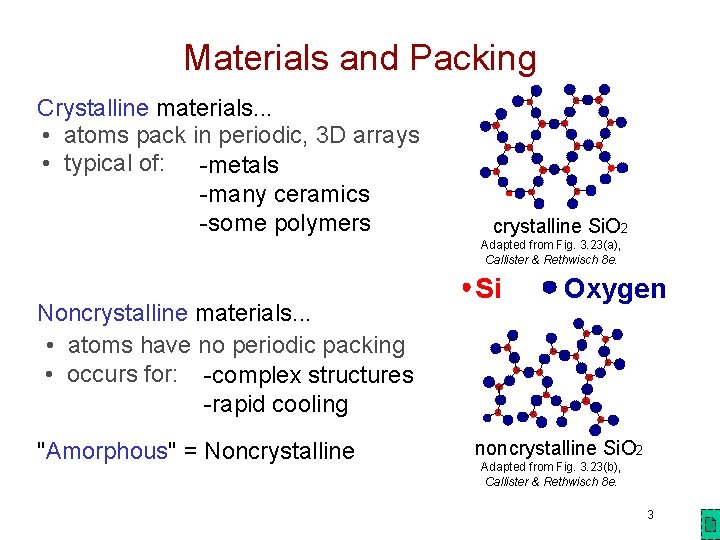
Materials and Packing Crystalline materials. . . • atoms pack in periodic, 3 D arrays • typical of: -metals -many ceramics -some polymers crystalline Si. O 2 Adapted from Fig. 3. 23(a), Callister & Rethwisch 8 e. Noncrystalline materials. . . • atoms have no periodic packing • occurs for: -complex structures -rapid cooling "Amorphous" = Noncrystalline Si Oxygen noncrystalline Si. O 2 Adapted from Fig. 3. 23(b), Callister & Rethwisch 8 e. 3

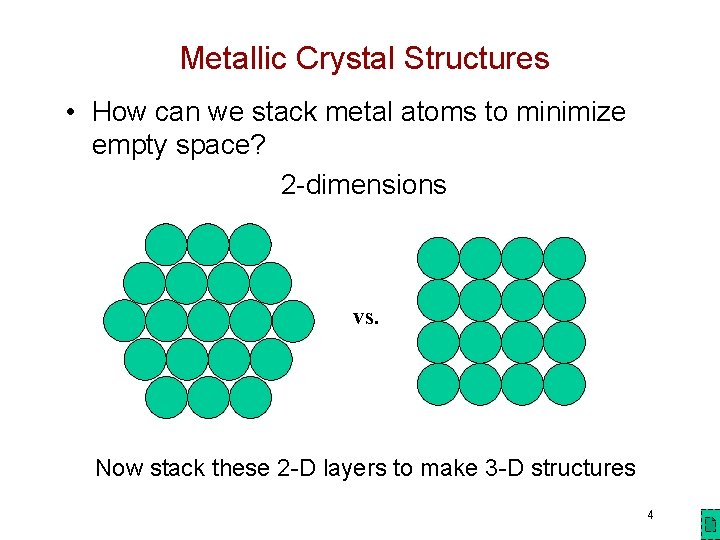
Metallic Crystal Structures • How can we stack metal atoms to minimize empty space? 2 -dimensions vs. Now stack these 2 -D layers to make 3 -D structures 4

Metallic Crystal Structures • Tend to be densely packed. • Reasons for dense packing: - Typically, only one element is present, so all atomic radii are the same. - Metallic bonding is not directional. - Nearest neighbor distances tend to be small in order to lower bond energy. - Electron cloud shields cores from each other • Have the simplest crystal structures. We will examine three such structures. . . 5

