The Crystalline Solid State Chapter 7 Crystalline Solid













- Slides: 58

The Crystalline Solid State Chapter 7

Crystalline Solid State • Many more “molecules” in the solid state. – We will focus on crystalline solids composed of atoms or ions. • Unit cell – structural component that, when repeated in all directions, results in a macroscopic (observable) crystal. – 14 possible crystal structures (Bravais lattices) – Discuss positions of atoms in the unit cell.

The Cubic Unit Cell (or Primitive) • 1 atom per unit cell (how? ). • What is the coordination number? Volume occupied? • Let’s calculate the length of the edge. What size of sphere would fit into the hole?

The Body-Centered Cubic • How many atoms per unit cell? • What is the length of the edge? This is a more complicated systems than the simple cubic.

Close-Packed Structures • How many atoms is each atom surrounded by in the same plane? • What is the coordination number? • Hexagonal close packing (hcp) – discuss the third layer (ABA). • Cubic close packing (ccp) or face-centered cubic (fcc) – discuss the third layer (ABC). • Two tetrahedral holes and one octahedral hole per atom. Can you see them?

Close-Packed Structures • The hcp has hexagonal prisms sharing vertical faces (Figure). – How many atoms per unit cell in the hcp structure? – What is the length of the cell edge? • The unit cell for the ccp or fcc is harder to see. – Need four close-packed layers to complete the cube. – What is the length of the cell edge? • In both close-packed structures, 74. 1% of the total volume is occupied.

Ionic Crystals • The tetrahedral and octahedral holes can have varying occupancies. • Holes are generally filled by smaller ions. – Tetrahedral holes – Octahedral holes • Na. Cl structure

Metallic Crystals • Most crystalize in bcc, ccp, and hcp structures. • Hard sphere model does not work well. – Depends on electronic structure. • Properties – Conductivity – Dislocations

Diamond • Each carbon atom is bonded tetrahedrally to four nearest neighbors (Figure). – Essentially the same strength in all directions.

Structures of Binary Compounds • Close-packed structures are generally defined by the larger ions (usually anions). The oppositelycharged ions occupy the holes. • Two important factors in considering the structure – Radius ratio (r+/r-) – Relative number of combining cations and anions.

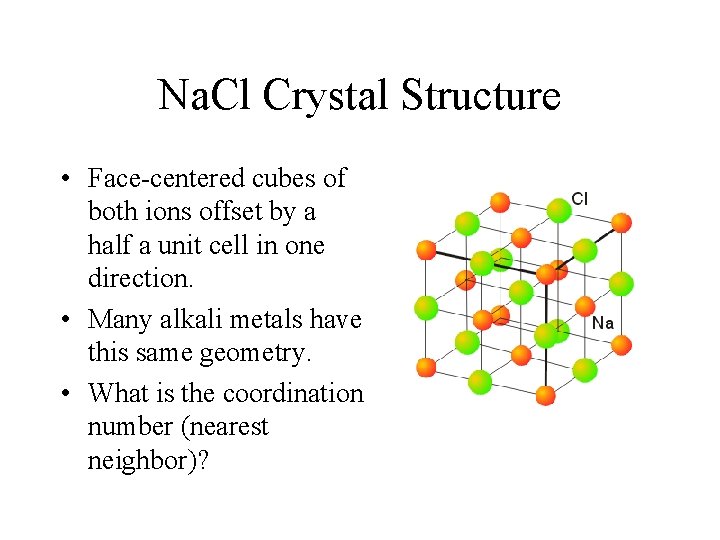
Na. Cl Crystal Structure • Face-centered cubes of both ions offset by a half a unit cell in one direction. • Many alkali metals have this same geometry. • What is the coordination number (nearest neighbor)?

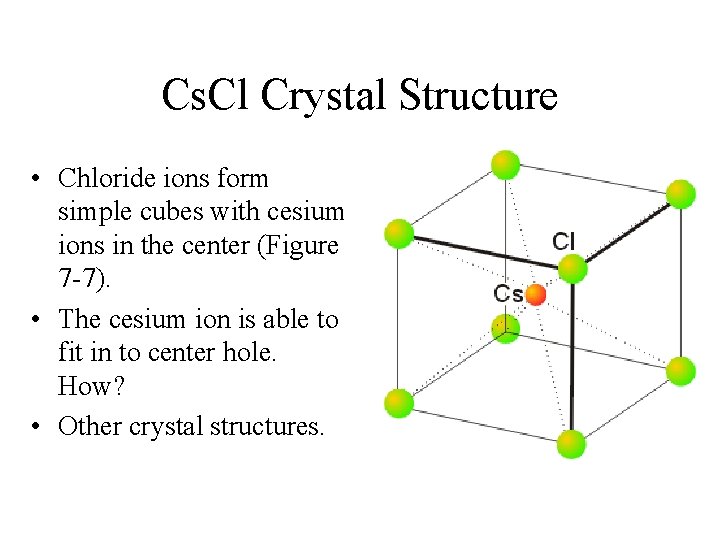
Cs. Cl Crystal Structure • Chloride ions form simple cubes with cesium ions in the center (Figure 7 -7). • The cesium ion is able to fit in to center hole. How? • Other crystal structures.

