Chapter 16 Solids Types of Solids Crystalline solids














- Slides: 82

Chapter 16 Solids

Types of Solids Crystalline solids 1. Shows a sharp melting point. 2. Have a regular, ordered structure composing of identical repeating units having the same orientation throughout the crystal.

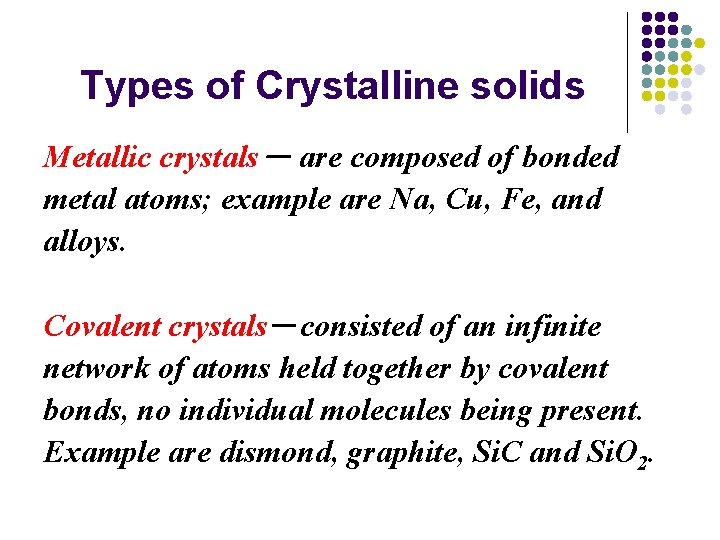
Types of Crystalline solids Metallic crystals- are composed of bonded metal atoms; example are Na, Cu, Fe, and alloys. Covalent crystals-consisted of an infinite network of atoms held together by covalent bonds, no individual molecules being present. Example are dismond, graphite, Si. C and Si. O 2.

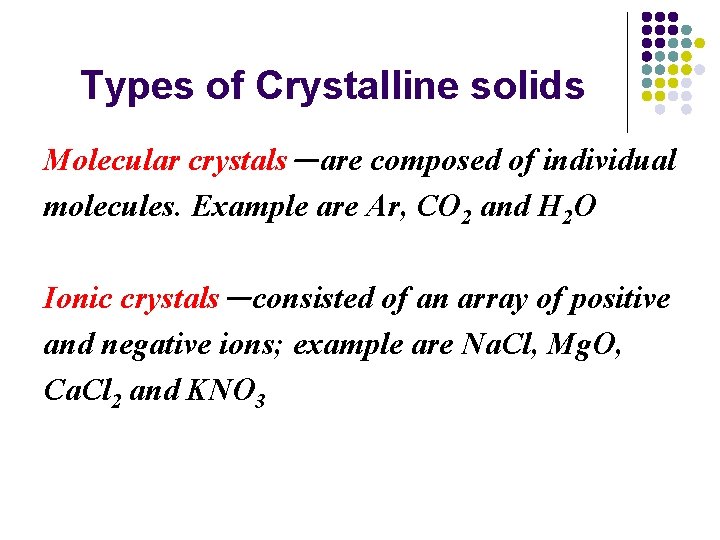
Types of Crystalline solids Molecular crystals-are composed of individual molecules. Example are Ar, CO 2 and H 2 O Ionic crystals-consisted of an array of positive and negative ions; example are Na. Cl, Mg. O, Ca. Cl 2 and KNO 3

Types of Solids Amorphous solids 1. An amorphous solid does not have a characteristic crystals shape. 2. When heated, it softens and melts over a wide temperature range.

Structure of Metals Simple Cubic (簡單立方) l Hexagonal Closest Packed (HCP) (六方最密堆積) l Face-Centered Cubic (FCC) (面心立方) l Body-Centered Cubic (BCC) (體心立方) l

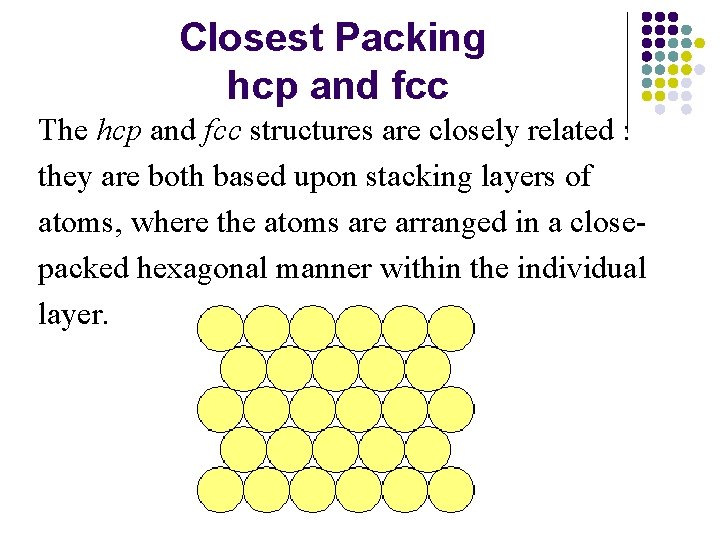
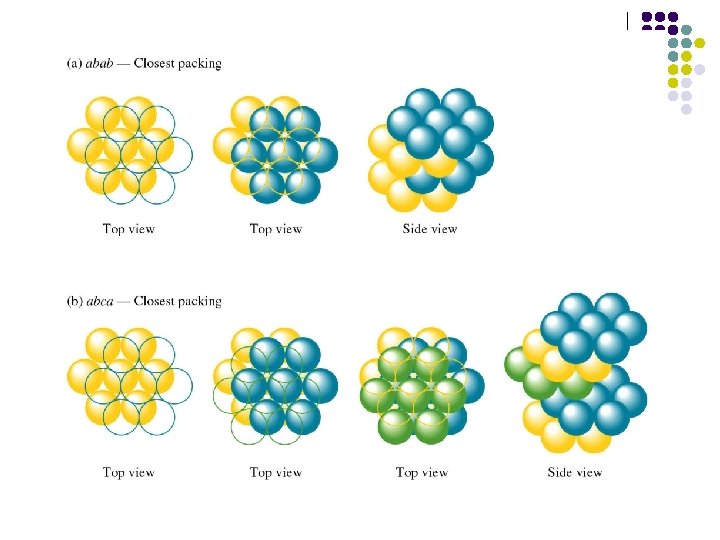
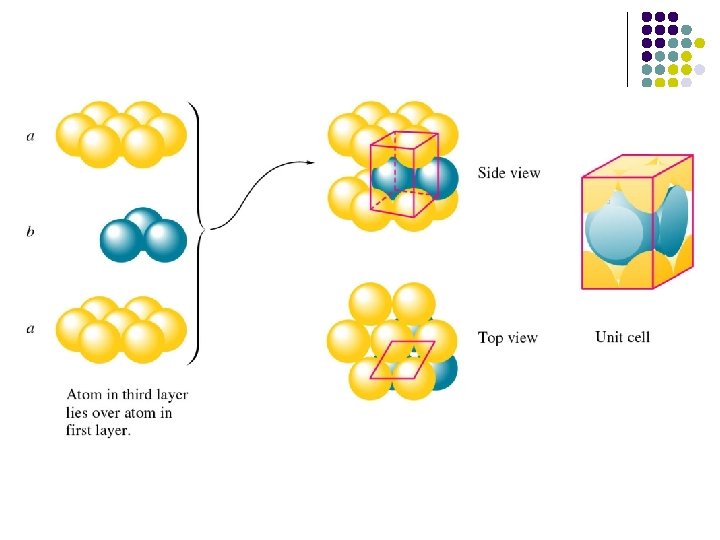
Closest Packing hcp and fcc The hcp and fcc structures are closely related : they are both based upon stacking layers of atoms, where the atoms are arranged in a closepacked hexagonal manner within the individual layer.

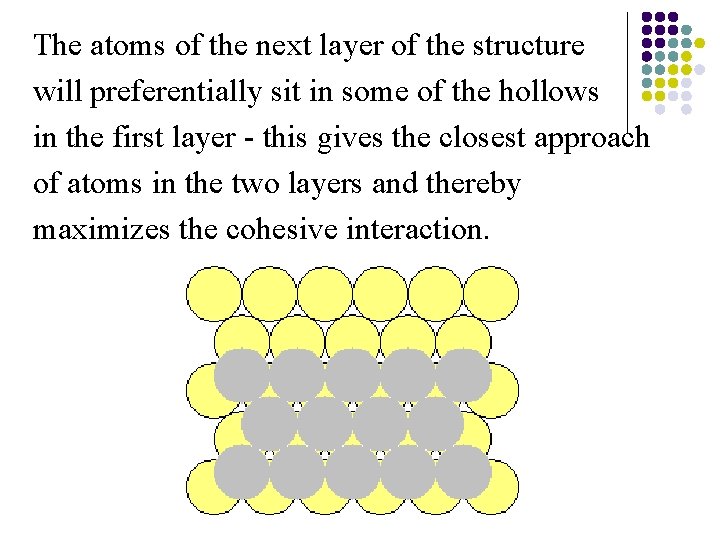
The atoms of the next layer of the structure will preferentially sit in some of the hollows in the first layer - this gives the closest approach of atoms in the two layers and thereby maximizes the cohesive interaction.

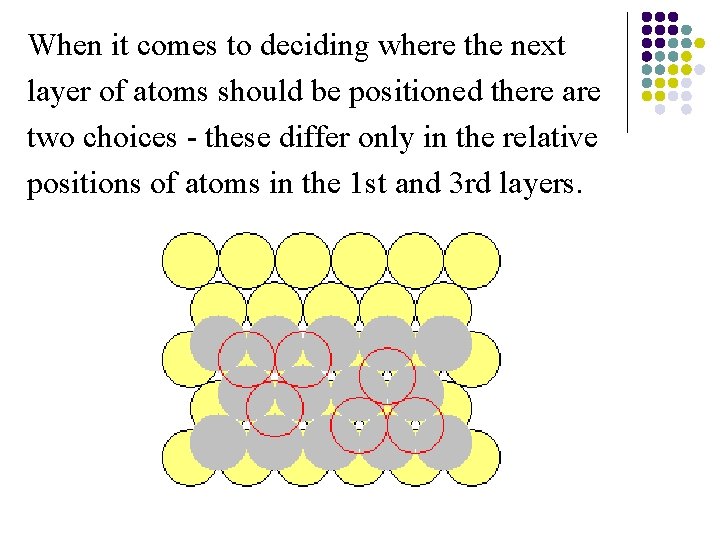
When it comes to deciding where the next layer of atoms should be positioned there are two choices - these differ only in the relative positions of atoms in the 1 st and 3 rd layers.