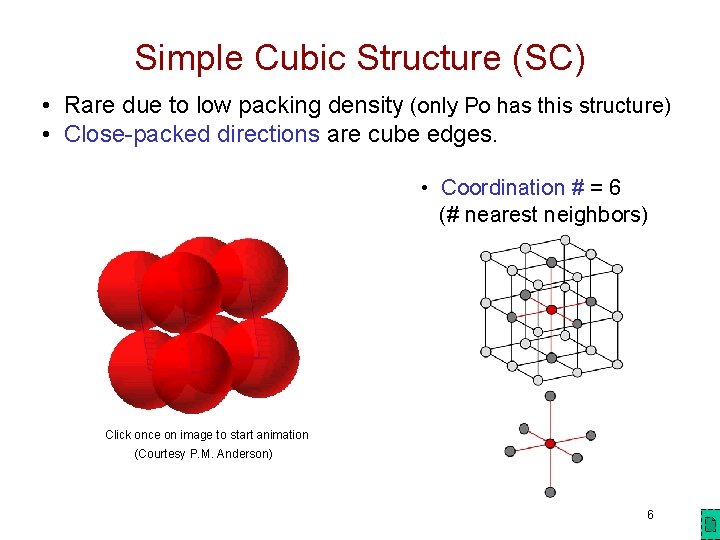
Simple Cubic Structure (SC) • Rare due to low packing density (only Po has this structure) • Close-packed directions are cube edges. • Coordination # = 6 (# nearest neighbors) Click once on image to start animation (Courtesy P. M. Anderson) 6

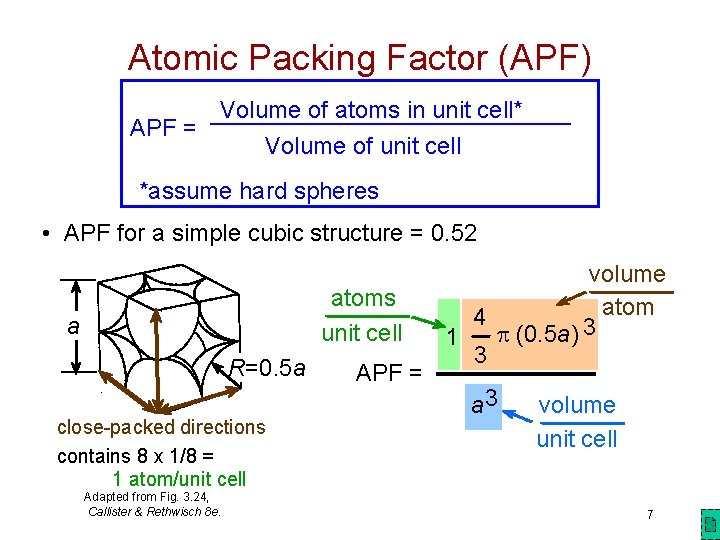
Atomic Packing Factor (APF) Volume of atoms in unit cell* APF = Volume of unit cell *assume hard spheres • APF for a simple cubic structure = 0. 52 atoms unit cell a R=0. 5 a close-packed directions contains 8 x 1/8 = 1 atom/unit cell Adapted from Fig. 3. 24, Callister & Rethwisch 8 e. APF = volume atom 4 p (0. 5 a) 3 1 3 a 3 volume unit cell 7

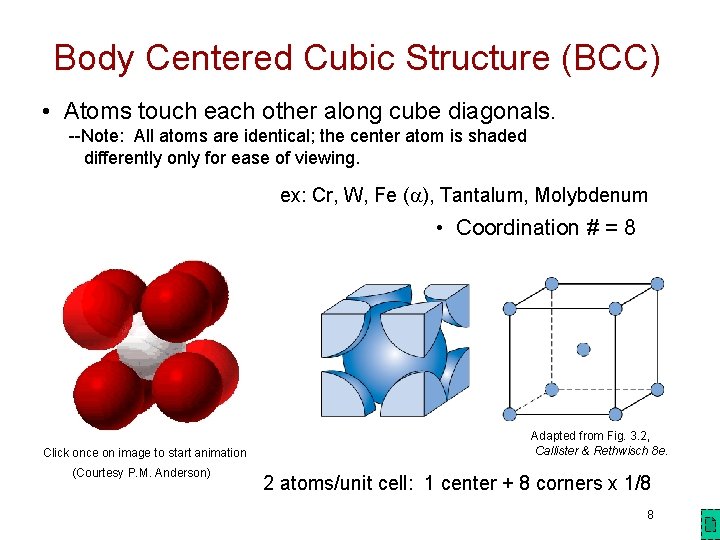
Body Centered Cubic Structure (BCC) • Atoms touch each other along cube diagonals. --Note: All atoms are identical; the center atom is shaded differently only for ease of viewing. ex: Cr, W, Fe ( ), Tantalum, Molybdenum • Coordination # = 8 Click once on image to start animation (Courtesy P. M. Anderson) Adapted from Fig. 3. 2, Callister & Rethwisch 8 e. 2 atoms/unit cell: 1 center + 8 corners x 1/8 8

