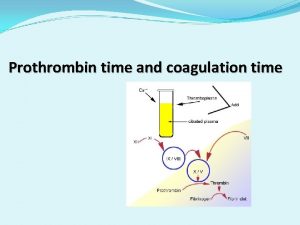
Coagulating and Peptization of Colloidal Precipitate Coagulation is







- Slides: 28

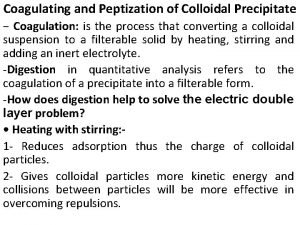
Coagulating and Peptization of Colloidal Precipitate − Coagulation: is the process that converting a colloidal suspension to a filterable solid by heating, stirring and adding an inert electrolyte. -Digestion in quantitative analysis refers to the coagulation of a precipitate into a filterable form. -How does digestion help to solve the electric double layer problem? • Heating with stirring: 1 - Reduces adsorption thus the charge of colloidal particles. 2 - Gives colloidal particles more kinetic energy and collisions between particles will be more effective in overcoming repulsions.

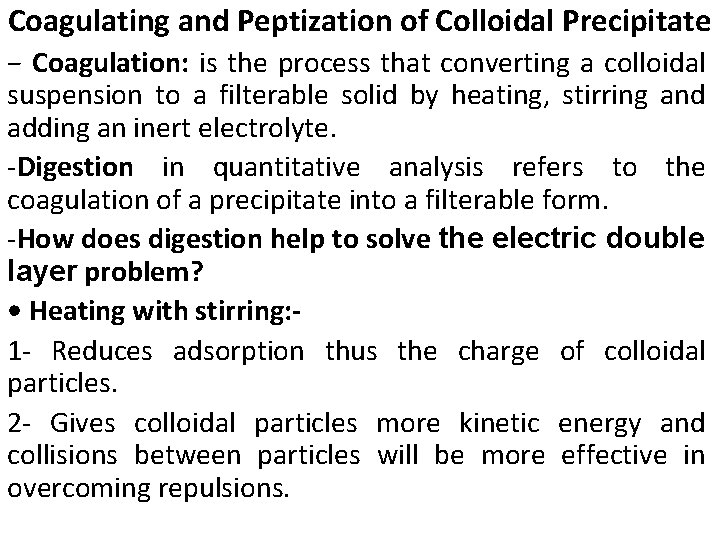
• Add inert electrolyte such as HNO 3 : 1 - Causes shrinkage of counter ion layer by increasing the concentration of ions of opposite charge to the adsorbed ions near the colloidal particle. 2 - Produces an insulating effect reducing the electrostatic repulsion between colloidal particles.

– Peptization : - is the process by which a coagulated colloidal precipitate is reconverted to colloidal suspension (re-suspension). -Colloidal precipitates can not be washed with pure water(why), because peptization occurs. (This is the reverse of coagulation). Peptization occurs when pure water is used to wash a colloidal precipitate (How)? The second electric layer is removed but the first layer remains on all particles with an electric charge of the same sign. The result is that there is a return to the repulsive state and begin once again to separate as colloidal particles. The process is called peptization Peptization can be minimized by washing a precipitate with a solution of an electrolyte that is volatilized during an ensuing drying step.