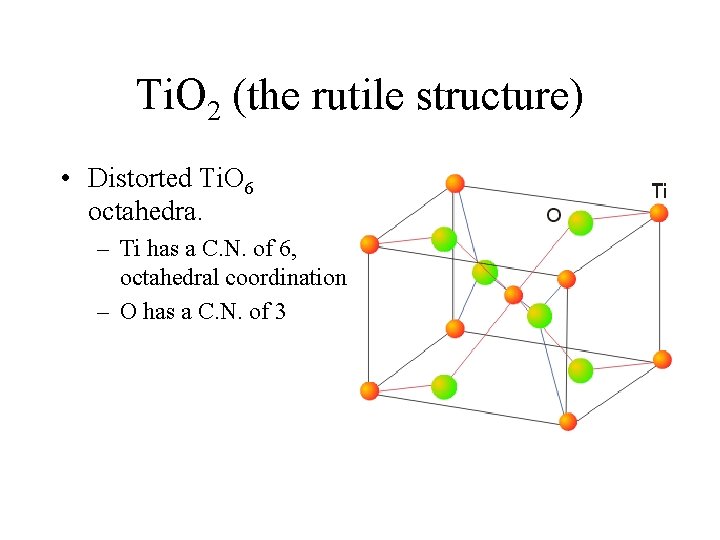
Ti. O 2 (the rutile structure) • Distorted Ti. O 6 octahedra. – Ti has a C. N. of 6, octahedral coordination – O has a C. N. of 3

Rationalization of Structure of Crystalline Solids • Predicting coordination number from radius ratio (r+/r-). – A hard sphere treatment of the ions. – Treats bonding as purely ionic. – Simply, as as the M+ ratio increases, more anions can pack around it. • Table 7 -1. Let’s look at a few (Na. Cl, Ca. F 2, and Ca. Cl 2).

Thermodynamics of Ionic Crystal Formation • A compound tends to adopt the crystal structure corresponding to lowest Gibbs energy. M+(g) + X-(g) MX(s) G = H - T S (standard state), 2 nd term can be ignored • Lattice enthalpy MX(s) M+(g) + X-(g) HL (standard molar enthalpy change) Currently, we are interested in lattice formation.

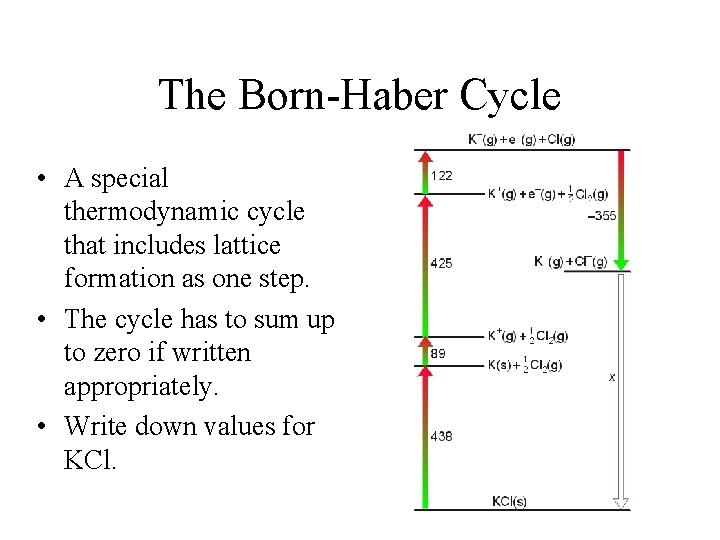
The Born-Haber Cycle • A special thermodynamic cycle that includes lattice formation as one step. • The cycle has to sum up to zero if written appropriately. • Write down values for KCl.

The Born-Haber Cycle • Calculate the lattice enthalpy for Mg. Br 2. • A discrepancy between this value and the real value may indicate the degree of covalent character. – We have assumed Coulombic interactions between ions. – The actual values for KCl and Mg. Br 2 are 701 and 2406 k. J/mol (versus 720 and 2451).

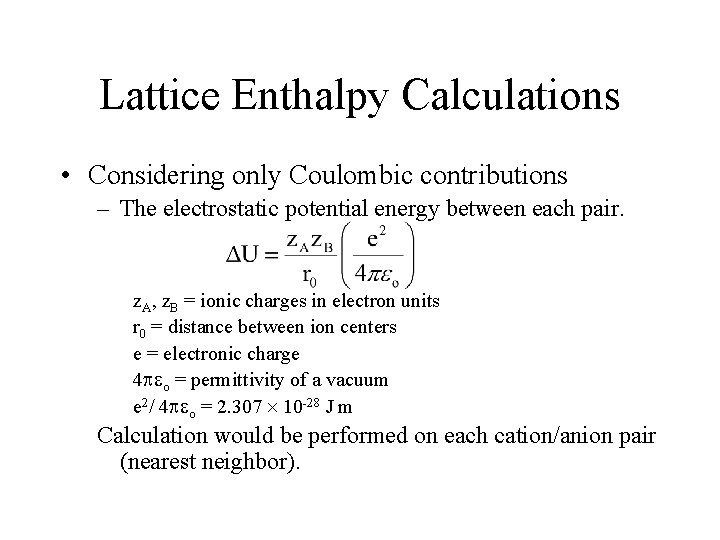
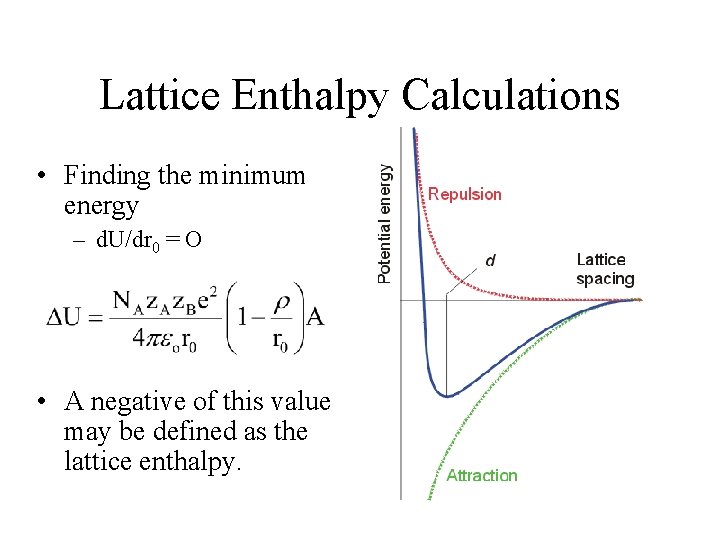
Lattice Enthalpy Calculations • Considering only Coulombic contributions – The electrostatic potential energy between each pair. z. A, z. B = ionic charges in electron units r 0 = distance between ion centers e = electronic charge 4 o = permittivity of a vacuum e 2/ 4 o = 2. 307 10 -28 J m Calculation would be performed on each cation/anion pair (nearest neighbor).