Atomic Packing Factor: BCC • APF for a body-centered cubic structure = 0. 68 3 a a 2 a Adapted from Fig. 3. 2(a), Callister & Rethwisch 8 e. R a Close-packed directions: length = 4 R = 3 a atoms volume 4 p ( 3 a/4) 3 2 unit cell atom 3 APF = volume 3 a unit cell 9

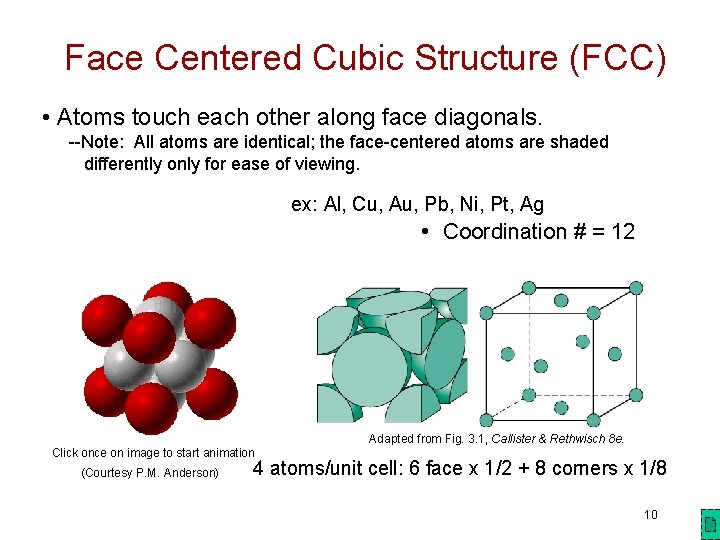
Face Centered Cubic Structure (FCC) • Atoms touch each other along face diagonals. --Note: All atoms are identical; the face-centered atoms are shaded differently only for ease of viewing. ex: Al, Cu, Au, Pb, Ni, Pt, Ag • Coordination # = 12 Adapted from Fig. 3. 1, Callister & Rethwisch 8 e. Click once on image to start animation (Courtesy P. M. Anderson) 4 atoms/unit cell: 6 face x 1/2 + 8 corners x 1/8 10

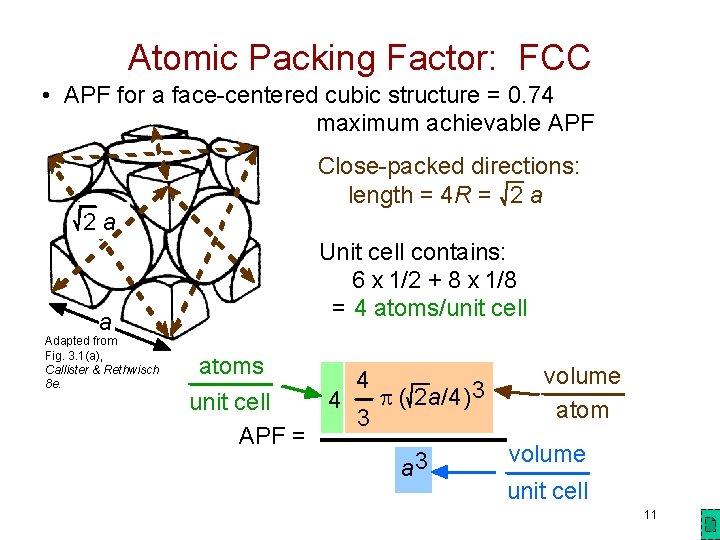
Atomic Packing Factor: FCC • APF for a face-centered cubic structure = 0. 74 maximum achievable APF 2 a a Adapted from Fig. 3. 1(a), Callister & Rethwisch 8 e. Close-packed directions: length = 4 R = 2 a Unit cell contains: 6 x 1/2 + 8 x 1/8 = 4 atoms/unit cell atoms volume 4 3 p ( 2 a/4) 4 unit cell atom 3 APF = volume 3 a unit cell 11