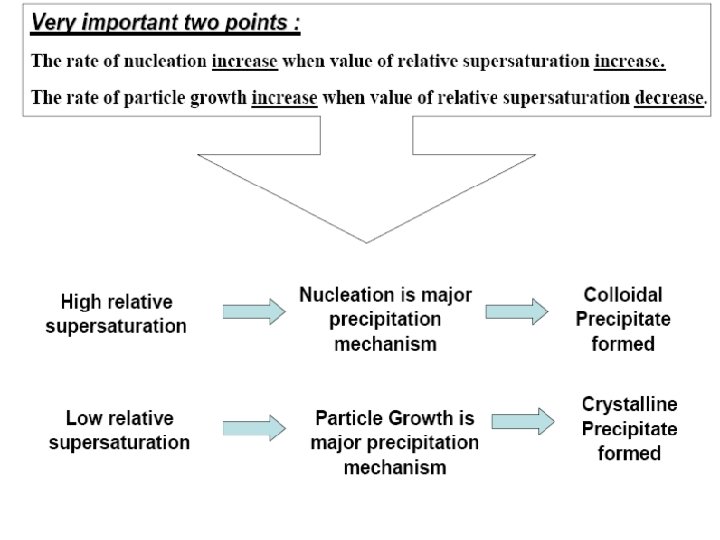
Some steps are used to produce a pure and filterable colloidal precipitate : 1 - Formation of the precipitate in a hot, stirred solution to aid coagulation process and also keeps the value of relative supersaturation as low as possible. 2 - Digestion process after precipitation allow the precipitate to sit in contact with the precipitating agent while solution still hot. This will remove weakly adsorbed ions and water molecules from the precipitate and produce a denser, easier to filter precipitate. 3 - Filtration the precipitate from hot solution this keeps the value of relative supersaturation as low as possible. 4 - Washing the precipitate with an electrolyte that will reduce the electrical double layer. 5 - Reprecipitation the compound which will minimize adsorption because there will be a lower concentration of adsorbing ions.



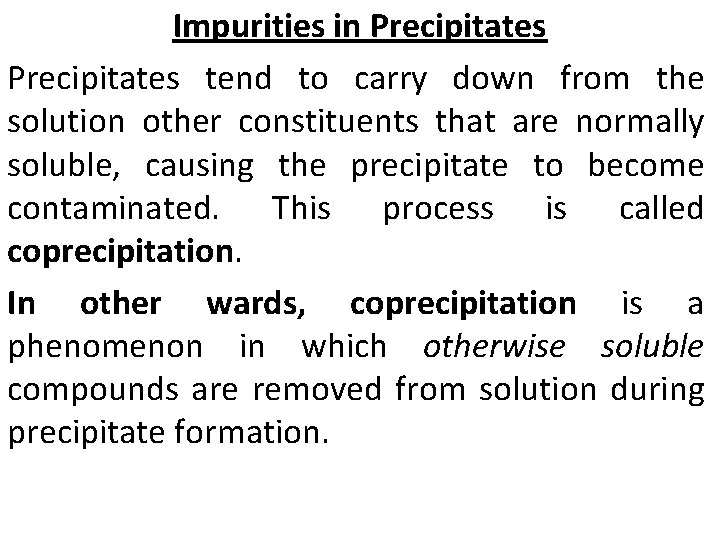
Impurities in Precipitates tend to carry down from the solution other constituents that are normally soluble, causing the precipitate to become contaminated. This process is called coprecipitation. In other wards, coprecipitation is a phenomenon in which otherwise soluble compounds are removed from solution during precipitate formation.

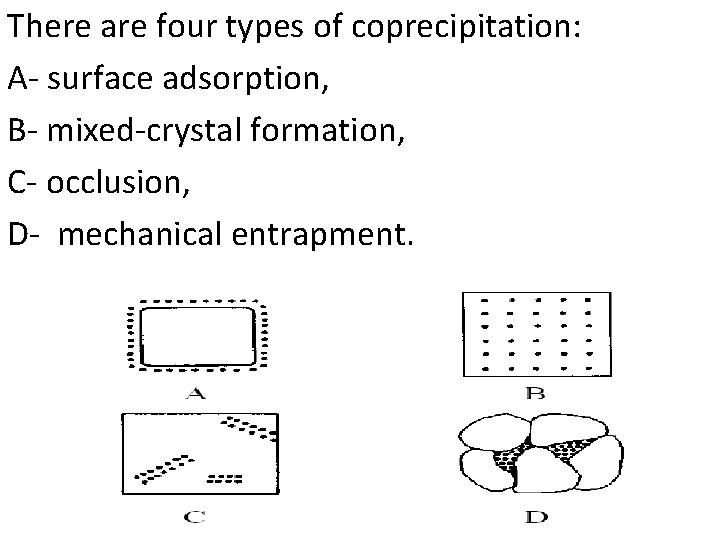
There are four types of coprecipitation: A- surface adsorption, B- mixed-crystal formation, C- occlusion, D- mechanical entrapment.

