The Solid State Types of solids Crystalline solids












- Slides: 19

The Solid State: Types of solids

Crystalline solids have a regular arrangement 1. Ionic Solids (Na. Cl) 2. Molecular solids (sucrose) 3. Atomic or elemental solids (graphite, diamond)

l Properties of a solid are determined by the nature of the forces between their particles (intermolecular/intramolecular)

Ionic Solids: oppositely charged ions are held together by strong forces of attraction l Conduct a current in solution as ions are free to move l Stable substances with high melting points (solids at room temp) l

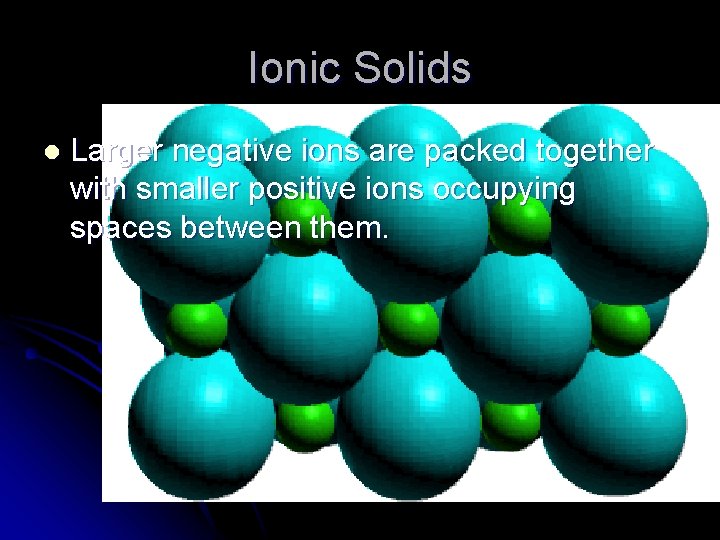
Ionic Solids l Larger negative ions are packed together with smaller positive ions occupying spaces between them.

Molecular Solids Carbon Dioxide (CO 2) Phosphorus (P 4) Sulfur (S 8)

l Forces are much weaker than in ionic solids l London dispersion forces l may have dipole-dipole attractions l melt at lower temperatures

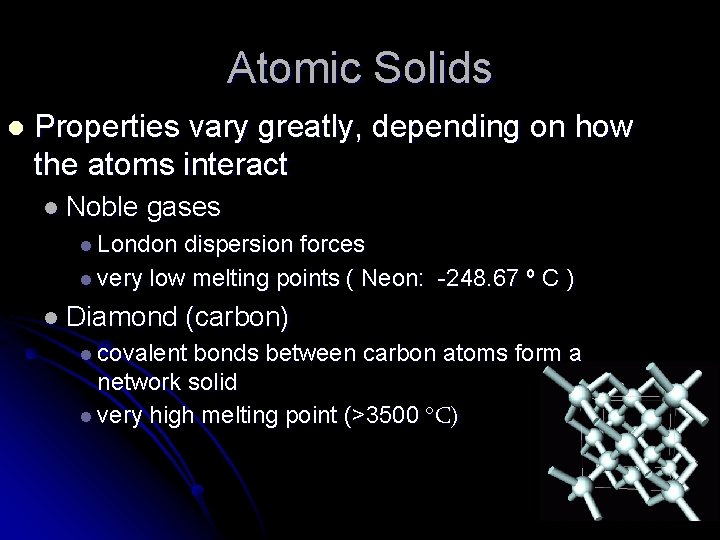
Atomic Solids l Properties vary greatly, depending on how the atoms interact l Noble gases l London dispersion forces l very low melting points ( Neon: -248. 67 º C ) l Diamond (carbon) l covalent bonds between carbon atoms form a network solid l very high melting point (>3500 C)

Bonding in Metals l Bonds are strong and nondirectional l difficult to separate atoms, but easy to slide them past each other

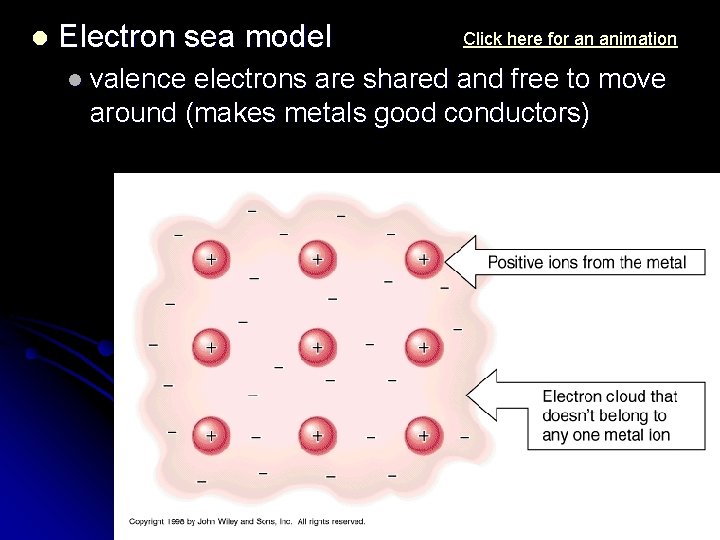
l Electron sea model l valence Click here for an animation electrons are shared and free to move around (makes metals good conductors)