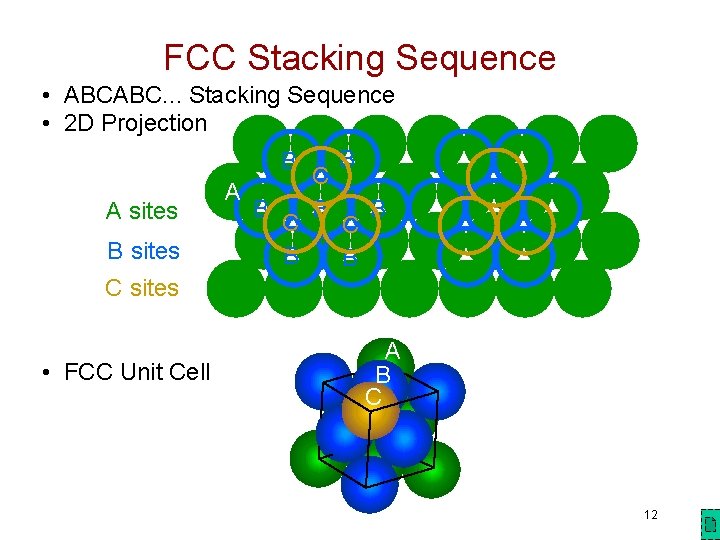
FCC Stacking Sequence • ABCABC. . . Stacking Sequence • 2 D Projection B B C A B B B A sites C C B sites B B C sites • FCC Unit Cell A B C 12

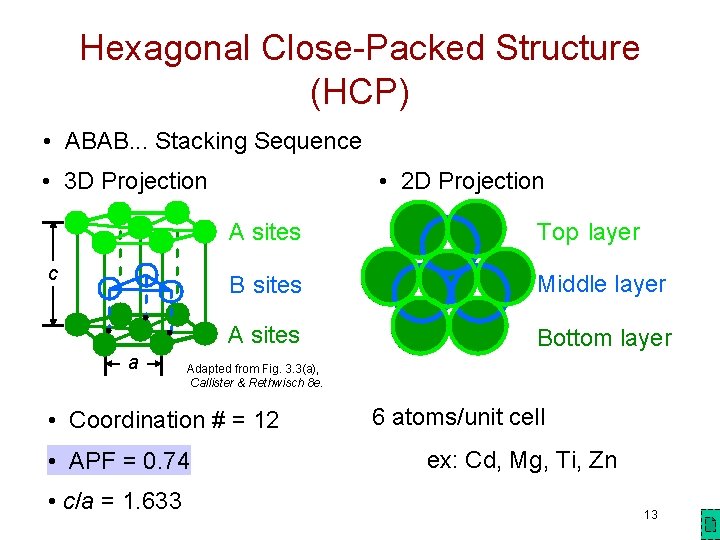
Hexagonal Close-Packed Structure (HCP) • ABAB. . . Stacking Sequence • 3 D Projection c a • 2 D Projection A sites Top layer B sites Middle layer A sites Bottom layer Adapted from Fig. 3. 3(a), Callister & Rethwisch 8 e. • Coordination # = 12 • APF = 0. 74 • c/a = 1. 633 6 atoms/unit cell ex: Cd, Mg, Ti, Zn 13