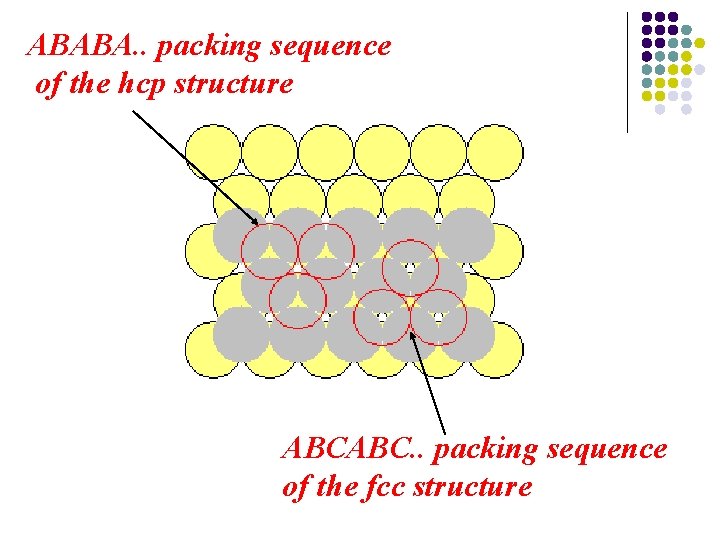
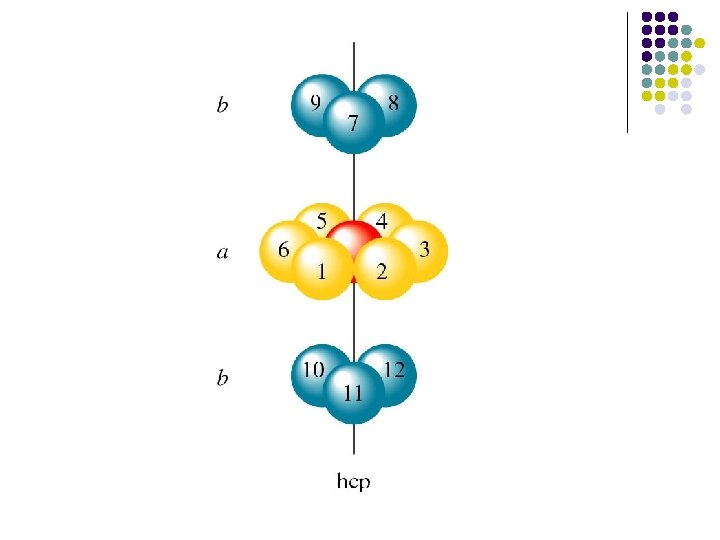
ABABA. . packing sequence of the hcp structure ABCABC. . packing sequence of the fcc structure


Closest Packing hcp and fcc These hcp and fcc structures share common features : (a) The atoms are close packed (b) Each atom has 12 nearest neighbours.

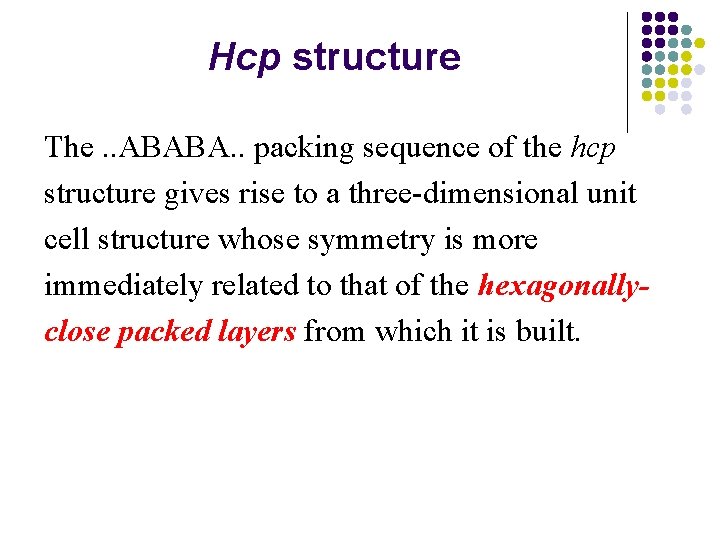
Hcp structure The. . ABABA. . packing sequence of the hcp structure gives rise to a three-dimensional unit cell structure whose symmetry is more immediately related to that of the hexagonallyclose packed layers from which it is built.

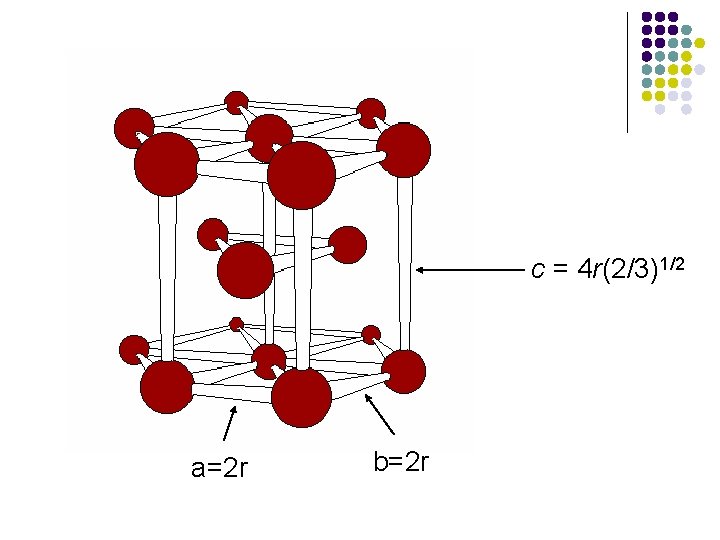
l l The unit cell for the hexagonal closest-packed structure has a diamond-shaped or hexagonal base with sides of equal length. The volume is the product of the area of the base and the height of the cell.

c = 4 r(2/3)1/2 a=2 r b=2 r


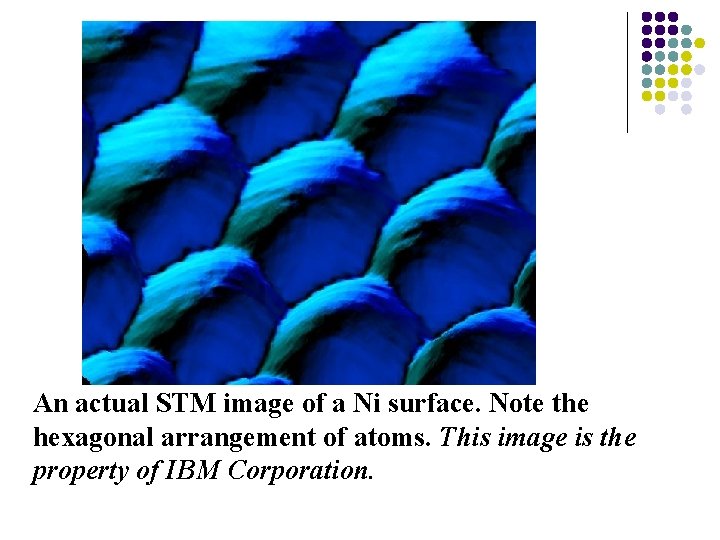
An actual STM image of a Ni surface. Note the hexagonal arrangement of atoms. This image is the property of IBM Corporation.

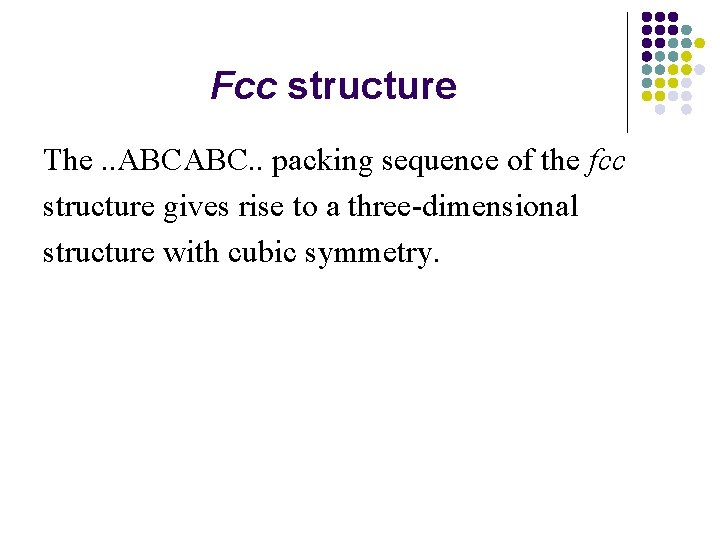
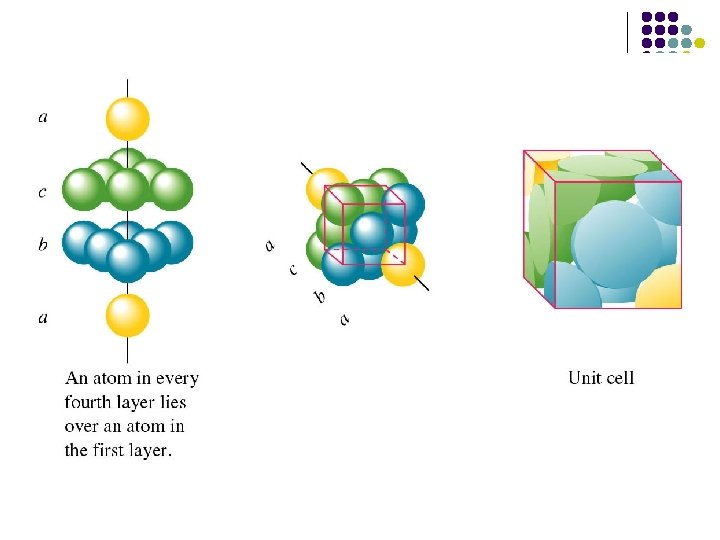
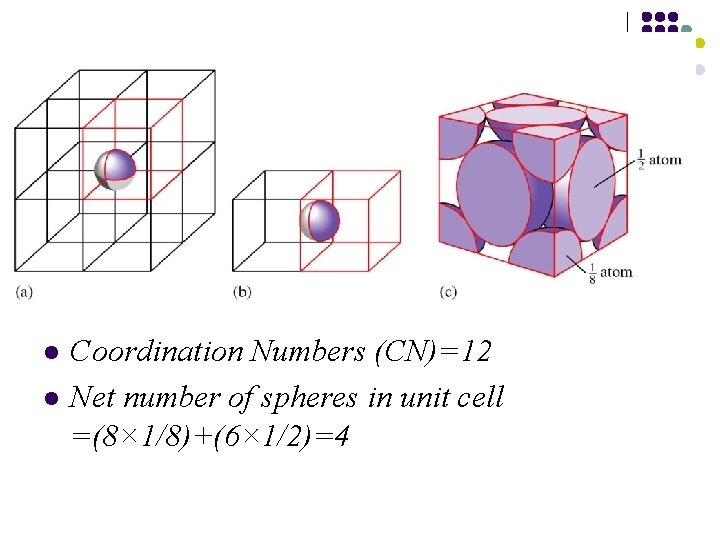
Fcc structure The. . ABCABC. . packing sequence of the fcc structure gives rise to a three-dimensional structure with cubic symmetry.