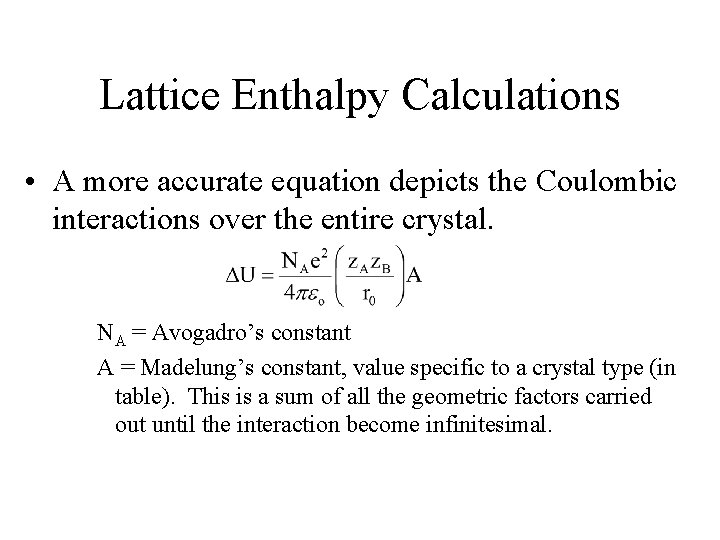
Lattice Enthalpy Calculations • A more accurate equation depicts the Coulombic interactions over the entire crystal. NA = Avogadro’s constant A = Madelung’s constant, value specific to a crystal type (in table). This is a sum of all the geometric factors carried out until the interaction become infinitesimal.

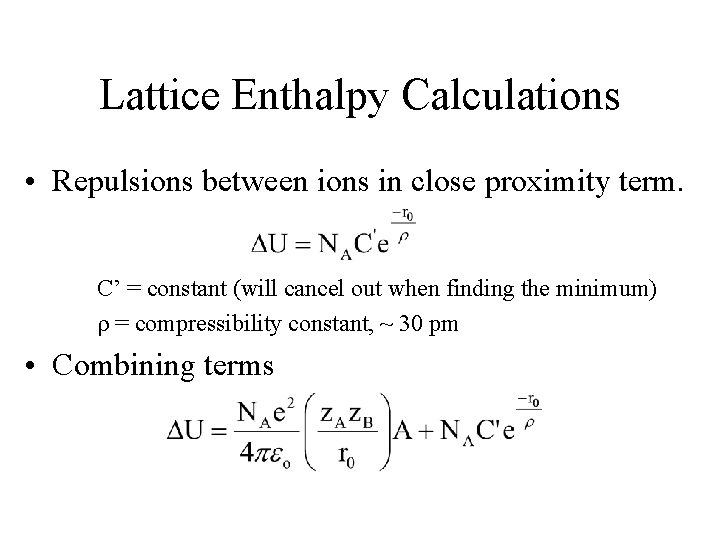
Lattice Enthalpy Calculations • Repulsions between ions in close proximity term. C’ = constant (will cancel out when finding the minimum) r = compressibility constant, ~ 30 pm • Combining terms

Lattice Enthalpy Calculations • Finding the minimum energy – d. U/dr 0 = O • A negative of this value may be defined as the lattice enthalpy.

Lattice Enthalpy Calculations • As the polarizability of the resultant ions increase the agreement with this ionic model worsens. – Polarizibility generally indicates more covalent character. Calculations Na. Cl and Ca. Br 2

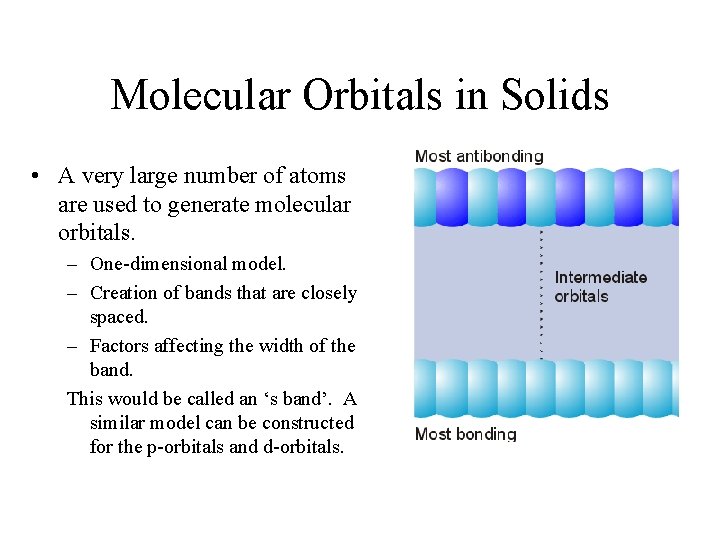
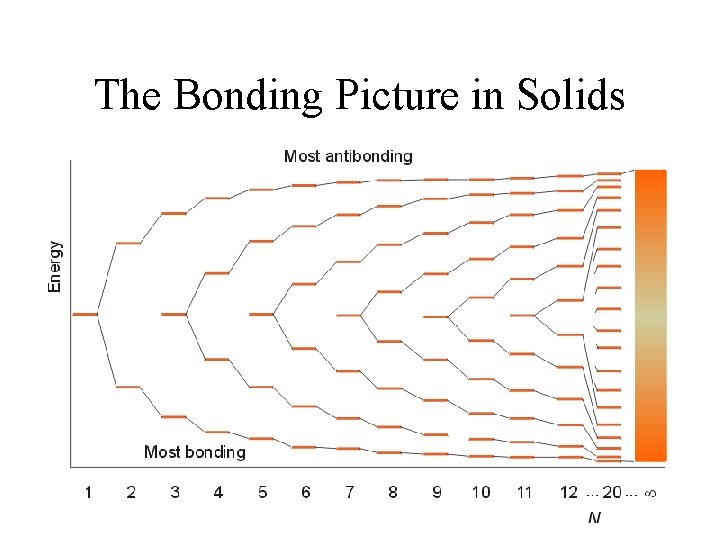
Molecular Orbitals in Solids • A very large number of atoms are used to generate molecular orbitals. – One-dimensional model. – Creation of bands that are closely spaced. – Factors affecting the width of the band. This would be called an ‘s band’. A similar model can be constructed for the p-orbitals and d-orbitals.