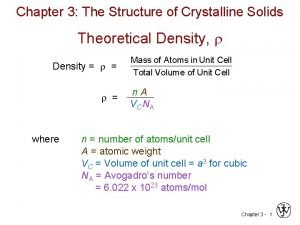
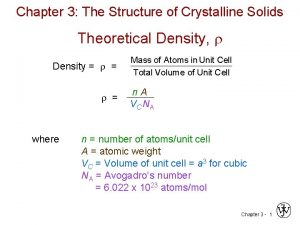
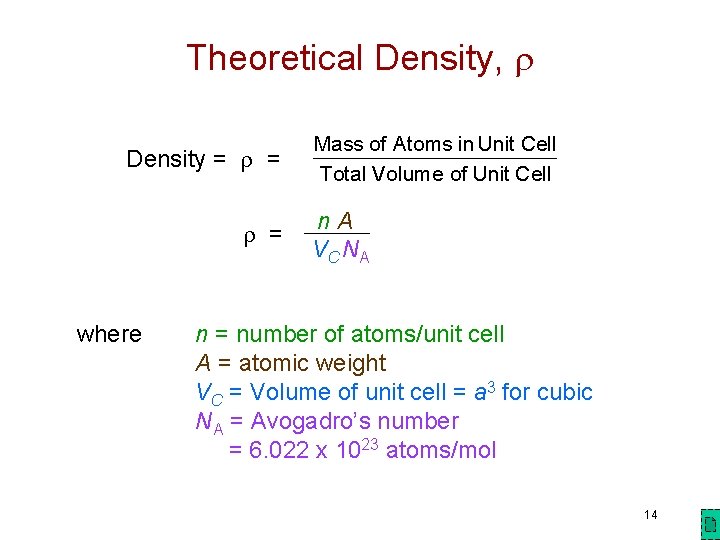
Theoretical Density, Density = = = Mass of Atoms in Unit Cell Total Volume of Unit Cell n A V C NA where n = number of atoms/unit cell A = atomic weight VC = Volume of unit cell = a 3 for cubic NA = Avogadro’s number = 6. 022 x 1023 atoms/mol 14

Theoretical Density, • Ex: Cr (BCC) A = 52. 00 g/mol R = 0. 125 nm n = 2 atoms/unit cell Adapted from Fig. 3. 2(a), Callister & Rethwisch 8 e. atoms unit cell = volume unit cell R a 2 52. 00 a 3 6. 022 x 1023 a = 4 R/ 3 = 0. 2887 nm g mol theoretical = 7. 18 g/cm 3 actual atoms mol = 7. 19 g/cm 3 15

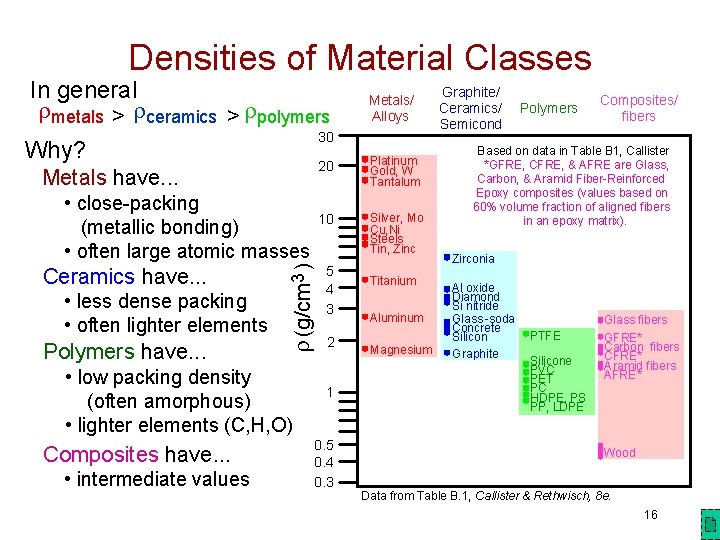
Densities of Material Classes In general metals > ceramics > polymers 30 Why? Ceramics have. . . 3 (g/cm ) Metals have. . . • close-packing (metallic bonding) • often large atomic masses • less dense packing • often lighter elements Polymers have. . . • low packing density (often amorphous) • lighter elements (C, H, O) Composites have. . . • intermediate values Metals/ Alloys 20 Platinum Gold, W Tantalum 10 Silver, Mo Cu, Ni Steels Tin, Zinc 5 4 3 2 1 0. 5 0. 4 0. 3 Graphite/ Ceramics/ Semicond Polymers Composites/ fibers Based on data in Table B 1, Callister *GFRE, CFRE, & AFRE are Glass, Carbon, & Aramid Fiber-Reinforced Epoxy composites (values based on 60% volume fraction of aligned fibers in an epoxy matrix). Zirconia Titanium Al oxide Diamond Si nitride Aluminum Glass -soda Concrete PTFE Silicon Magnesium Graphite Silicone PVC PET PC HDPE, PS PP, LDPE Glass fibers GFRE* Carbon fibers CFRE* Aramid fibers AFRE* Wood Data from Table B. 1, Callister & Rethwisch, 8 e. 16

