THE STRUCTURE OF CRYSTALLINE SOLID CONTENT v FUNDAMENTAL




![EXAMPLE: Crystallographic Direction [? ? ? ] = A: B: C: D: E: F: EXAMPLE: Crystallographic Direction [? ? ? ] = A: B: C: D: E: F:](https://slidetodoc.com/presentation_image/21f6152038d1033db126615d2a21e613/image-24.jpg)
![CRYSTALLOGRAPHIC DIRECTION: Families of Directions Equivalence of directions <123> Family of directions ü [123], CRYSTALLOGRAPHIC DIRECTION: Families of Directions Equivalence of directions <123> Family of directions ü [123],](https://slidetodoc.com/presentation_image/21f6152038d1033db126615d2a21e613/image-25.jpg)

![EXAMPLE 1. Calculate the linear density of the [100] direction for BCC 2. Calculate EXAMPLE 1. Calculate the linear density of the [100] direction for BCC 2. Calculate](https://slidetodoc.com/presentation_image/21f6152038d1033db126615d2a21e613/image-31.jpg)

- Slides: 32

THE STRUCTURE OF CRYSTALLINE SOLID CONTENT: v FUNDAMENTAL CONCEPTS v UNITS CELLS v METALLIC CRYSTAL STRUCTURES The Face-centered Cubic Crystal Structure The Body-centered Cubic Crystal Structure The Hexagonal Close-packed Crystal Structure v DENSITY v POLYMORHISM AND ALLOTROPY v CRYSTALOGRAPHIC DIRECTION AND PLANE v LINEAR AND PLANER ATOMIC DENSITIES

FUNDAMENTAL CONCEPTS Solid materials may be classified according to the regularity with which atoms or irons are arranged with respect to one another Crystalline: – The atoms are situated in a repeating or periodic array over large atomic distance; long-range order exists (upon solidification), atoms will position themselves in a repetitive three-dimensional pattern, each atom bonded to its nearestneighbor atom – All metal, many ceramic and certain polymers form crystalline under normal solidification condition – For those do not crystalline (noncrystalline or amorphous) – long-range atomic is absent

FUNDAMENTAL CONCEPTS Crystal structure: – The manner in which atoms, ions or molecules are spatially arranged – Atoms / ions are thought of as being solid spheres having welldefined diameters – atomic hard sphere model (figure) in which spheres representing nearest neighbor atoms touch one another – ‘lattice’ – three-dimensional array of points coinciding with atom positions

UNIT CELL • In describing crystal structures, it is often convenient to subdivide the structure into small repeat entities called unit cells. • Unit cell is chosen to represent the symmetry of the crystal structure , wherein all the atom positions in the crystal may be generated by translations of the unit cell integral distance along each of its edges. • Unit cell is the basic structural init or building block of the crystal structure & defines the crystal structure by virtue of its geometry & the atom positions within.

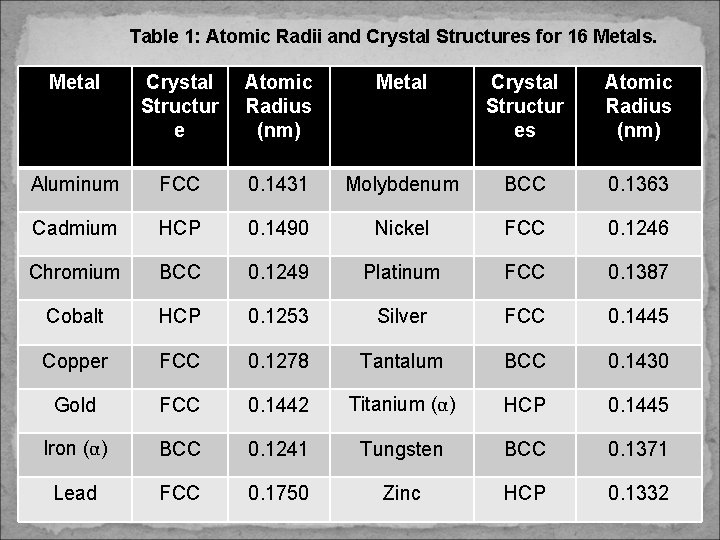
METALLIC CRYSTAL STRUCTURE The atomic bonding in this group of materials is: ü metallic & nondirectional in nature. ü no restrictions as to the number & position of nearest-neighbor atoms – lead to relatively large numbers of nearest neighbors & dense atomic packing for most metallic crystal structures. Table 1 presents the atomic radii for a number of metals. Three relatively simple structure are found for most of the common metals: 1) Face-Centered Cubic, (FCC) 2) Body-Centered Cubic (BCC), and 3) Hexagonal Close-Packed (HCP)