FCC structure



l l Coordination Numbers (CN)=12 Net number of spheres in unit cell =(8× 1/8)+(6× 1/2)=4

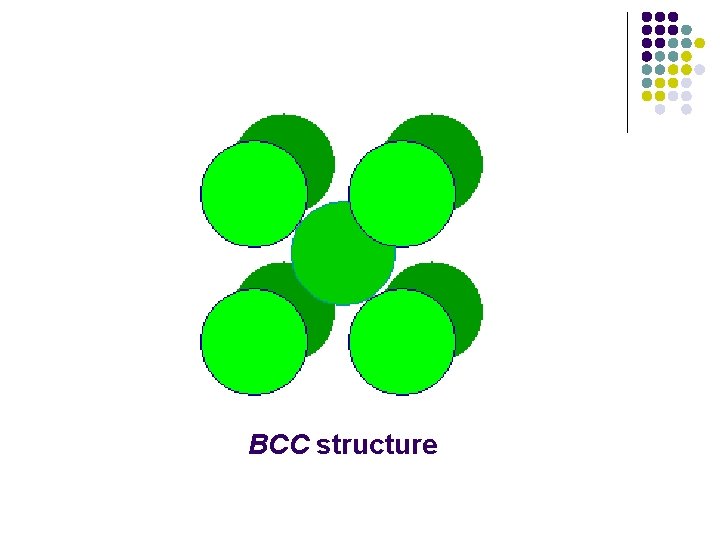
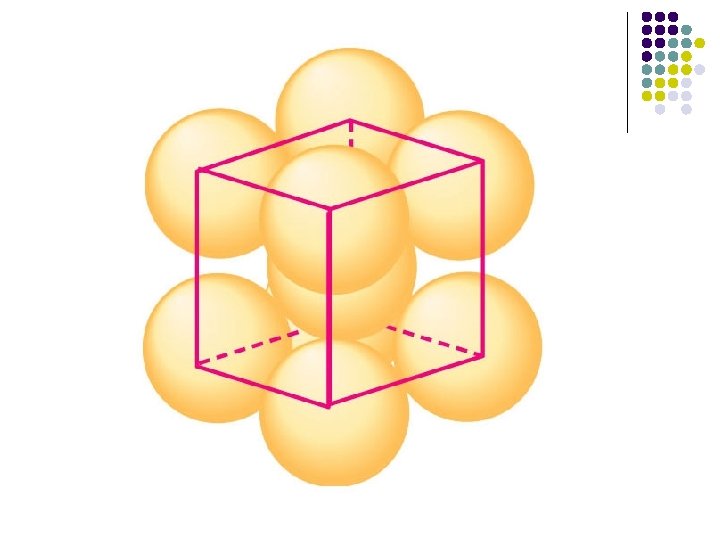
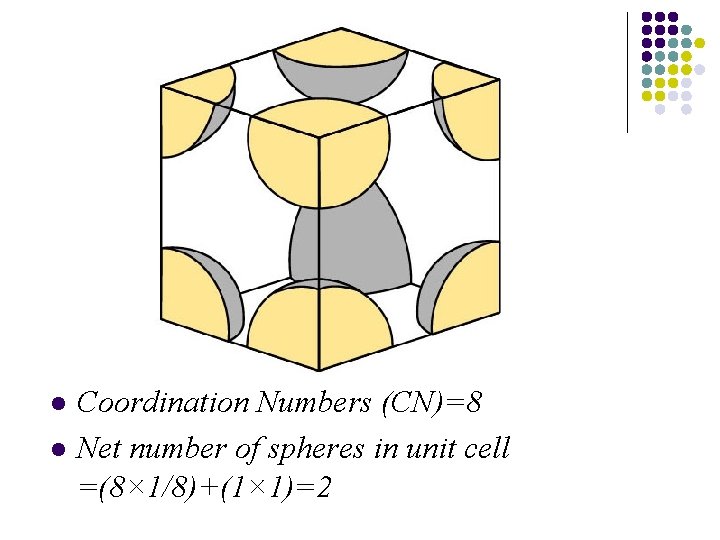
Bcc structure l l The bcc structure has very little in common with the fcc structure - except the cubic nature of the unit cell. Most importantly, it differs from the hcp and fcc structures in that it is not a close-packed structure. The structure of the alkali metals are cheracterized by a bcc unit cell.

BCC structure


l l Coordination Numbers (CN)=8 Net number of spheres in unit cell =(8× 1/8)+(1× 1)=2


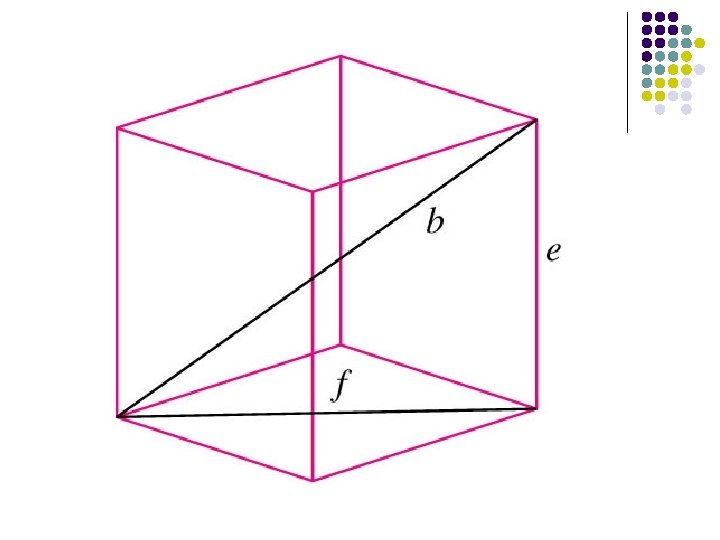
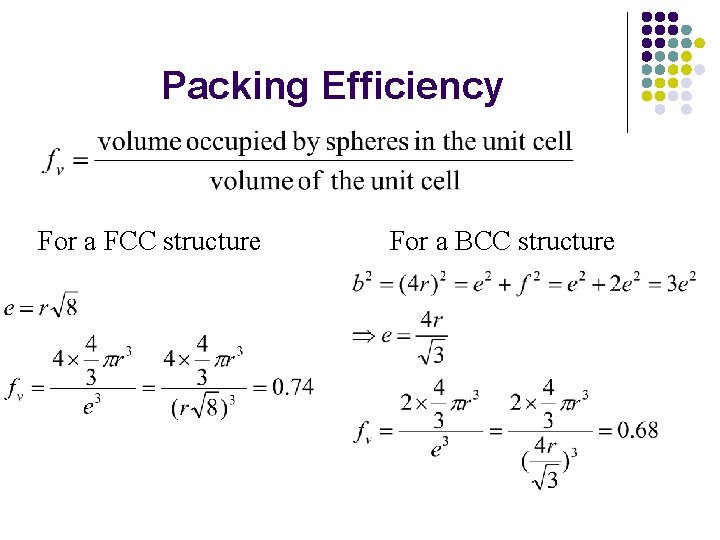
Packing Efficiency For a FCC structure For a BCC structure

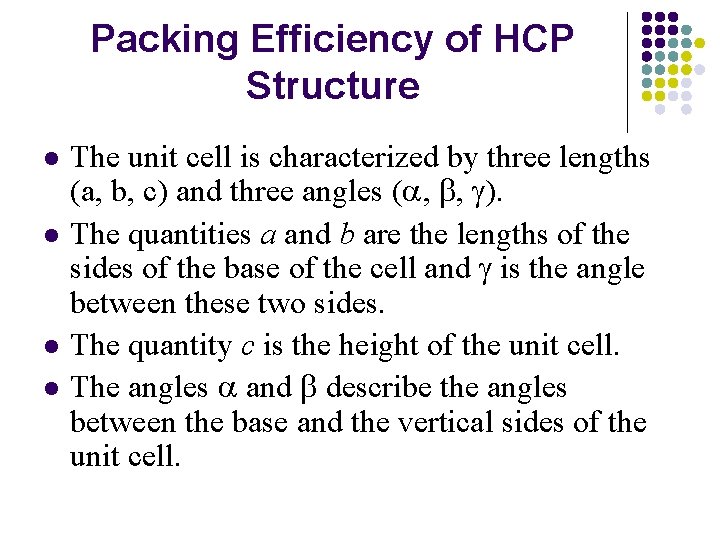
Packing Efficiency of HCP Structure l l The unit cell is characterized by three lengths (a, b, c) and three angles (a, b, g). The quantities a and b are the lengths of the sides of the base of the cell and g is the angle between these two sides. The quantity c is the height of the unit cell. The angles a and b describe the angles between the base and the vertical sides of the unit cell.