The Bonding Picture in Solids

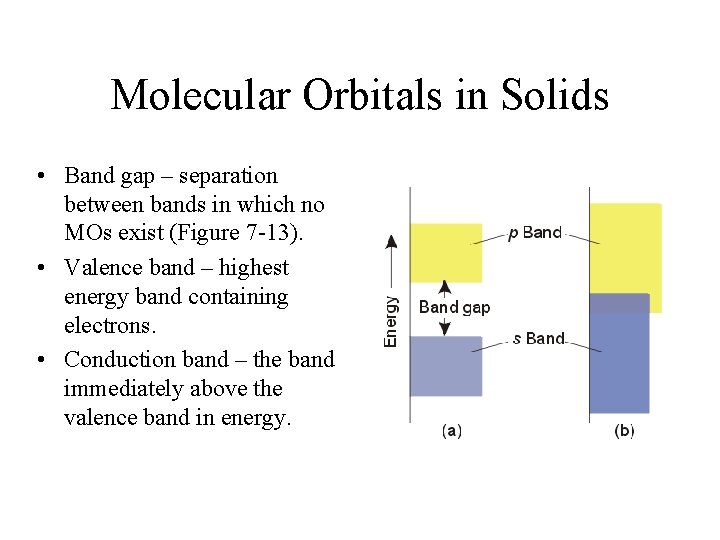
Molecular Orbitals in Solids • Band gap – separation between bands in which no MOs exist (Figure 7 -13). • Valence band – highest energy band containing electrons. • Conduction band – the band immediately above the valence band in energy.

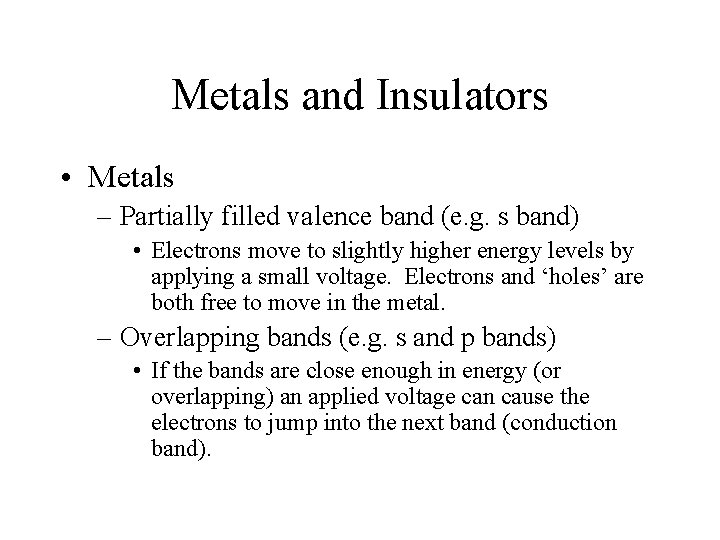
Metals and Insulators • Metals – Partially filled valence band (e. g. s band) • Electrons move to slightly higher energy levels by applying a small voltage. Electrons and ‘holes’ are both free to move in the metal. – Overlapping bands (e. g. s and p bands) • If the bands are close enough in energy (or overlapping) an applied voltage can cause the electrons to jump into the next band (conduction band).

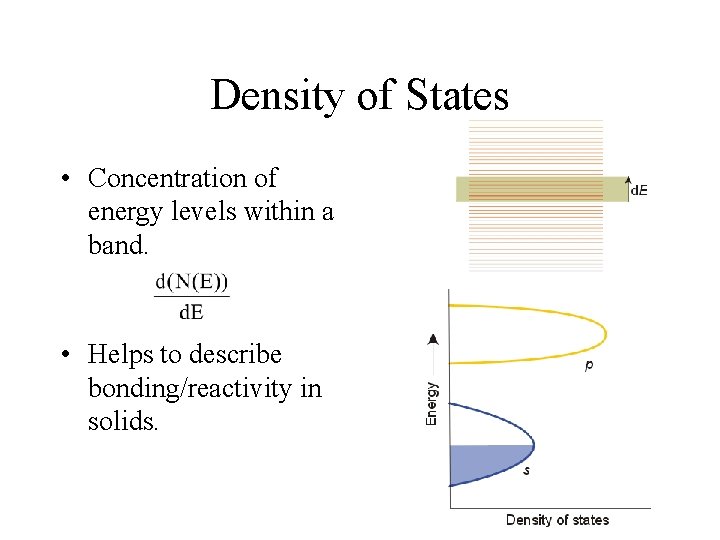
Density of States • Concentration of energy levels within a band. • Helps to describe bonding/reactivity in solids.