Table 1: Atomic Radii and Crystal Structures for 16 Metals. Metal Crystal Structur e Atomic Radius (nm) Metal Crystal Structur es Atomic Radius (nm) Aluminum FCC 0. 1431 Molybdenum BCC 0. 1363 Cadmium HCP 0. 1490 Nickel FCC 0. 1246 Chromium BCC 0. 1249 Platinum FCC 0. 1387 Cobalt HCP 0. 1253 Silver FCC 0. 1445 Copper FCC 0. 1278 Tantalum BCC 0. 1430 Gold FCC 0. 1442 Titanium (α) HCP 0. 1445 Iron (α) BCC 0. 1241 Tungsten BCC 0. 1371 Lead FCC 0. 1750 Zinc HCP 0. 1332

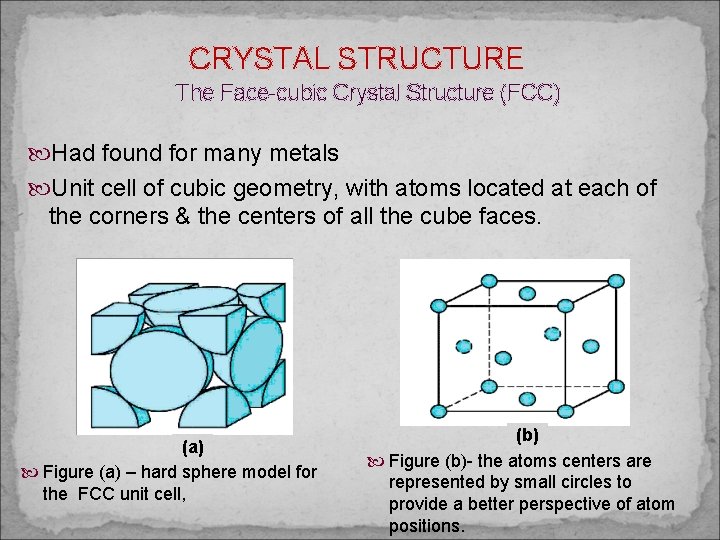
CRYSTAL STRUCTURE The Face-cubic Crystal Structure (FCC) Had found for many metals Unit cell of cubic geometry, with atoms located at each of the corners & the centers of all the cube faces. (a) Figure (a) – hard sphere model for the FCC unit cell, (b) Figure (b)- the atoms centers are represented by small circles to provide a better perspective of atom positions.

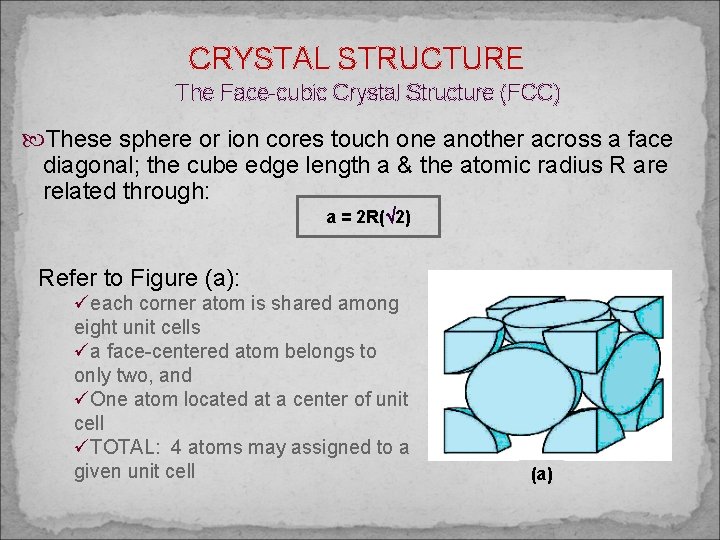
CRYSTAL STRUCTURE The Face-cubic Crystal Structure (FCC) These sphere or ion cores touch one another across a face diagonal; the cube edge length a & the atomic radius R are related through: a = 2 R( 2) Refer to Figure (a): üeach corner atom is shared among eight unit cells üa face-centered atom belongs to only two, and üOne atom located at a center of unit cell üTOTAL: 4 atoms may assigned to a given unit cell (a)