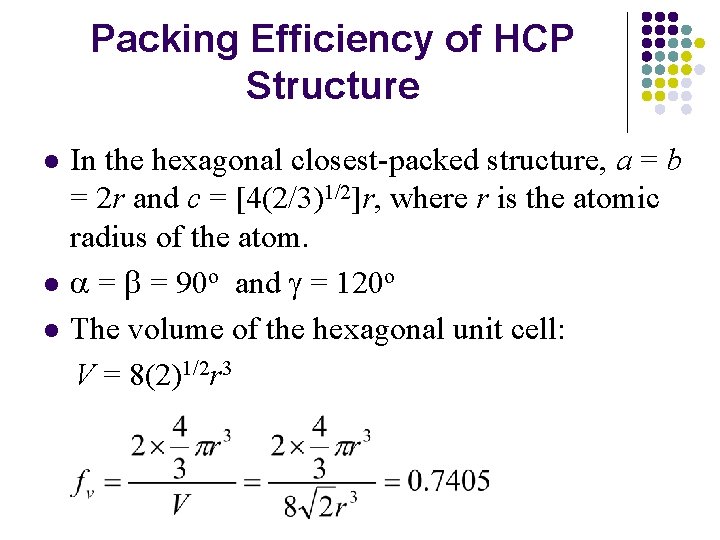
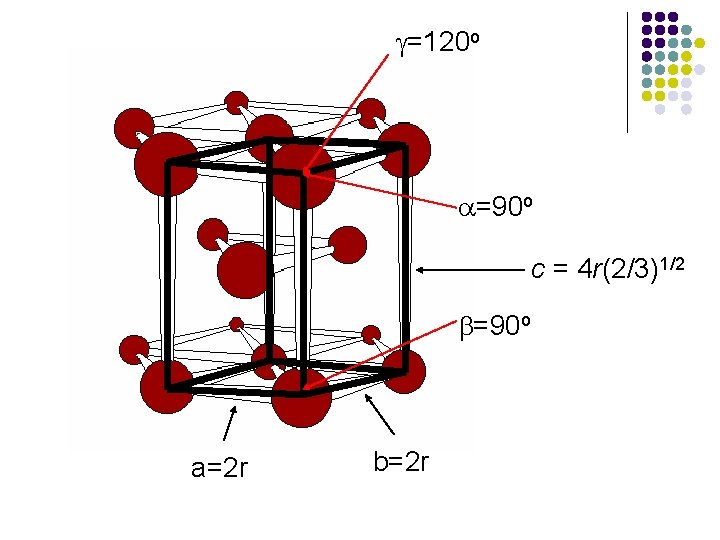
Packing Efficiency of HCP Structure l l l In the hexagonal closest-packed structure, a = b = 2 r and c = [4(2/3)1/2]r, where r is the atomic radius of the atom. a = b = 90 o and g = 120 o The volume of the hexagonal unit cell: V = 8(2)1/2 r 3

g=120 o a=90 o c = 4 r(2/3)1/2 b=90 o a=2 r b=2 r

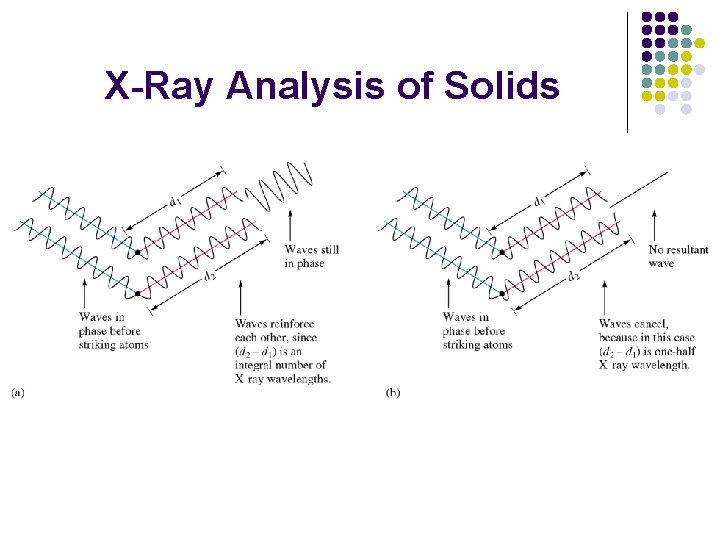
X-Ray Analysis of Solids

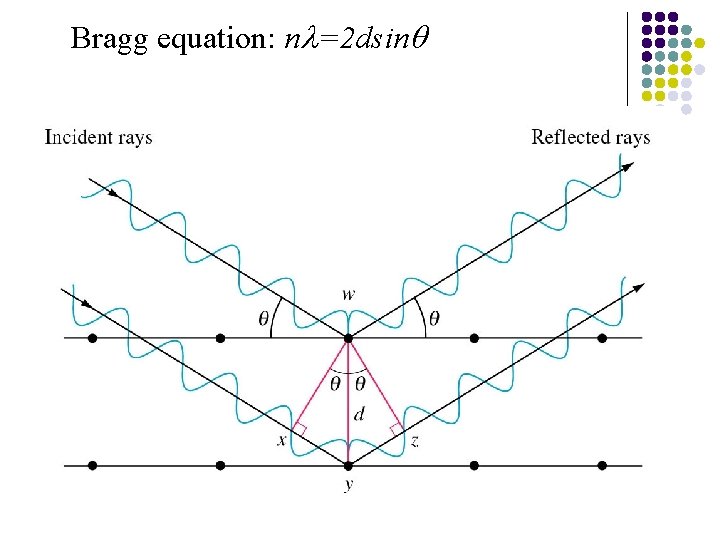
Bragg equation: nl=2 dsinq

X-ray crystal diffraction

X-ray power diffraction Cu(111) Cu(100)

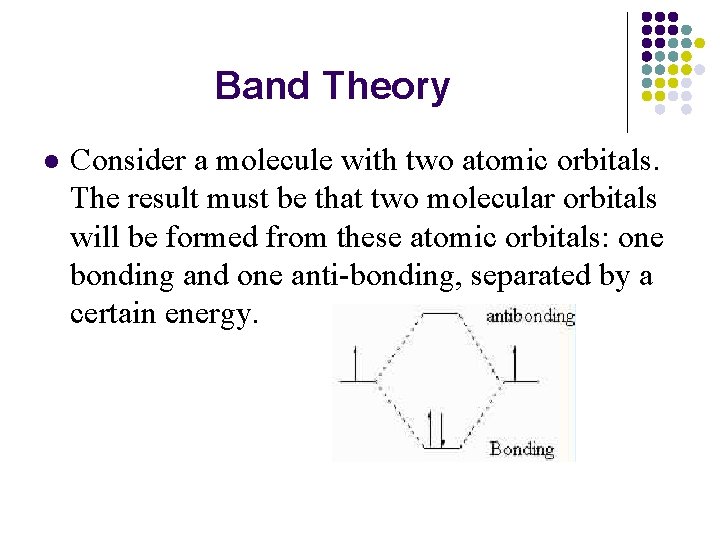
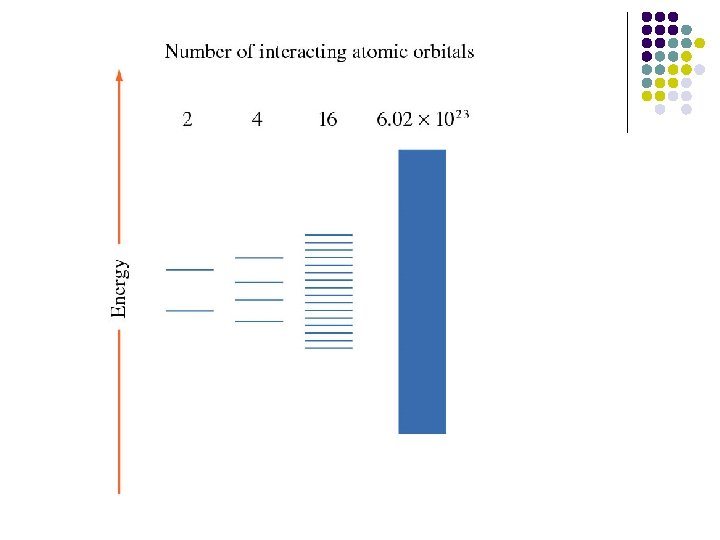
Band Theory l Consider a molecule with two atomic orbitals. The result must be that two molecular orbitals will be formed from these atomic orbitals: one bonding and one anti-bonding, separated by a certain energy.

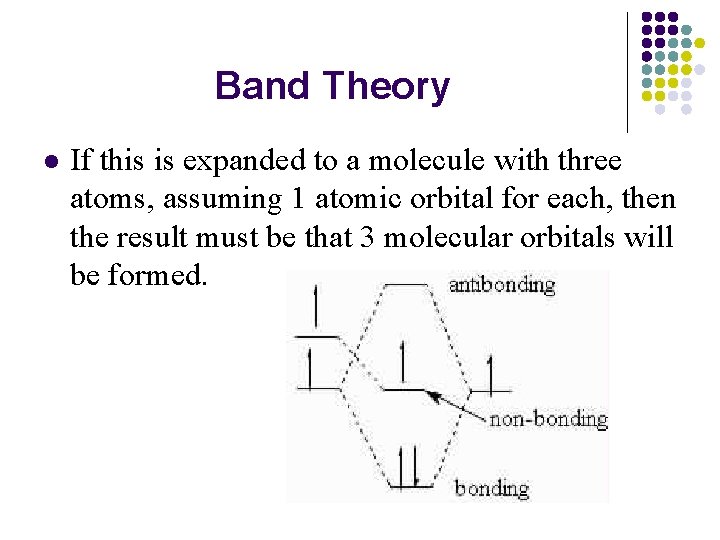
Band Theory l If this is expanded to a molecule with three atoms, assuming 1 atomic orbital for each, then the result must be that 3 molecular orbitals will be formed.

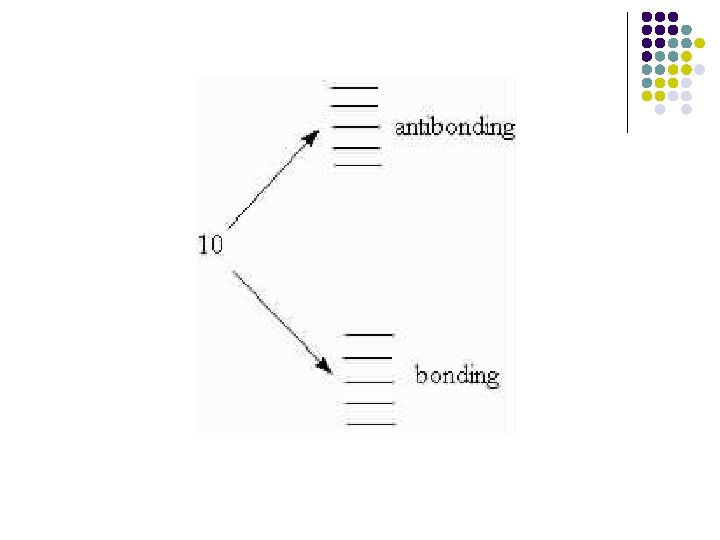
• Now , let's take it to 10 atoms. This will produce 10 molecular orbitals: 5 bonding and 5 antibonding. As the number of molecular orbitals increases, the energy difference between the lowest bonding and the highest anti-bondig increases, while the space between each individual orbital decreases.