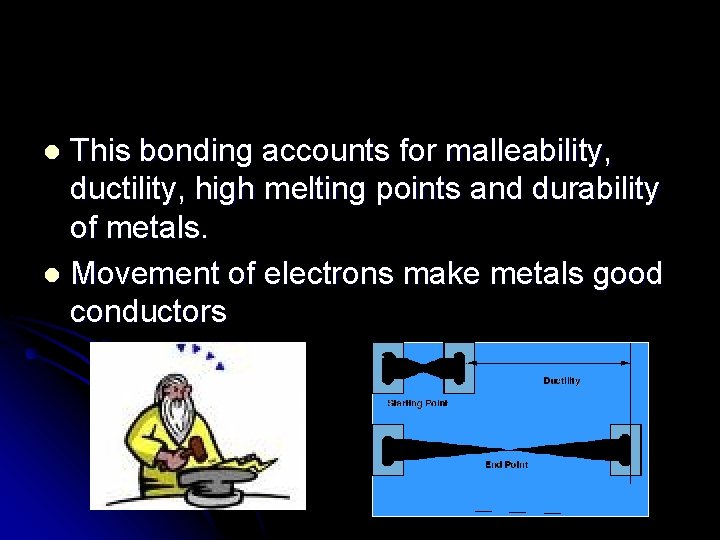
This bonding accounts for malleability, ductility, high melting points and durability of metals. l Movement of electrons make metals good conductors l

Alloys substances that contain a mixture of elements and have metallic properties l There are 2 types of alloys l l substitutional alloy l interstitial alloy

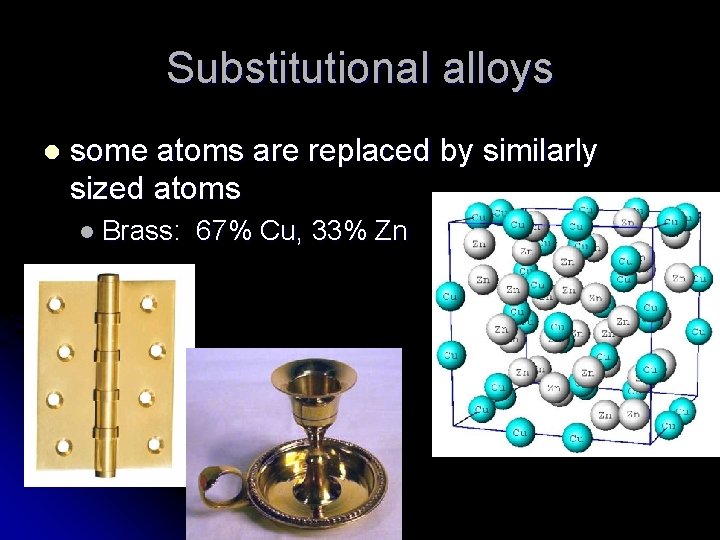
Substitutional alloys l some atoms are replaced by similarly sized atoms l Brass: 67% Cu, 33% Zn

l Sterling silver: 93% Ag, 7% Cu

Interstitial alloy Inter: between; stitial: spaces l Smaller atoms fit in “holes” (interstices) between atoms l Steel: Iron and carbon l l Carbon atoms form directional bonds, which give strength, increase hardness and decrease ductility

l Amount of carbon affects type of steel l Mild steels (<0. 2%C): nails, cables, chains

l Medium rails steels (0. 2 -0. 6%C): structural steel,

l High-carbon tools, cutlery steels (0. 6 -1. 5%C): springs,

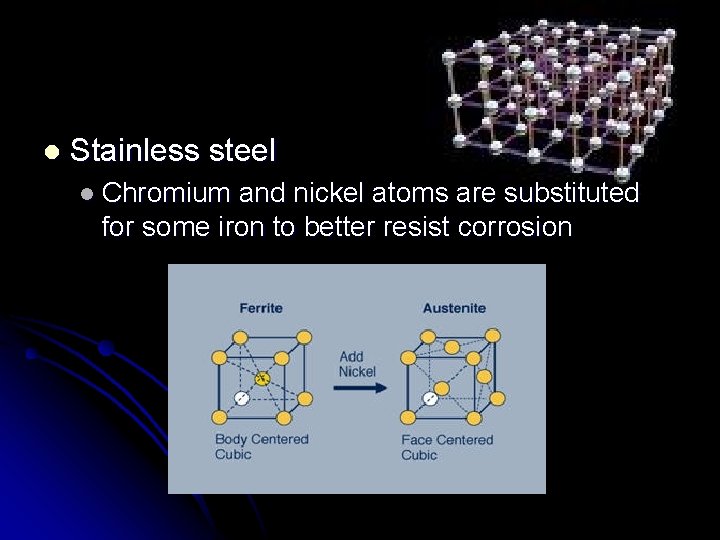
l Stainless steel l Chromium and nickel atoms are substituted for some iron to better resist corrosion