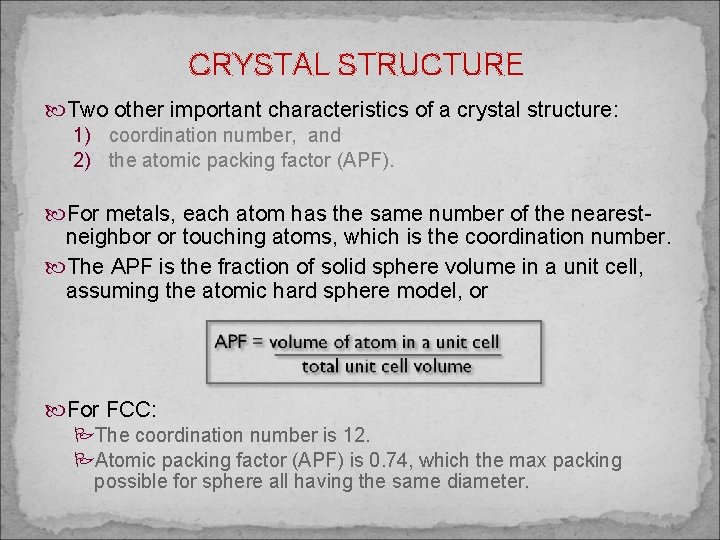
CRYSTAL STRUCTURE Two other important characteristics of a crystal structure: 1) coordination number, and 2) the atomic packing factor (APF). For metals, each atom has the same number of the nearestneighbor or touching atoms, which is the coordination number. The APF is the fraction of solid sphere volume in a unit cell, assuming the atomic hard sphere model, or For FCC: PThe coordination number is 12. PAtomic packing factor (APF) is 0. 74, which the max packing possible for sphere all having the same diameter.

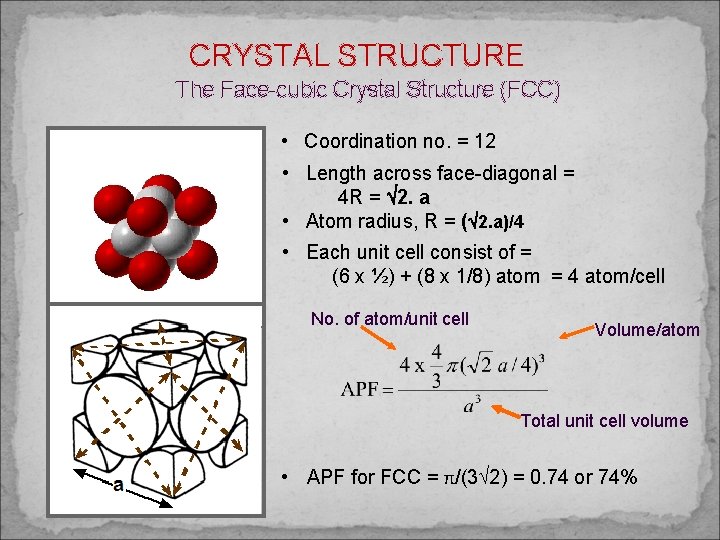
CRYSTAL STRUCTURE The Face-cubic Crystal Structure (FCC) • Coordination no. = 12 • Length across face-diagonal = 4 R = 2. a • Atom radius, R = ( 2. a)/4 • Each unit cell consist of = (6 x ½) + (8 x 1/8) atom = 4 atom/cell No. of atom/unit cell Volume/atom Total unit cell volume • APF for FCC = π/(3 2) = 0. 74 or 74%