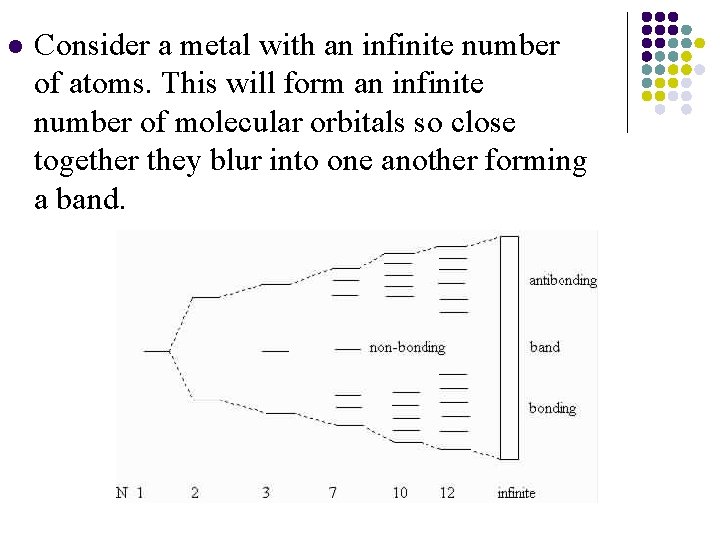
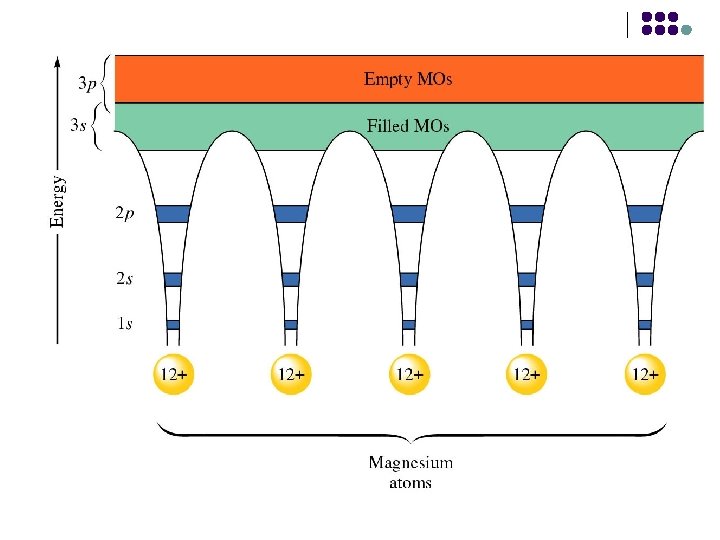
l Consider a metal with an infinite number of atoms. This will form an infinite number of molecular orbitals so close together they blur into one another forming a band.

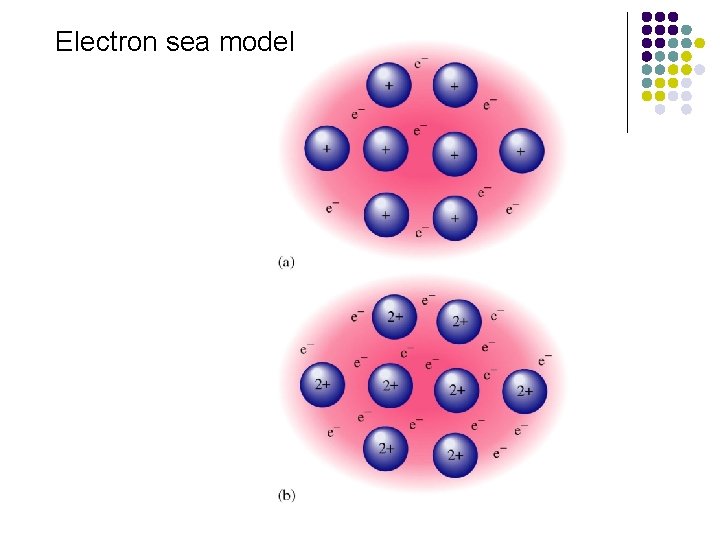
Electron sea model



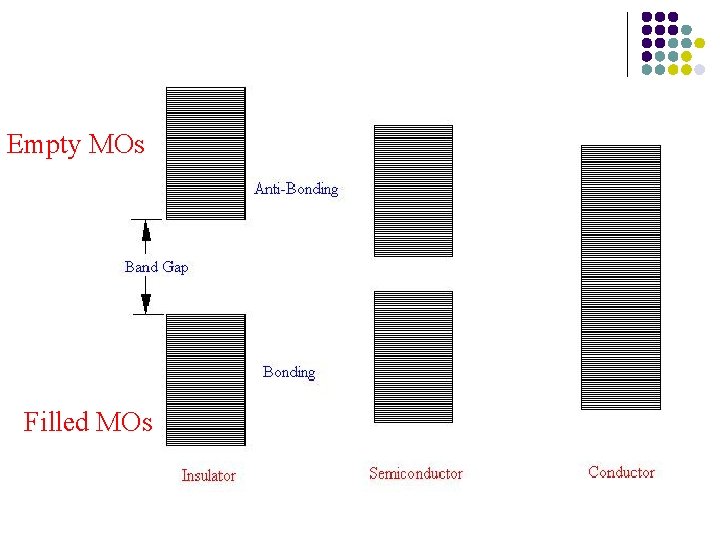
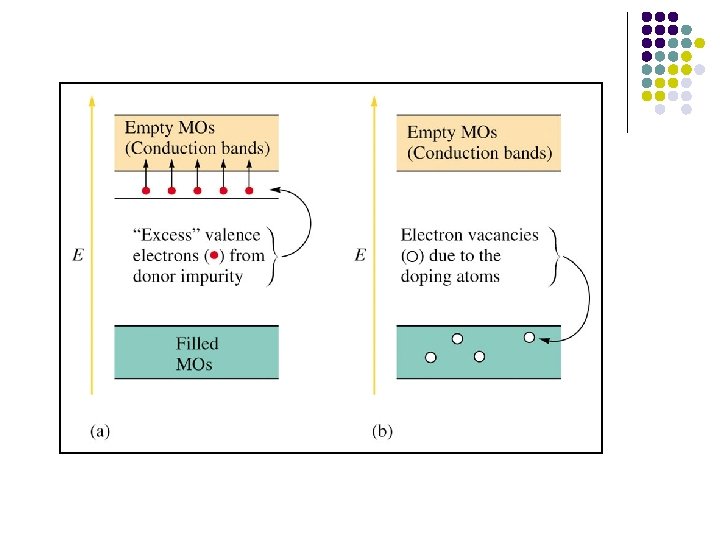
Empty MOs Filled MOs


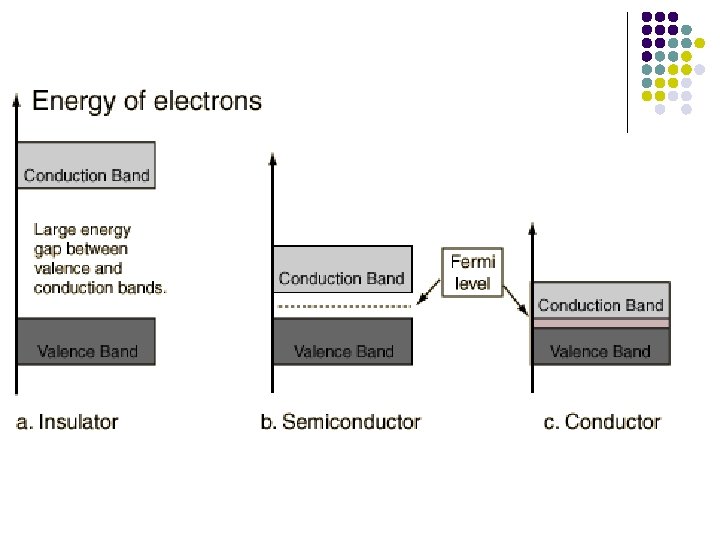
Fermi Level/ Fermi Energy l l At absolute zero, electrons pack into the lowest available energy states and build up a "Fermi sea" of electron energy states. The top of that "Fermi sea" of electrons is called the Fermi energy or Fermi level. The Fermi level is the surface of that sea at absolute zero where no electrons will have enough energy to rise above the surface.

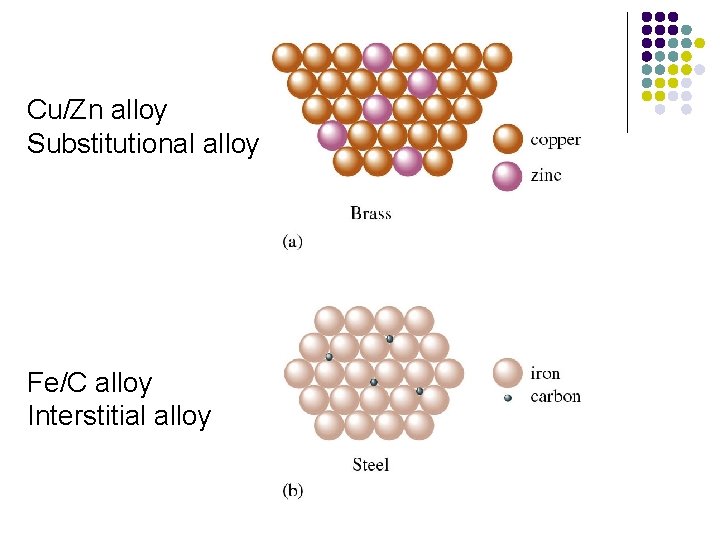
Metal Alloys l l l Definition: A substance that contains a mixture of elements and has metallic properties. Substitutional alloy Interstitial alloy

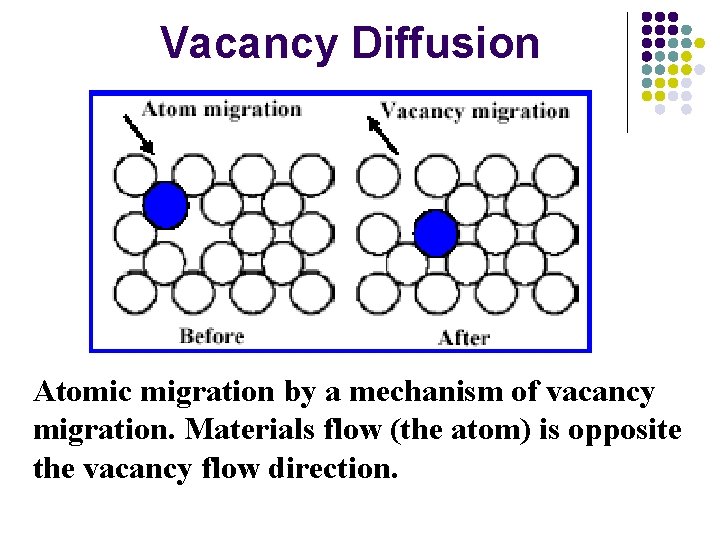
Substitutional alloy Definition: Some of the host metal atoms are replaced by other metal atoms of similar size. l Vacancy Diffusion: Vacancy diffusion involves the migration of an atom from a typical lattice position to a vacancy lattice site. l

Vacancy Diffusion Atomic migration by a mechanism of vacancy migration. Materials flow (the atom) is opposite the vacancy flow direction.