Crystals as Building Blocks • Some engineering applications require single crystals: -- diamond single crystals for abrasives (Courtesy Martin Deakins, GE Superabrasives, Worthington, OH. Used with permission. ) -- turbine blades Fig. 8. 33(c), Callister & Rethwisch 8 e. (Fig. 8. 33(c) courtesy of Pratt and Whitney). • Properties of crystalline materials often related to crystal structure. -- Ex: Quartz fractures more easily along some crystal planes than others. (Courtesy P. M. Anderson) 17

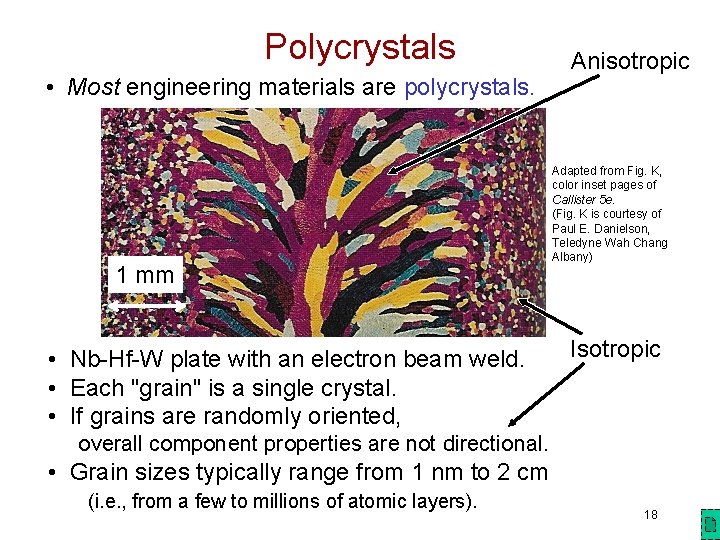
Polycrystals • Most engineering materials are polycrystals. 1 mm • Nb-Hf-W plate with an electron beam weld. • Each "grain" is a single crystal. • If grains are randomly oriented, Anisotropic Adapted from Fig. K, color inset pages of Callister 5 e. (Fig. K is courtesy of Paul E. Danielson, Teledyne Wah Chang Albany) Isotropic overall component properties are not directional. • Grain sizes typically range from 1 nm to 2 cm (i. e. , from a few to millions of atomic layers). 18