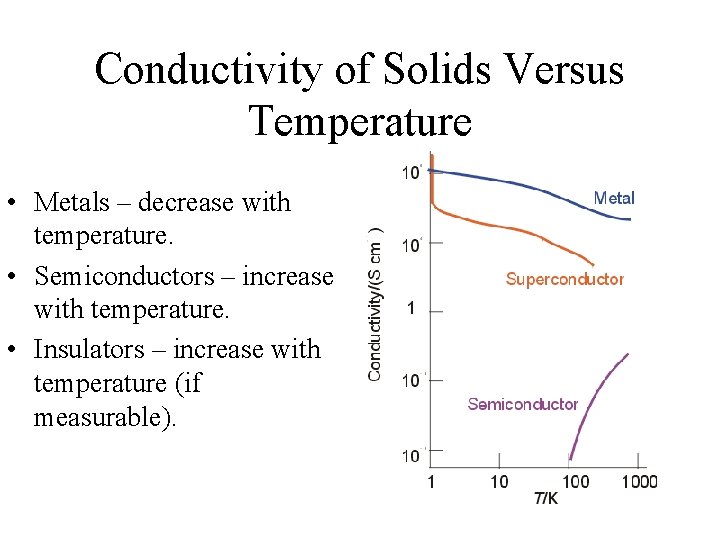
Conductivity of Solids Versus Temperature • Metals – decrease with temperature. • Semiconductors – increase with temperature. • Insulators – increase with temperature (if measurable).

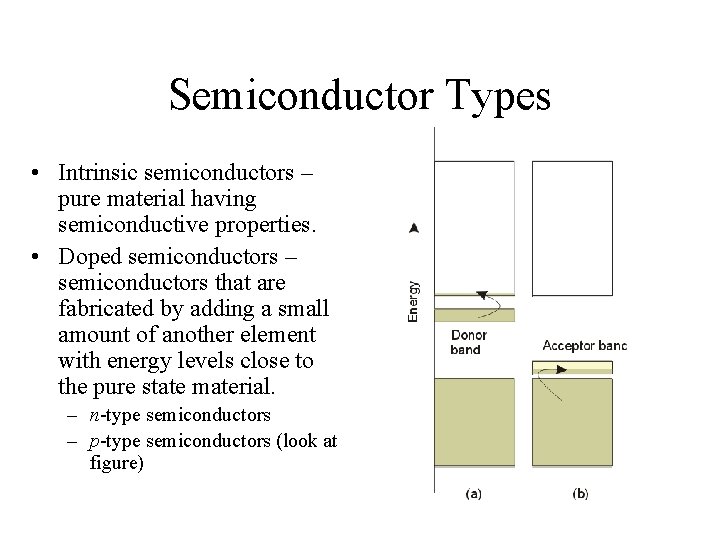
Semiconductor Types • Intrinsic semiconductors – pure material having semiconductive properties. • Doped semiconductors – semiconductors that are fabricated by adding a small amount of another element with energy levels close to the pure state material. – n-type semiconductors – p-type semiconductors (look at figure)

Semiconductors • Fermi-level (semiconductor) – the energy at which an electron is equally likely to be in each of two levels (Figure). • Effects of dopants on the Fermi level. – n-type and p-type.

Diodes (creating p-n junctions) • Migration of electrons from the n-type material to the p-type material. – Equilibrium is established due to charge transfer. • Application of a negative potential to the ntype material and a positive potential to the ptype material. – Discuss (Figure 7 -16).

Superconductivity • No resistance to flow of electrons. – Currents started in a loop will continue to flow indefinitely. • Type I superconductors – expel all magnetic fields below a critical temperature, Tc (Meisner effect). • Type II superconductors – below a critical temperature exclude all magnetic fields completely. Between this temperature and a second critical temperature, they allow partial penetration by the magnetic field. – Levitation experiment works well.

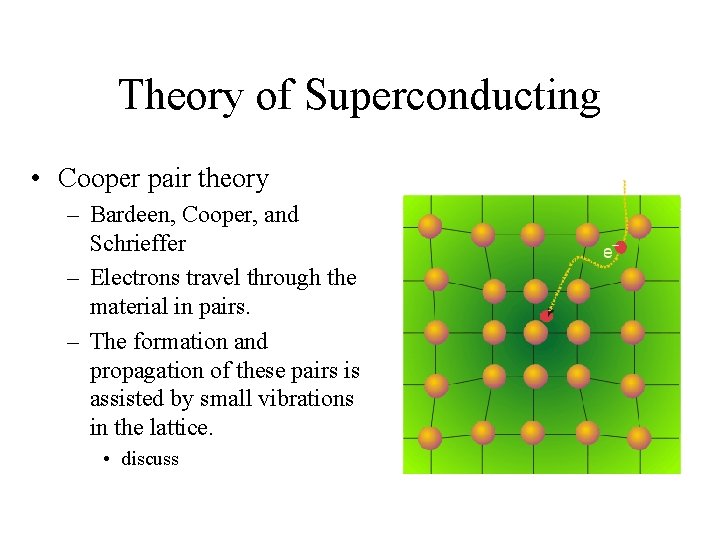
Theory of Superconducting • Cooper pair theory – Bardeen, Cooper, and Schrieffer – Electrons travel through the material in pairs. – The formation and propagation of these pairs is assisted by small vibrations in the lattice. • discuss

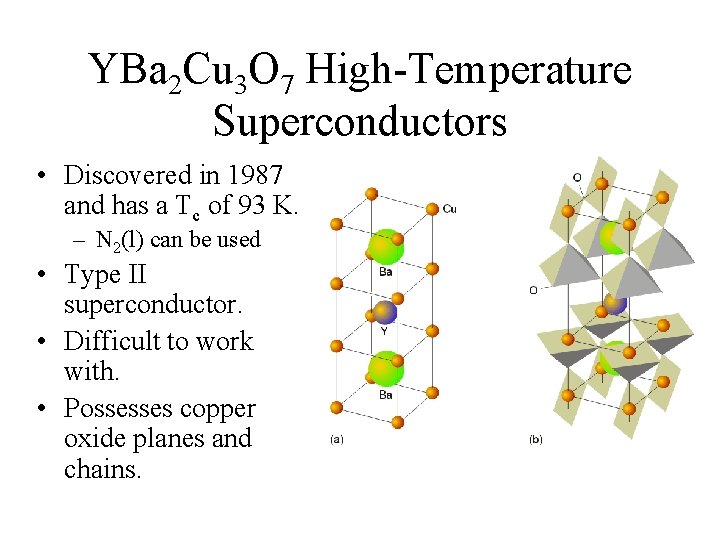
YBa 2 Cu 3 O 7 High-Temperature Superconductors • Discovered in 1987 and has a Tc of 93 K. – N 2(l) can be used • Type II superconductor. • Difficult to work with. • Possesses copper oxide planes and chains.