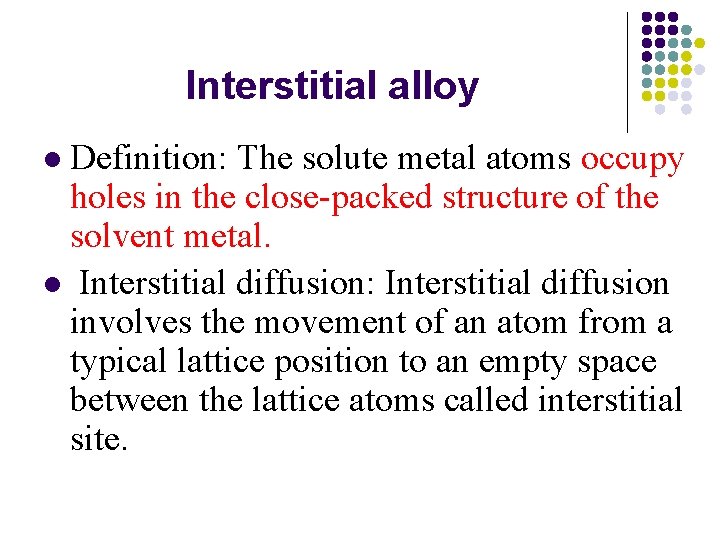
Interstitial alloy Definition: The solute metal atoms occupy holes in the close-packed structure of the solvent metal. l Interstitial diffusion: Interstitial diffusion involves the movement of an atom from a typical lattice position to an empty space between the lattice atoms called interstitial site. l

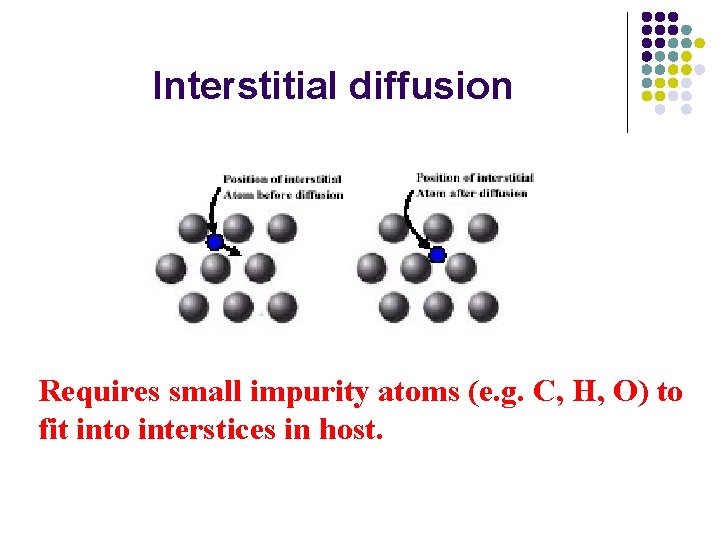
Interstitial diffusion Requires small impurity atoms (e. g. C, H, O) to fit into interstices in host.

Cu/Zn alloy Substitutional alloy Fe/C alloy Interstitial alloy

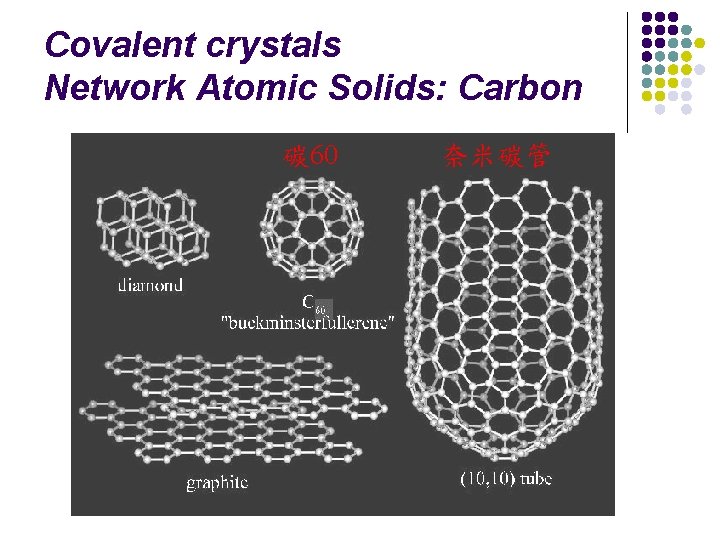
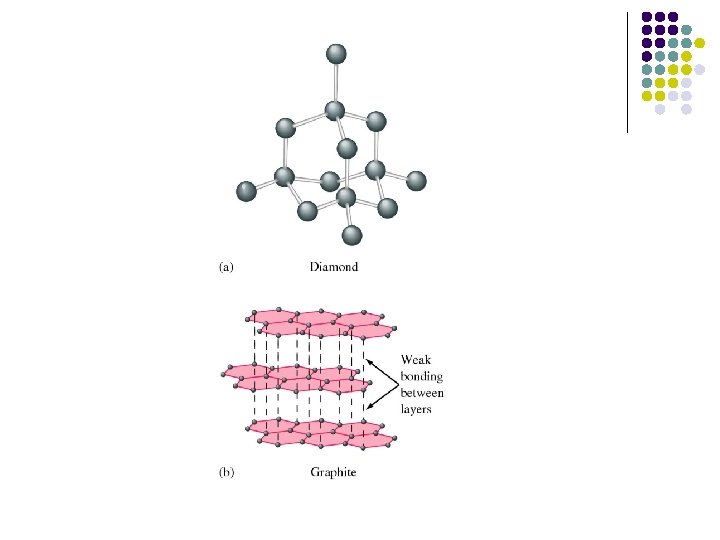
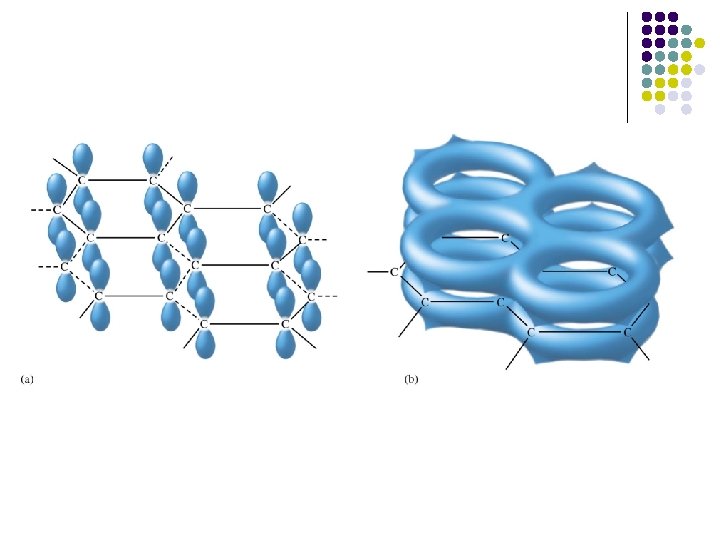
Covalent crystals Network Atomic Solids: Carbon 碳 60 奈米碳管



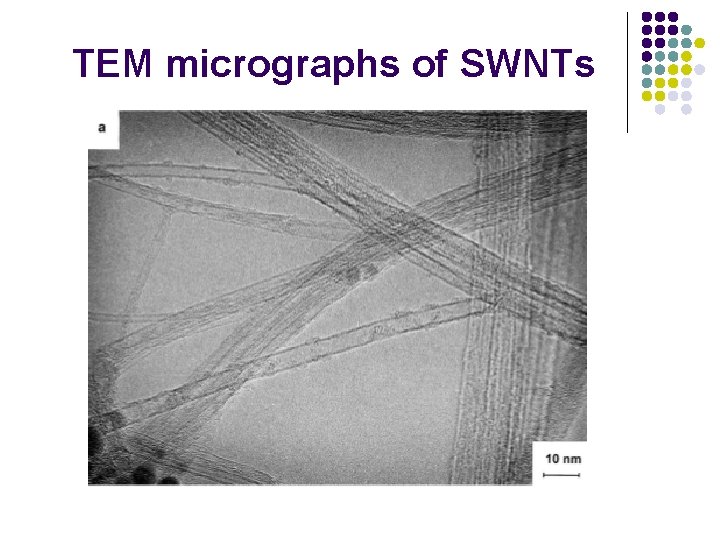
TEM micrographs of SWNTs

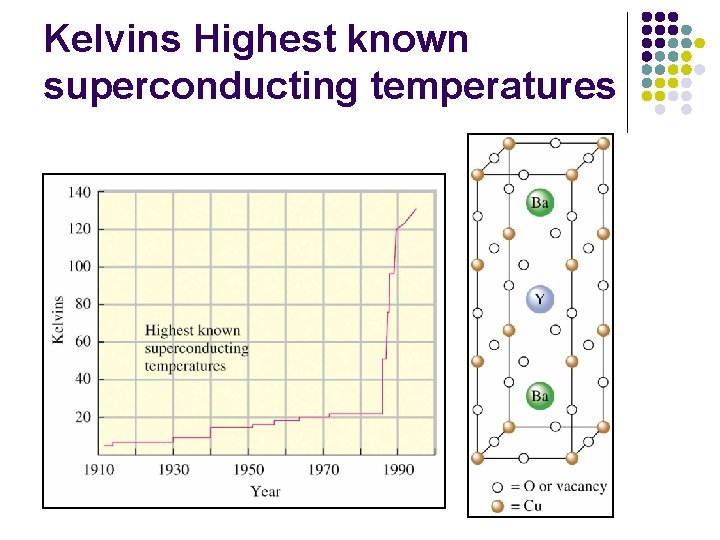
Superconductivity l l l Electrical resistance is zero. No wasted heat energy 1911 mercury-4 K Niobium alloy – 23 K High-temperature superconductor-perovskites YBa 2 Cu 3 Ox (x=6. 527)