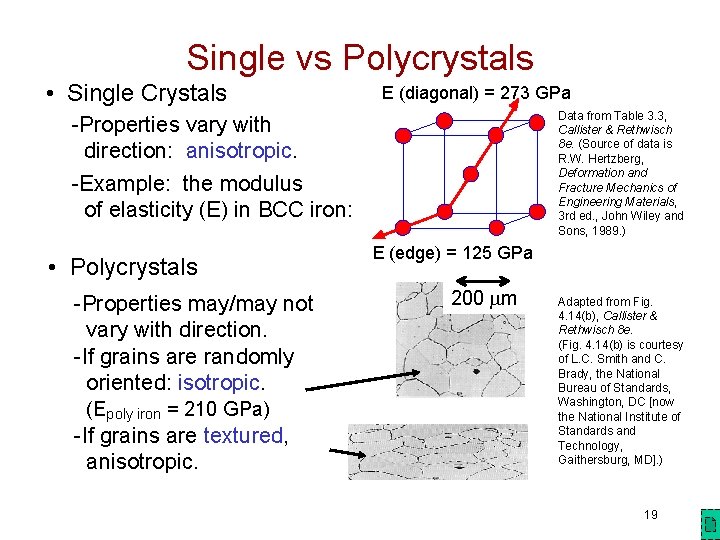
Single vs Polycrystals • Single Crystals E (diagonal) = 273 GPa Data from Table 3. 3, Callister & Rethwisch 8 e. (Source of data is R. W. Hertzberg, Deformation and Fracture Mechanics of Engineering Materials, 3 rd ed. , John Wiley and Sons, 1989. ) -Properties vary with direction: anisotropic. -Example: the modulus of elasticity (E) in BCC iron: • Polycrystals -Properties may/may not vary with direction. -If grains are randomly oriented: isotropic. (Epoly iron = 210 GPa) -If grains are textured, anisotropic. E (edge) = 125 GPa 200 mm Adapted from Fig. 4. 14(b), Callister & Rethwisch 8 e. (Fig. 4. 14(b) is courtesy of L. C. Smith and C. Brady, the National Bureau of Standards, Washington, DC [now the National Institute of Standards and Technology, Gaithersburg, MD]. ) 19

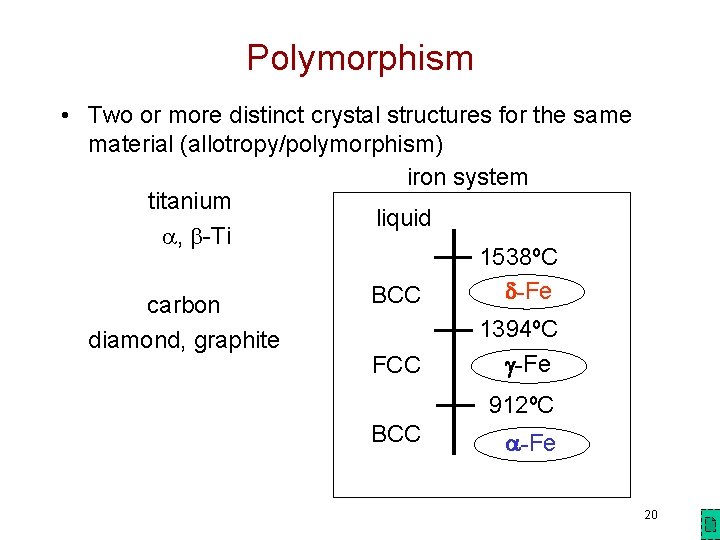
Polymorphism • Two or more distinct crystal structures for the same material (allotropy/polymorphism) iron system titanium liquid , -Ti 1538ºC -Fe BCC carbon 1394ºC diamond, graphite -Fe FCC 912ºC BCC -Fe 20

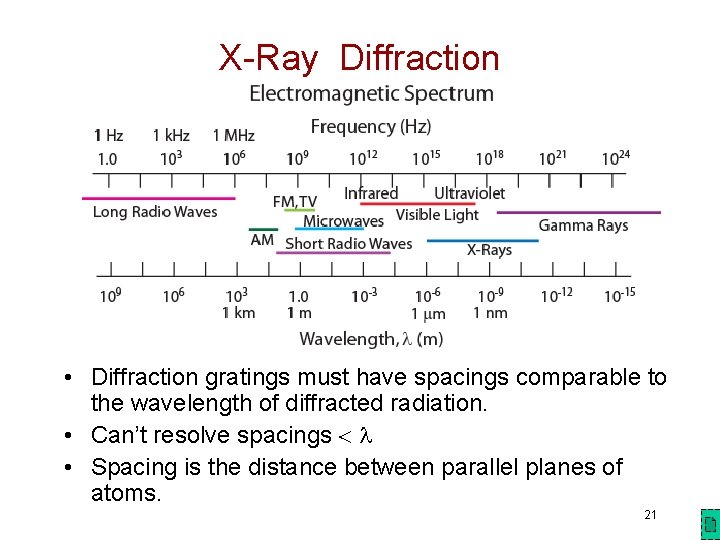
X-Ray Diffraction • Diffraction gratings must have spacings comparable to the wavelength of diffracted radiation. • Can’t resolve spacings • Spacing is the distance between parallel planes of atoms. 21