1 - Surface adsorption is a common source of coprecipitation and is likely to cause significant contamination of precipitates. The net effect of surface adsorption is therefore the carrying down of an otherwise soluble compound as a surface contaminant. In order to minimizing adsorbed impurities on colloids: 1 - Using digestion process to improve the purity. 2 - Washing a coagulated colloid with a solution containing a volatile electrolyte. The adsorbed layers can often be removed by washing. 3 - Reprecipitation is effective way to minimize the effects of adsorption.

2 - Mixed-crystal formation, one of the ions in the crystal lattice of a solid is replaced by an ion of another element. For this exchange to occur, it is necessary that the two ions have the same charge and that their sizes differ by no more than about 5%. This problem occur with both colloidal suspensions and crystalline precipitates. Example: - (Pb ion replace some of the barium ion). In order to minimizing this type of coprecipitation: 1 - The interfering ion may have to be separated before the final precipitation step. 2 - a different precipitating reagent that does not give mixed crystals with the ions interested may be used.

1. Occlusion • The Occlusion occurs when materials that are not part of the crystal structure are trapped within the crystal. Example, water may be trapped in pockets when Ag. N 03 crystals are formed. Occluded impurities are difficult to remove. The impurities cannot be removed by washing. Occlusion can be minimumize by Digestion and Reprecipitation (How)? Temperature of digestion open up the pockets and allow the impurities to escape into the solution.

4 - Mechanical entrapment occurs when crystals lie close together during growth. Here, several crystals grow together and in so doing trap a portion of the solution in a tiny pocket. Mechanical entrapment can be minimumize when the rate of precipitate formation is low— that is under conditions of low supersaturation. In addition, digestion is often remarkably helpful in reducing this types of coprecipitation.

• Precipitation from Homogenous Solution – Precipitation from homogenous solution is a technique in which a precipitating agent is generated in a solution of the analyte by a slow chemical reaction. - ( precipitation from homogeneous solution is a method to get pure and fine precipitate. . . that is the quality of the precipitate is increased through several parameters. . . in the precipitate formation we have two reactants. . one is precipitating agent another one is analyte. . in this method we are slowly producing any one of the above reactants through some chemical reactions. . hence the excess super saturation decreases and crystal growth happens slowly. . . as a result fine crystals are formed)

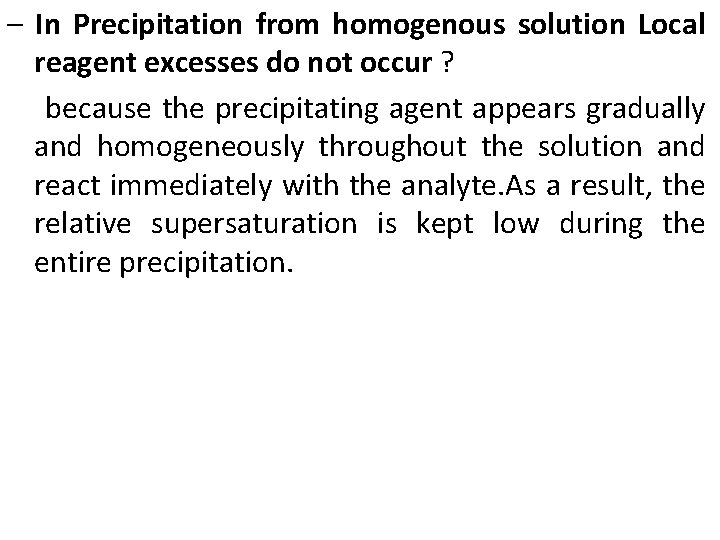
– In Precipitation from homogenous solution Local reagent excesses do not occur ? because the precipitating agent appears gradually and homogeneously throughout the solution and react immediately with the analyte. As a result, the relative supersaturation is kept low during the entire precipitation.