Kelvins Highest known superconducting temperatures

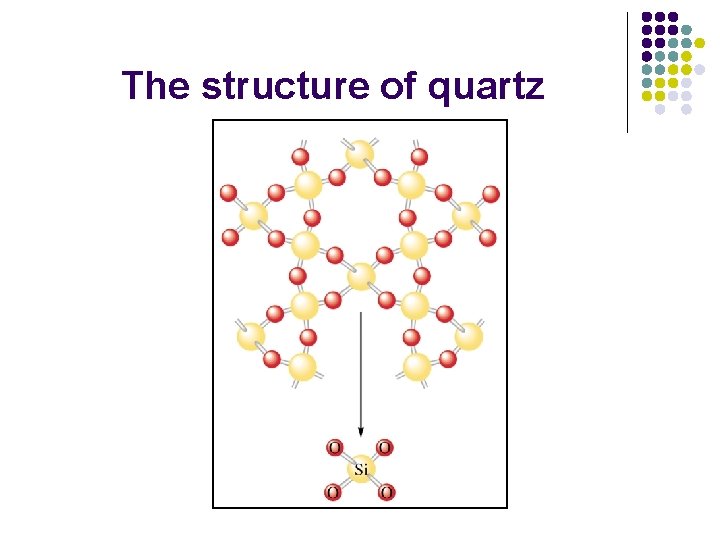
The structure of quartz

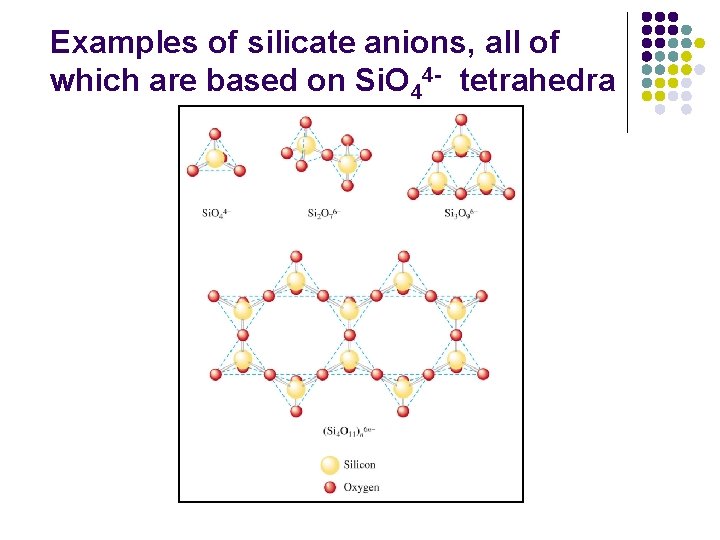
Examples of silicate anions, all of which are based on Si. O 44 - tetrahedra


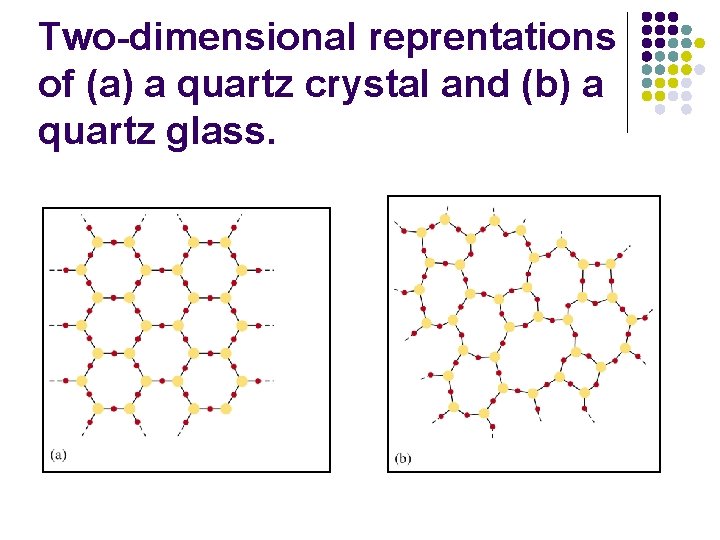
Two-dimensional reprentations of (a) a quartz crystal and (b) a quartz glass.

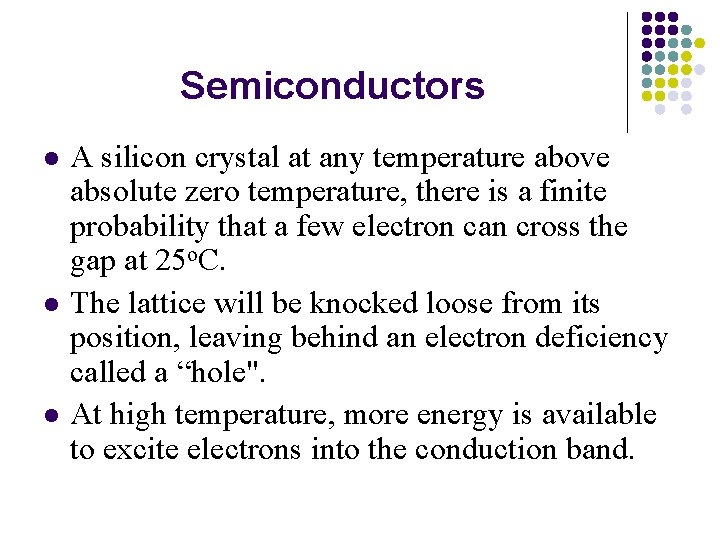
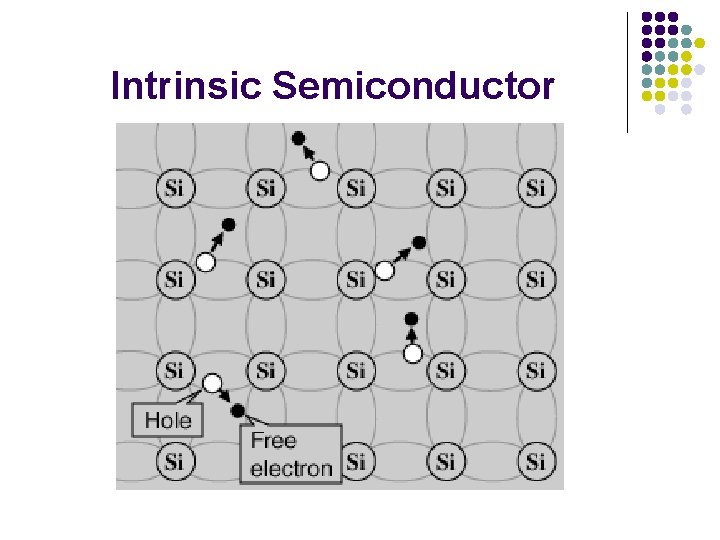
Semiconductors l l l A silicon crystal at any temperature above absolute zero temperature, there is a finite probability that a few electron can cross the gap at 25 o. C. The lattice will be knocked loose from its position, leaving behind an electron deficiency called a “hole". At high temperature, more energy is available to excite electrons into the conduction band.

Intrinsic Semiconductor

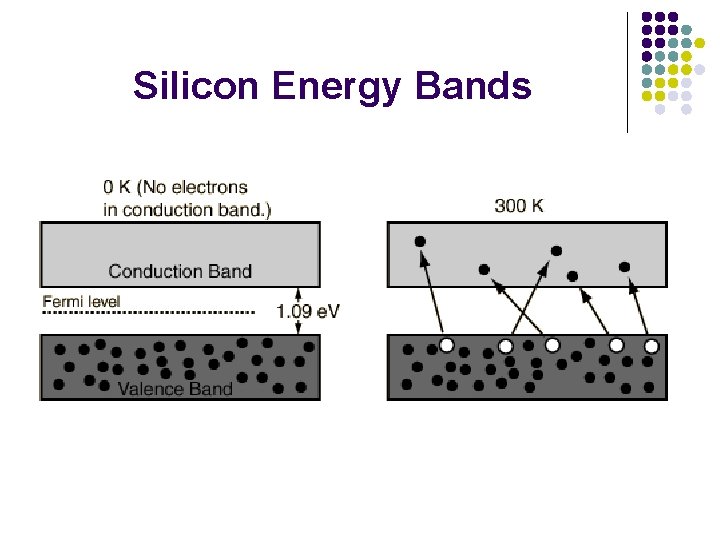
Silicon Energy Bands

The Doped Semiconductors l The addition of a small percentage of foreign atoms in the regular crystal lattice of silicon or germanium produces dramatic changes in their electrical properties, producing n-type and ptype semiconductors.

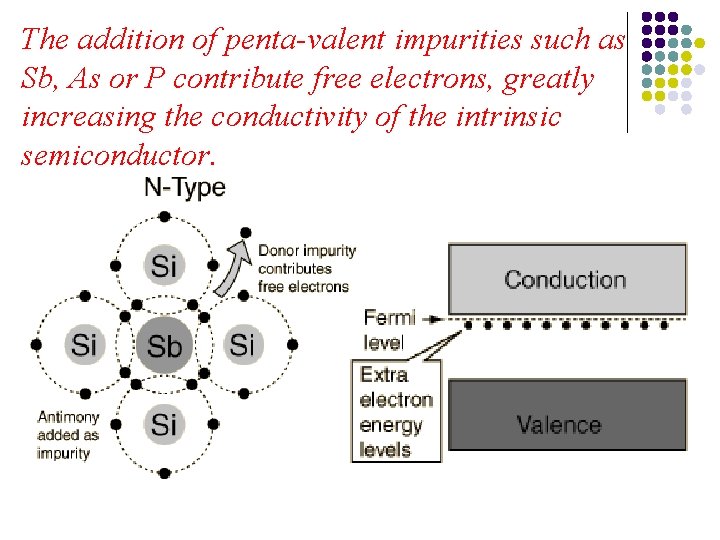
The addition of penta-valent impurities such as Sb, As or P contribute free electrons, greatly increasing the conductivity of the intrinsic semiconductor.

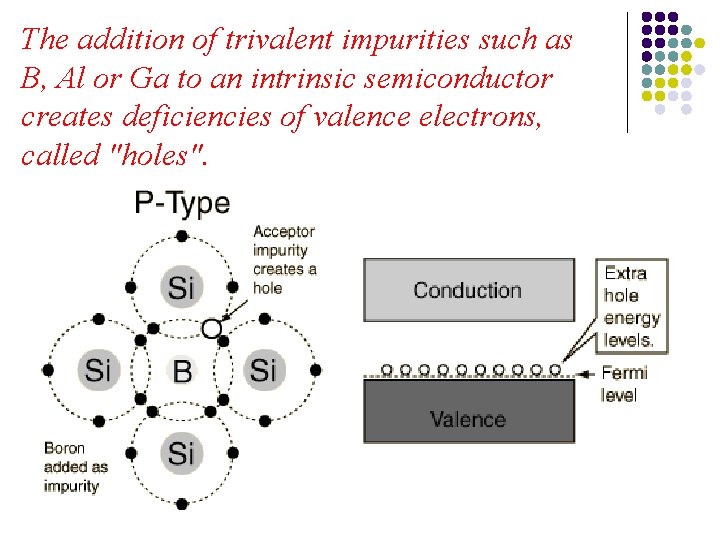
The addition of trivalent impurities such as B, Al or Ga to an intrinsic semiconductor creates deficiencies of valence electrons, called "holes".


Ionic Solid l l l Stable High melting substance Held by the strong electrostatic forces that exists between oppositely charged ions.