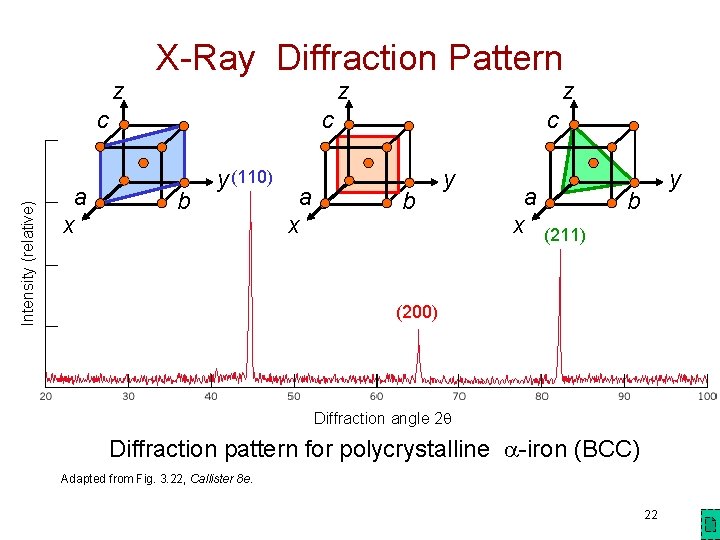
z X-Ray Diffraction Pattern z Intensity (relative) c a x z c b y (110) a x c b y a x (211) y b (200) Diffraction angle 2 q Diffraction pattern for polycrystalline -iron (BCC) Adapted from Fig. 3. 22, Callister 8 e. 22

SUMMARY • Atoms may assemble into crystalline or amorphous structures. • Common metallic crystal structures are FCC, BCC, and HCP. Coordination number and atomic packing factor are the same for both FCC and HCP crystal structures. • We can predict the density of a material, provided we know the atomic weight, atomic radius, and crystal geometry (e. g. , FCC, BCC, HCP). • Crystallographic points, directions and planes are specified in terms of indexing schemes. Crystallographic directions and planes are related to atomic linear densities and planar densities. 23

SUMMARY • Materials can be single crystals or polycrystalline. Material properties generally vary with single crystal orientation (i. e. , they are anisotropic), but are generally non-directional (i. e. , they are isotropic) in polycrystals with randomly oriented grains. • Some materials can have more than one crystal structure. This is referred to as polymorphism (or allotropy). • X-ray diffraction is used for crystal structure and interplanar spacing determinations. 24
 Crystalline vs non crystalline
Crystalline vs non crystalline Crystalline and non crystalline materials
Crystalline and non crystalline materials Crystalline solid
Crystalline solid Logical address
Logical address Crystalline or amorphous
Crystalline or amorphous Anisotropy
Anisotropy Crystalline substances
Crystalline substances Crystaline candy
Crystaline candy Polycrystalline solids
Polycrystalline solids Difference between colloidal and crystalline precipitate
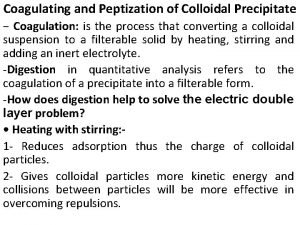
Difference between colloidal and crystalline precipitate Peptization
Peptization Difference between colloidal and crystalline precipitate
Difference between colloidal and crystalline precipitate Inorganic precipitating agents examples
Inorganic precipitating agents examples Snap head rivet diagram
Snap head rivet diagram Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Crystalline silicate clays
Crystalline silicate clays Definition of crystalline
Definition of crystalline Crystalline solid
Crystalline solid Destiny 2 crystalline formations
Destiny 2 crystalline formations Ccl
Ccl Explain how dishes on a menu address environmental issues
Explain how dishes on a menu address environmental issues Wjec level 1/2 hospitality and catering
Wjec level 1/2 hospitality and catering Structure of metallic solids
Structure of metallic solids Types of ip address
Types of ip address Address calculation in data structure
Address calculation in data structure