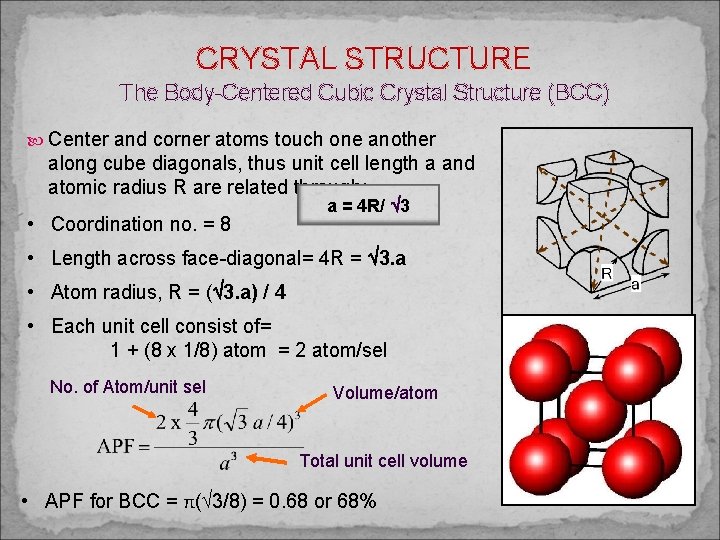
CRYSTAL STRUCTURE The Body-Centered Cubic Crystal Structure (BCC) Center and corner atoms touch one another along cube diagonals, thus unit cell length a and atomic radius R are related through: • Coordination no. = 8 a = 4 R/ 3 • Length across face-diagonal= 4 R = 3. a • Atom radius, R = ( 3. a) / 4 • Each unit cell consist of= 1 + (8 x 1/8) atom = 2 atom/sel No. of Atom/unit sel Volume/atom Total unit cell volume • APF for BCC = π( 3/8) = 0. 68 or 68%

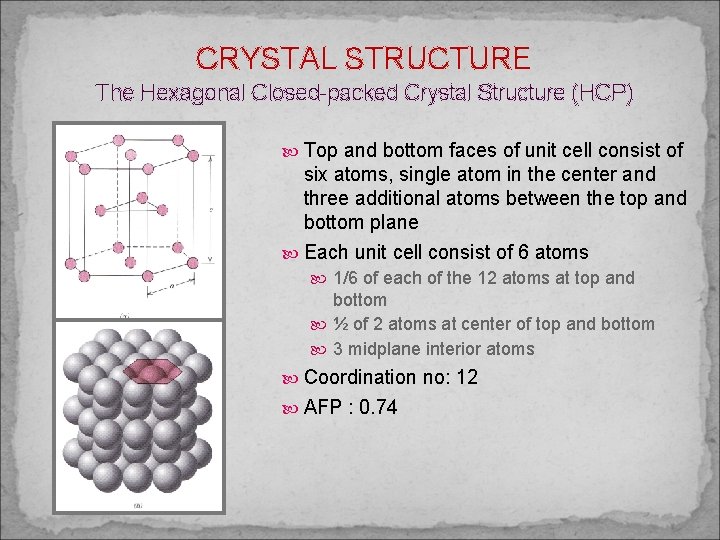
CRYSTAL STRUCTURE The Hexagonal Closed-packed Crystal Structure (HCP) Top and bottom faces of unit cell consist of six atoms, single atom in the center and three additional atoms between the top and bottom plane Each unit cell consist of 6 atoms 1/6 of each of the 12 atoms at top and bottom ½ of 2 atoms at center of top and bottom 3 midplane interior atoms Coordination no: 12 AFP : 0. 74

EXAMPLE: Crystal Structure Computation 1) Calculate the volume of an FCC unit cell in terms of the atomic radius R. 2) Show that the atomic packing factor for the FCC crystal structure is 0. 74.