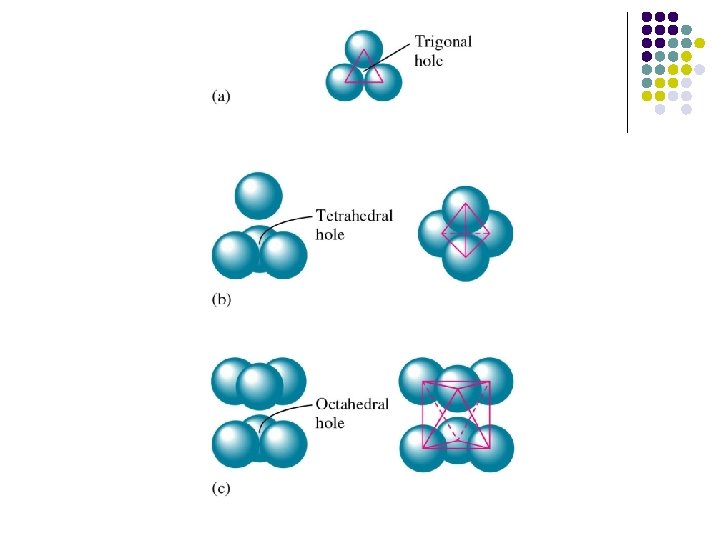
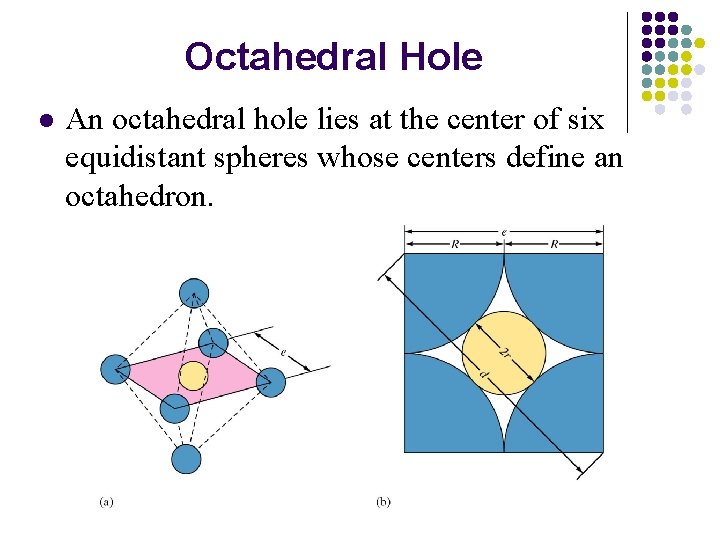
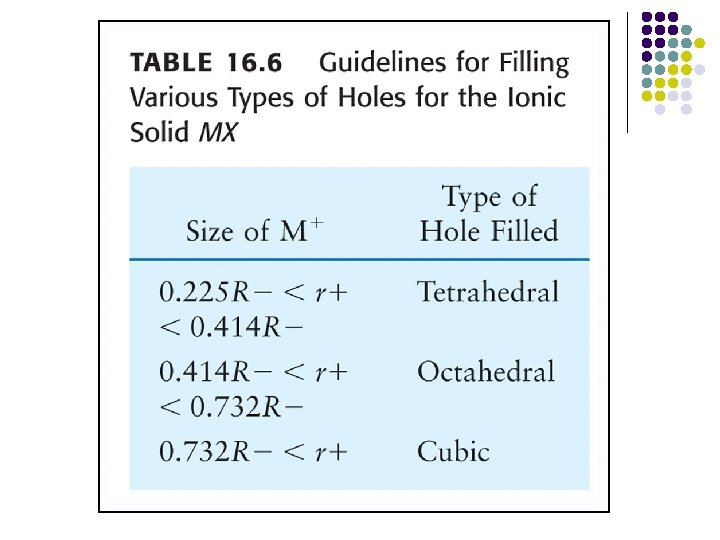
Octahedral Hole l An octahedral hole lies at the center of six equidistant spheres whose centers define an octahedron.

This result shows that an octahedral hole in a closest packed structure has a radius that is 0. 414 times the radius of the packed spheres.

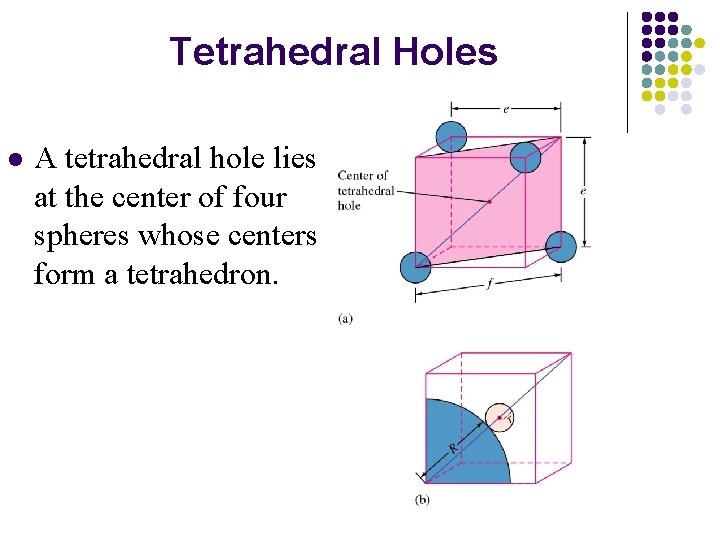
Tetrahedral Holes l A tetrahedral hole lies at the center of four spheres whose centers form a tetrahedron.

In a closest packed structure, a tetrahedral hole has a radius that is 0. 225 times the radius of the packed spheres.

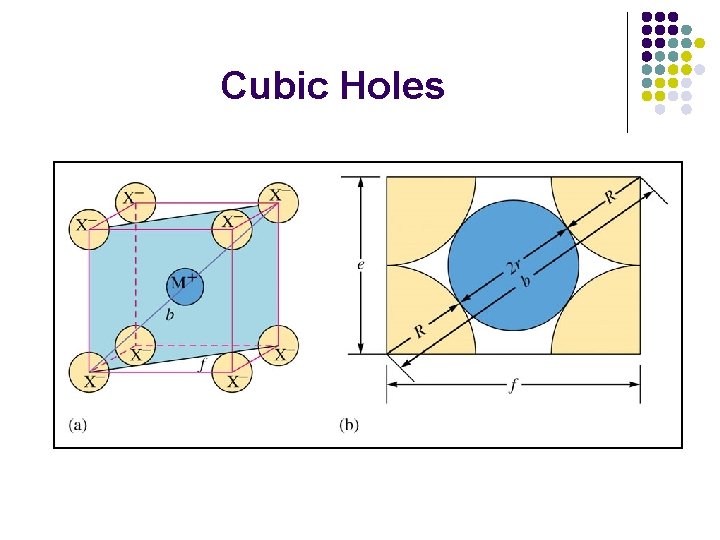
Cubic Holes



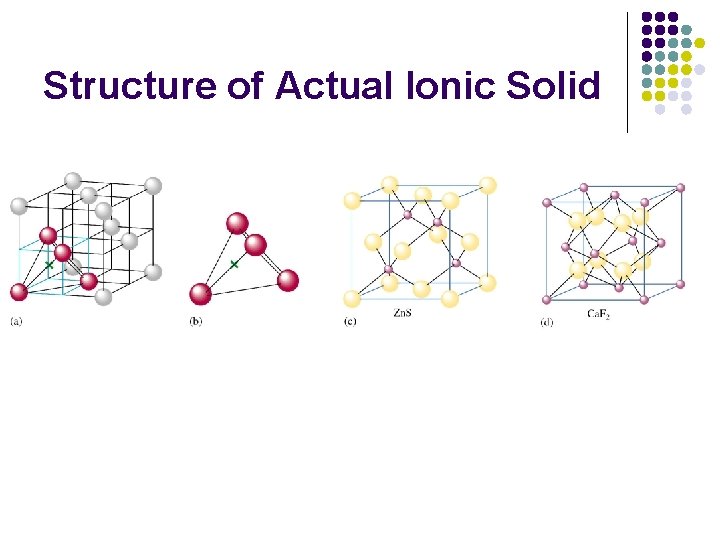
Structure of Actual Ionic Solid

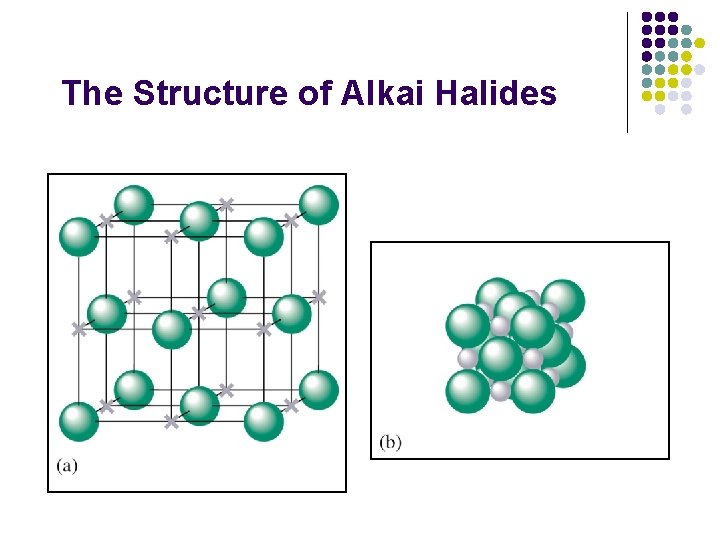
The Structure of Alkai Halides

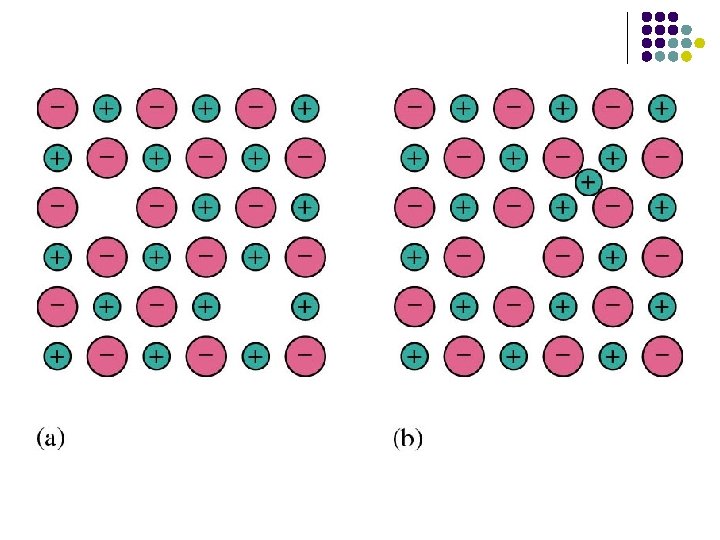
Lattice Defects Point Defects l Schottky defects: A crystal with missing particles l Frenkel defect: Crystals in which particles have migrated to nonstandard positions.