• The hydrated oxides of iron (III) and aluminum formed by direct addition of a base are gelatinous masses that are heavily contaminated and difficult to filter. In contrast, when these same precipitates are produced by the homogenous generation of hydroxide ion, they are dense and easily filtered and have higher purity (why).

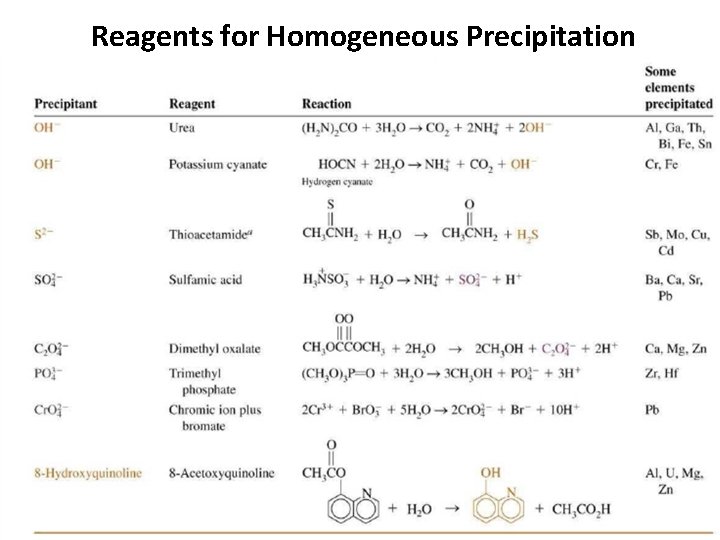
Reagents for Homogeneous Precipitation

• Drying and Ignition of Precipitates After filtration, a gravimetric precipitate is heated until its mass becomes constant. -Heating removes the solvent and any volatile species carried down with the precipitate. --Some precipitates are also ignited to decompose the solid and form a compound of known composition. This new Compound is often called the weighing form.