Bonding in Solid State Structures • The hard-sphere model is too simplistic. – Deviations are observed in ion sizes. – Sharing of electrons (or transfer back to the cation) can vary depending upon the polarizability. • Li. I versus Na. Cl (which structure would exhibit more covalent character? )

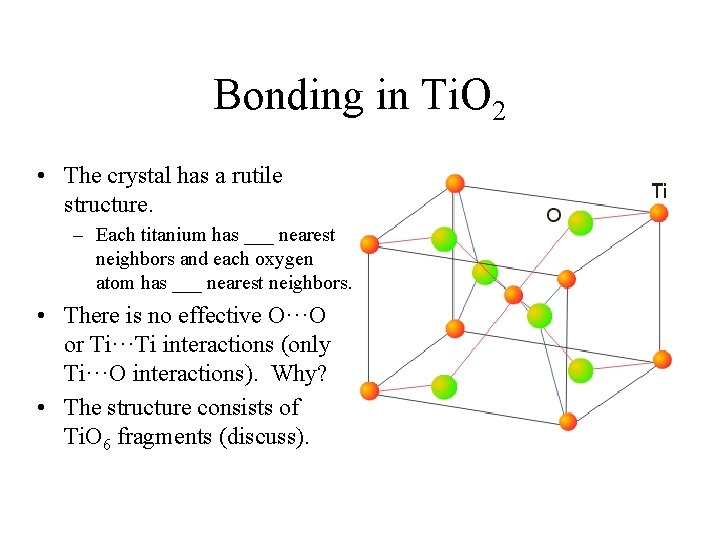
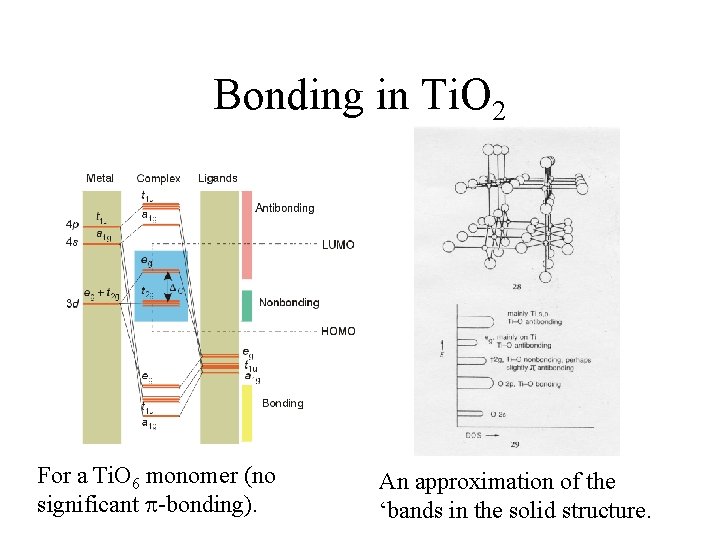
Bonding in Ti. O 2 • The crystal has a rutile structure. – Each titanium has ___ nearest neighbors and each oxygen atom has ___ nearest neighbors. • There is no effective O···O or Ti···Ti interactions (only Ti···O interactions). Why? • The structure consists of Ti. O 6 fragments (discuss).

Bonding in Ti. O 2 For a Ti. O 6 monomer (no significant -bonding). An approximation of the ‘bands in the solid structure.

Bonding in Ti. O 2 • The calculated DOS curve in 3 -d space is slightly more complicated. • The O 2 s, O 2 p, Ti t 2 g, and eg bands are well separate. The separation predicts that this material has ‘insulator-like’ properties.

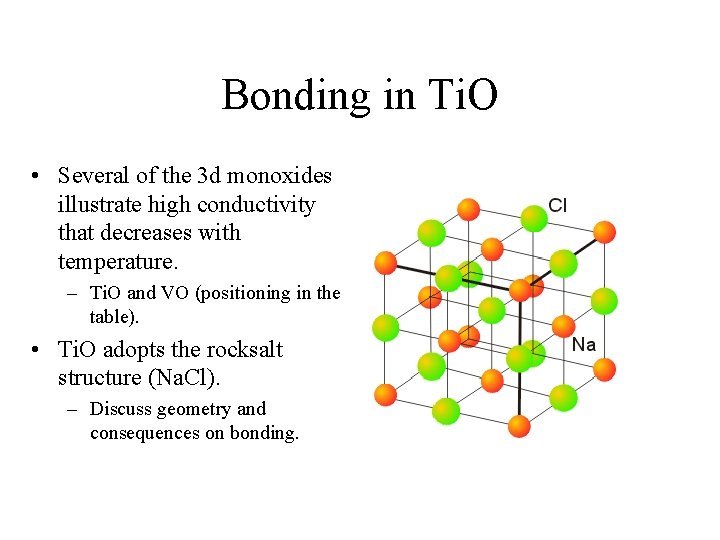
Bonding in Ti. O • Several of the 3 d monoxides illustrate high conductivity that decreases with temperature. – Ti. O and VO (positioning in the table). • Ti. O adopts the rocksalt structure (Na. Cl). – Discuss geometry and consequences on bonding.