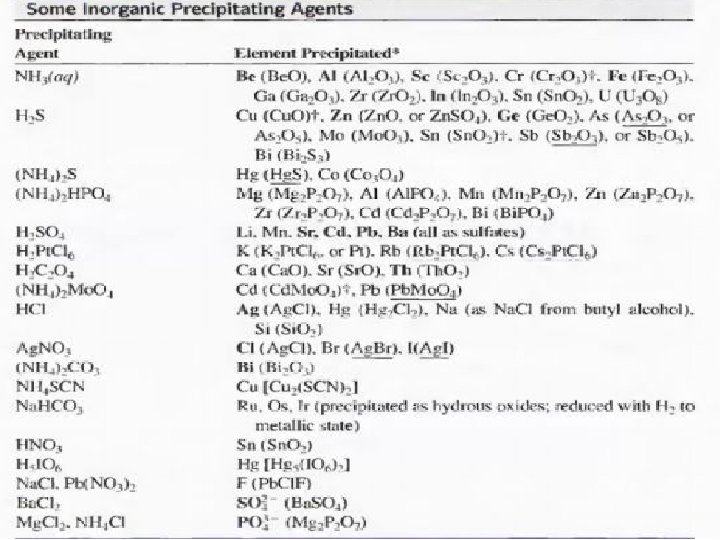
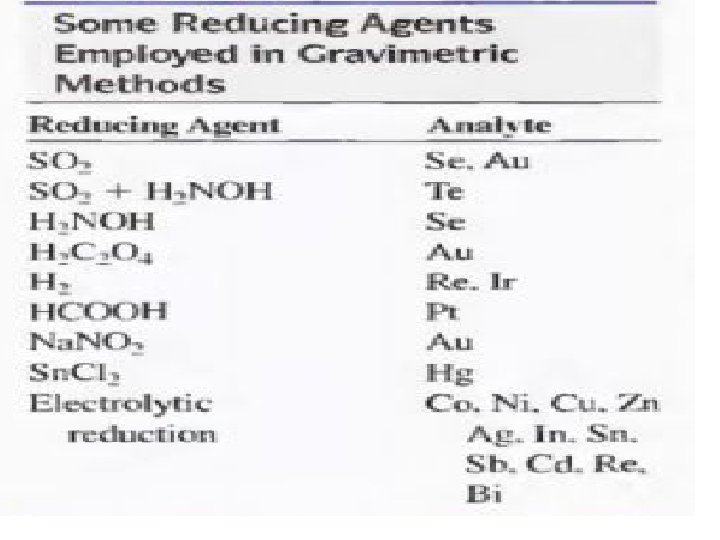
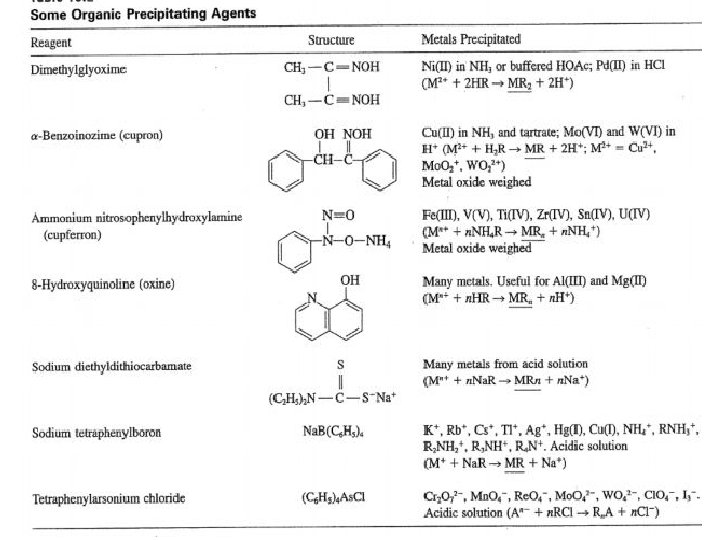
• Types of Precipitating Agents 1 - Inorganic Precipitating Agents These reagents typically form slightly soluble salts or hydrous oxides with the analyte. 2 - Reducing Agents This type of reagents convert an analyte to its elemental form for weighing 3 - Organic Precipitating Agents two types of organic reagents. One forms slightly soluble non-ionic products called coordination compounds; the other forms products in which the bonding between the inorganic species and the reagent is largely ionic.

• Organic precipitating agents have the advantages of: – Some of organic precipitating agents are very selective, and others are very broad in the number of elements they will precipitate. – Giving precipitates with very low solubility in water. – Give a favorable gravimetric factor.




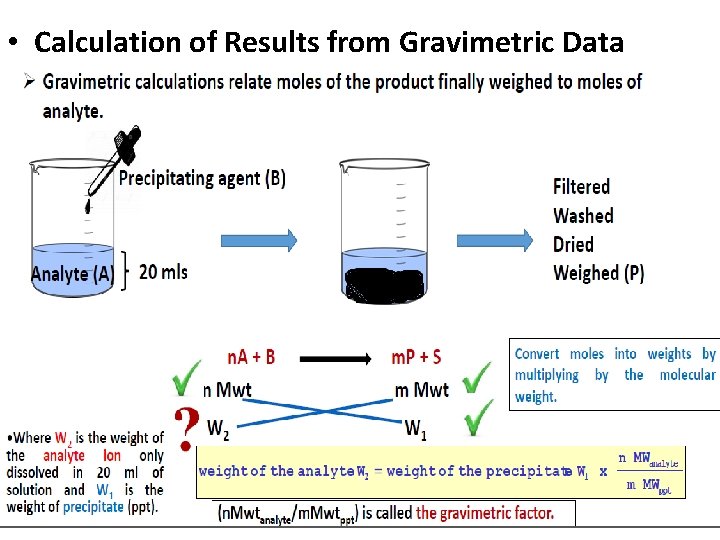
• Calculation of Results from Gravimetric Data


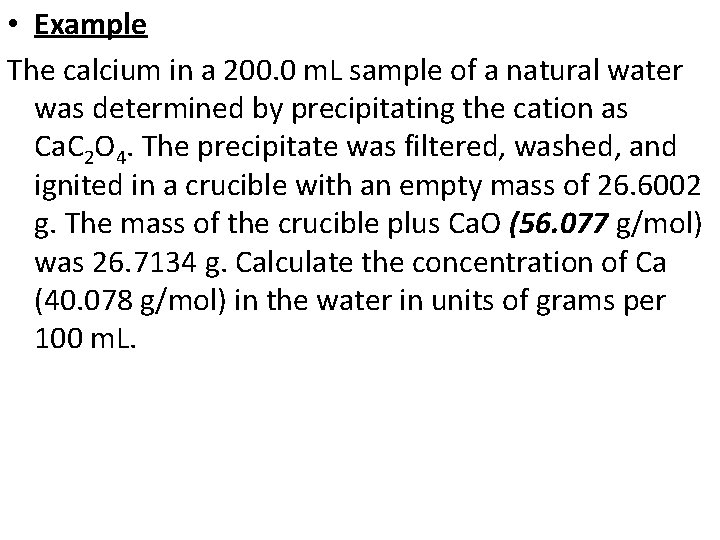
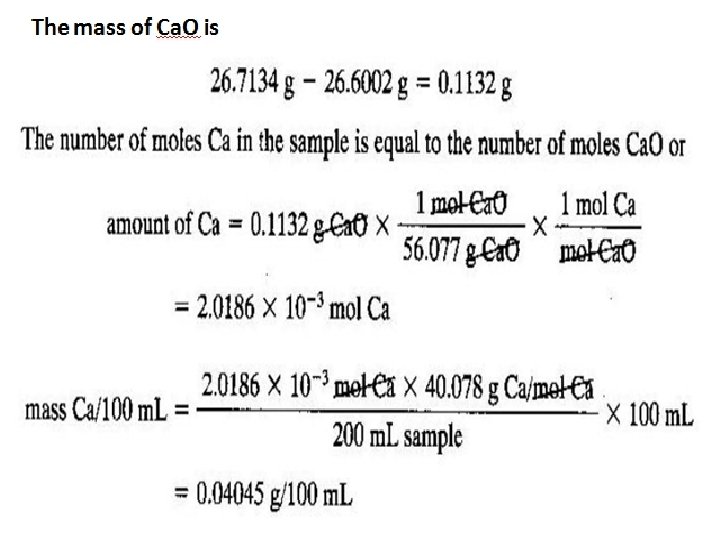
• Example The calcium in a 200. 0 m. L sample of a natural water was determined by precipitating the cation as Ca. C 2 O 4. The precipitate was filtered, washed, and ignited in a crucible with an empty mass of 26. 6002 g. The mass of the crucible plus Ca. O (56. 077 g/mol) was 26. 7134 g. Calculate the concentration of Ca (40. 078 g/mol) in the water in units of grams per 100 m. L.