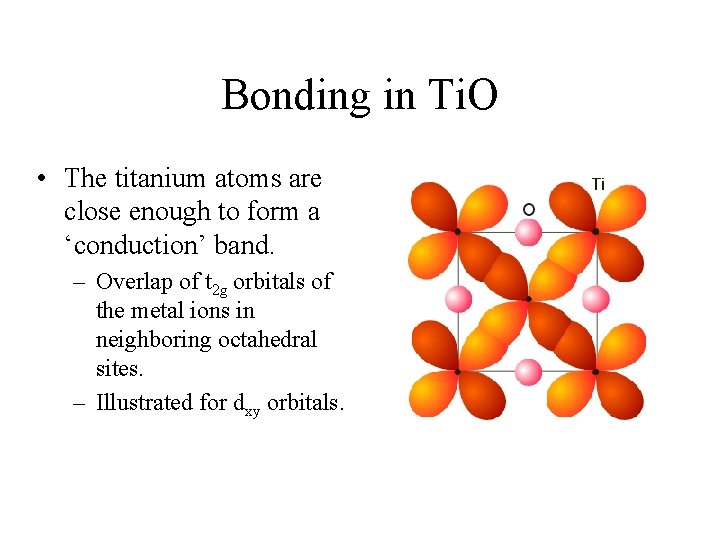
Bonding in Ti. O • The titanium atoms are close enough to form a ‘conduction’ band. – Overlap of t 2 g orbitals of the metal ions in neighboring octahedral sites. – Illustrated for dxy orbitals.

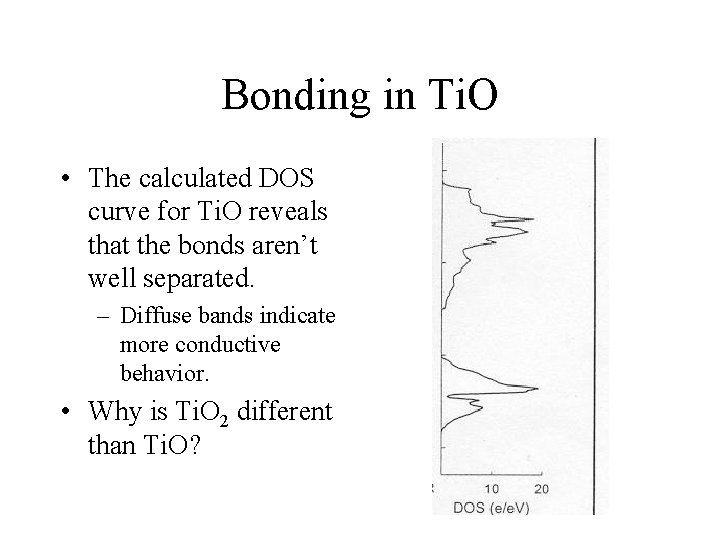
Bonding in Ti. O • The calculated DOS curve for Ti. O reveals that the bonds aren’t well separated. – Diffuse bands indicate more conductive behavior. • Why is Ti. O 2 different than Ti. O?

Bonding in Ti. O • Mn. O, Fe. O, Co. O, and Ni. O do not conduct, but they have the same basic structure. Why?

Imperfections in Solids • All crystalline solids possess imperfections. – Crystal growth occurring at many sites causes boundaries to form. – Vacancies and self-interstitials – Substitutions – Dislocations

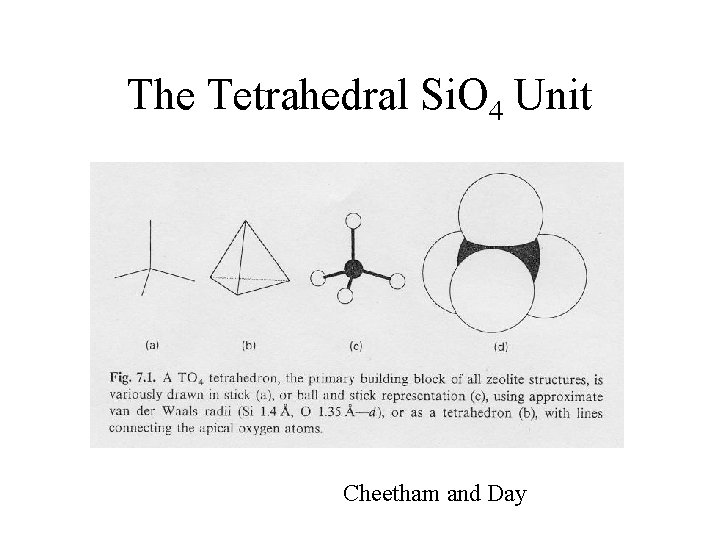
Silicates • The earth’s crustal rocks (clays, soils, and sands) are composed almost entirely (~95%) of silicate minerals and silica (O, Si, and Al). – There exist many structural types with widely varying stoichiometries (replacement of Si by Al is common). Consequences? • Common to all: – Si. O 4 tetrahedra units • Si is coordinated tetrahedrally to 4 oxygens http: //www. soils. wisc. edu/virtual_museum/displays. html http: //mineral. galleries. com/minerals/silicate/class. htm

The Tetrahedral Si. O 4 Unit Cheetham and Day

Structures with the Si. O 4 Unit • Discrete structural units which commonly contain cations for charge balance. • Corner sharing of O atoms into larger units. – O lattice is usually close-packed (near) – Charge balance is obtained by presence of cations. Individual units, chains, multiple chains (ribbons), rings, sheets and 3 -d networks.

Structure Containing Discrete Units • Nesosilicates – no O atoms are shared. – Contain individual Si. O 44 - units. – Zr. Si. O 4 (zircon) – illustrate with softwares • Stoichiometry dictates 8 -fold coordination of the cation. – (Mg 3 or Fe 3)Al 2 Si 3 O 12 (garnet) – illustrate with softwares • 8 -fold coordination for Mg or Fe and 6 -fold coordination for the Al.

Structure Containing Discrete Units • The sorosilicates (disilicates) – 1 O atom is shared. – Contain Si 2 O 76 - units – Show Epidote (Ca 2 Fe. Al 2(Si. O 4)(Si 2 O 7)O(OH)) with softwares. • Epidote contains Si. O 44 - and Si 2 O 76 - units – Near linear Si-O-Si bond angle between tetrahedra.