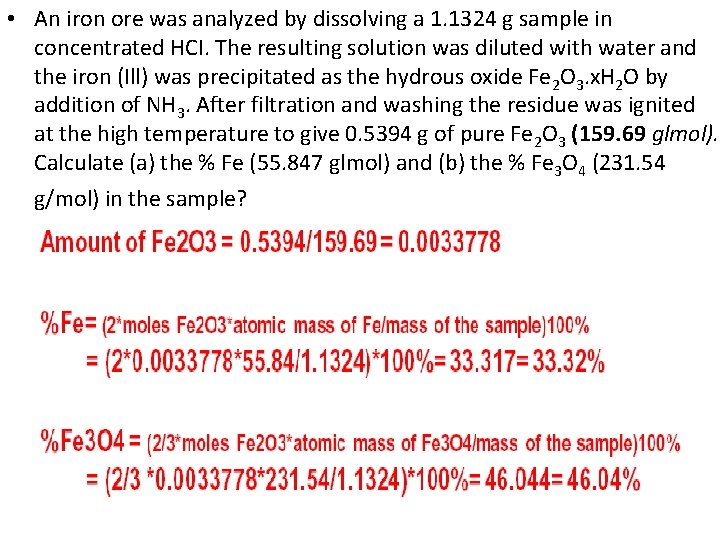
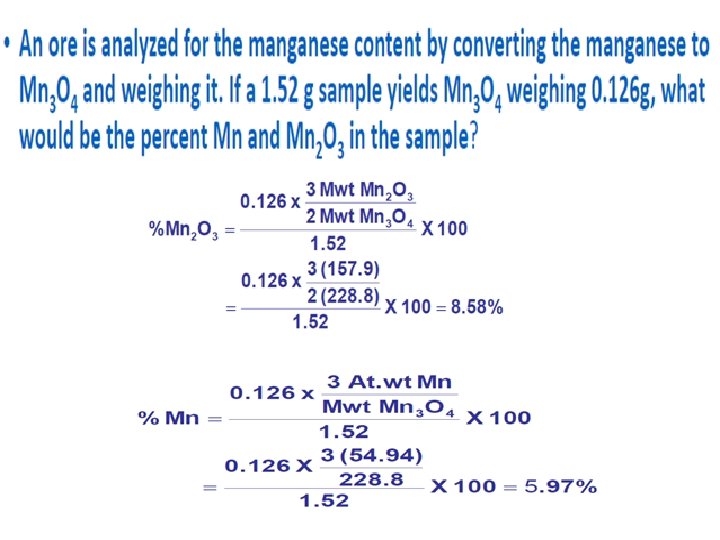
• An iron ore was analyzed by dissolving a 1. 1324 g sample in concentrated HCI. The resulting solution was diluted with water and the iron (Ill) was precipitated as the hydrous oxide Fe 2 O 3. x. H 2 O by addition of NH 3. After filtration and washing the residue was ignited at the high temperature to give 0. 5394 g of pure Fe 2 O 3 (159. 69 glmol). Calculate (a) the % Fe (55. 847 glmol) and (b) the % Fe 3 O 4 (231. 54 g/mol) in the sample?

 Cengage
Cengage Difference between occlusion and mixed-crystal formation
Difference between occlusion and mixed-crystal formation Difference between colloidal and crystalline precipitate
Difference between colloidal and crystalline precipitate Occlusion and mixed-crystal formation
Occlusion and mixed-crystal formation Copper sulfate and potassium iodide
Copper sulfate and potassium iodide Predicting precipitation reactions
Predicting precipitation reactions Histoplasma blastomyces
Histoplasma blastomyces Chemical change formation of precipitate
Chemical change formation of precipitate Chemical change formation of precipitate
Chemical change formation of precipitate Nalgonda technique steps
Nalgonda technique steps Electrodes used in surgical diathermy
Electrodes used in surgical diathermy Disseminated intravascular coagulation pathophysiology
Disseminated intravascular coagulation pathophysiology Clotting time capillary method
Clotting time capillary method Coagulation made easy
Coagulation made easy Coagulation profile test
Coagulation profile test Dégénérescence hydropique
Dégénérescence hydropique Hemostasis
Hemostasis Coagulation factors list
Coagulation factors list Dextrinization of starch
Dextrinization of starch Composition of eggs
Composition of eggs Coagulation profile test
Coagulation profile test Plasma and serum
Plasma and serum Lines of zahn gross
Lines of zahn gross D'alessio
D'alessio Antifibrinolytic drugs
Antifibrinolytic drugs Coagulation disorders
Coagulation disorders Denaturation simple definition
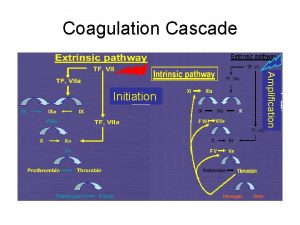
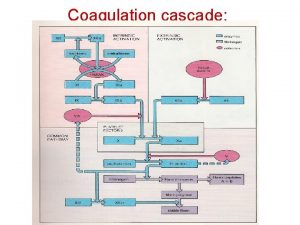
Denaturation simple definition Coagulation cascade
Coagulation cascade Dicoumoral
Dicoumoral