Cyclosilicates (discrete cyclic units) • Each Si. O 4 units shares two O atoms with neighboring Si. O 4 tetrahedra. – Formula – Si. O 32 - or [(Si. O 3)n]2 n- (n=3 -6 are the most common. – Beryl – six-linked Si. O 4 tetrahedra (show with softwares). • Be 3 Al 2(Si. O 3)6 – contains Si 6 O 1812 - cyclic units • The impurities produce its colors. – Wadeite – three-linked Si. O 4 tetrahedra (don’t have an actual picture) • K 2 Zr. Si 3 O 9

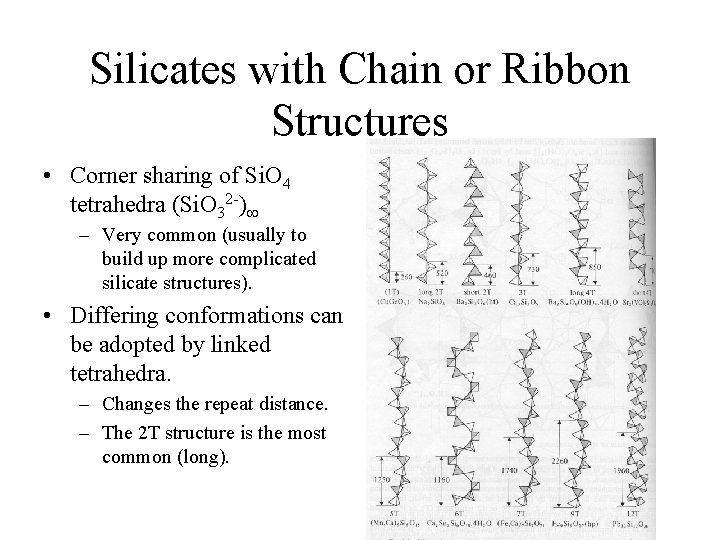
Silicates with Chain or Ribbon Structures • Corner sharing of Si. O 4 tetrahedra (Si. O 32 -) – Very common (usually to build up more complicated silicate structures). • Differing conformations can be adopted by linked tetrahedra. – Changes the repeat distance. – The 2 T structure is the most common (long).

Silicates with Chain or Ribbon Structures • The chains are usually packed parallel to provide sites of 6 and 8 coordination for the cations. – Jadeite [Na. Al. Si 2 O 6] • Illustrate the different repeat units. • What is the repeat unit?

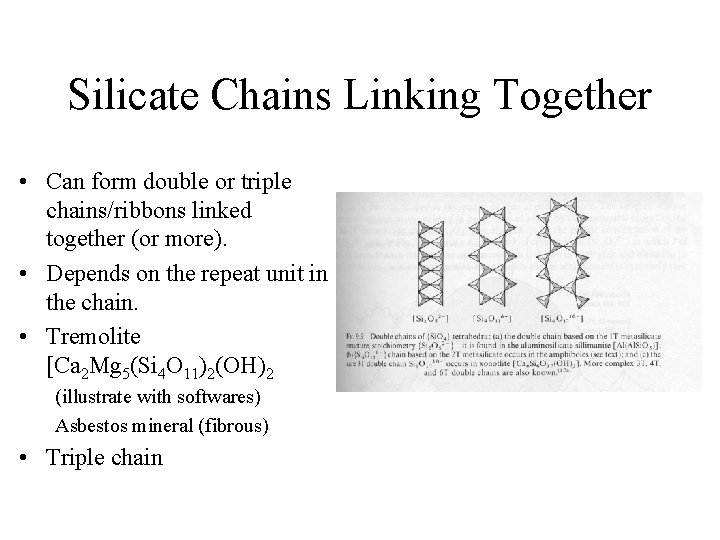
Silicate Chains Linking Together • Can form double or triple chains/ribbons linked together (or more). • Depends on the repeat unit in the chain. • Tremolite [Ca 2 Mg 5(Si 4 O 11)2(OH)2 (illustrate with softwares) Asbestos mineral (fibrous) • Triple chain

Phyllosilicates (Silicates with Layer Structures) • Clay minerals, micas, talc, soapstone. • Individual layers are formed by sharing 3 of the 4 atoms of each tetrahedron. • Simplest structure is made up of a 2 T network of silicate chains to give a network composition of Si 2 O 52 -. – This is exhibited with kaolinite (illustrate the silicon tetrahedral layer).

Creation of Layers in the Phyllosilicates • Can be formed by sharing the fourth O atom between pairs of tetrahedra. – Produces an Si. O 2 stoichiometry (neutral) – Replacing Si with Al • Al 2 Si 2 O 82 -; requires charge balance. The cations connect the double layers.

Creation of Layers in the Phyllosilicates • Double layers can be produced by interleaving layers of the gibbsite Al(OH)3 or brucite Mg(OH)2 structure. – Incorporation of gibbsite produces kaolinite, [Al 2(OH)4 Si 2 O 5] (China clay); illustrate with software the different layers present. – Placing a Si. O layer on the other side of the Al. O layer produces pyrophyllite, [Al 2(OH)2 Si 4 O 10]. • Illustrate both with software.

More Layered Structures • The Al can be replaced by Mg (2: 3) ratio. – Kaolinite serpentine asbestos – Pyrophyllite talc • Charged layers can also result by replacing the framework Si with Al or other cations. For charge balance these layers can be interleaved with M(+1) or M(+2) to give micas (illite) or by layers of hydrated cations to give montmorillonite. – Illustrate both.

The Tectosilicates • Each oxygen atom is shared by 2 tetrahedra (Si. O 2 formula). • Silica ( -quartz; one crystalline form) – Si-O-Si bond angles are ~144 degrees. – Contains helical chains of Si. O 4. • Six combine to form hexagonal shape (illustrate).

The Tectosilicates (Zeolites aluminosilicates) • A large fraction of the Si atoms are replaced with Al (other metals can also be used). – Charge balance will be required (Si, Al)n. O 2 n. • Contain cavities that allow molecules to enter. – Able to tailor electronic and physical properties. • Pore structure and cation exchange. • Illustrate with software.