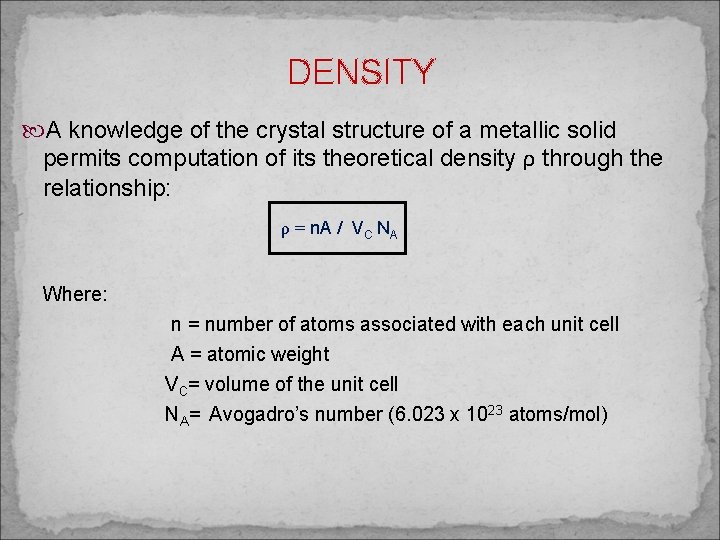
DENSITY A knowledge of the crystal structure of a metallic solid permits computation of its theoretical density ρ through the relationship: ρ = n. A / VC NA Where: n = number of atoms associated with each unit cell A = atomic weight VC= volume of the unit cell NA= Avogadro’s number (6. 023 x 1023 atoms/mol)

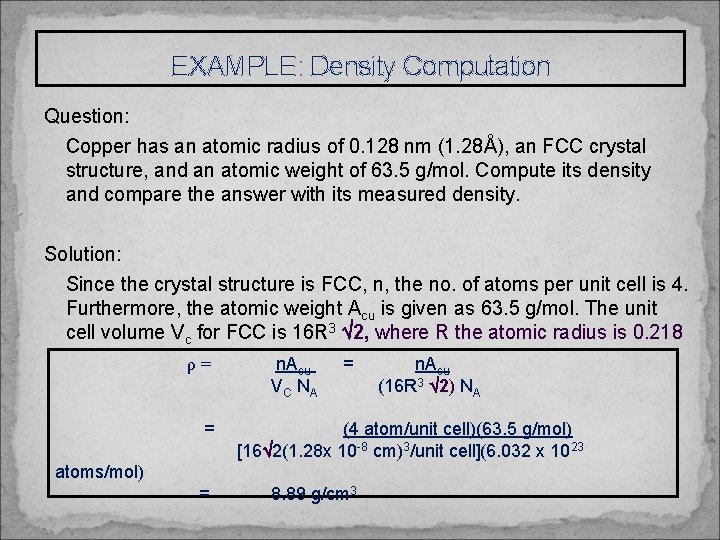
EXAMPLE: Density Computation Question: Copper has an atomic radius of 0. 128 nm (1. 28Å), an FCC crystal structure, and an atomic weight of 63. 5 g/mol. Compute its density and compare the answer with its measured density. Solution: Since the crystal structure is FCC, n, the no. of atoms per unit cell is 4. Furthermore, the atomic weight Acu is given as 63. 5 g/mol. The unit cell volume Vc for FCC is 16 R 3 2, where R the atomic radius is 0. 218 nm. ρ= n. A cu VC NA = cu 2) NA (4 atom/unit cell)(63. 5 g/mol) [16 2(1. 28 x 10 -8 cm)3/unit cell](6. 032 x 1023 atoms/mol) = (16 R 3 8. 89 g/cm 3

POLYMOPRHISM AND ALLOTROPHY Polymorphism – a phenomenon of some metal & nonmetals have more than one crystal structure. In the elemental solids, the condition is often termed allotropy. The prevailing crystal structure depends on both: 1) the temperature & 2) the external pressure. Example: i. ii. carbon: graphite is a stable polymorph at ambient conditions, whereas diamond is formed at extremely high pressures. Iron: Has a BCC crystal structure at room temperature, but changes to FCC iron at 912°C. Most a modification of density and other physical properties will lead to polymorphic transformation.

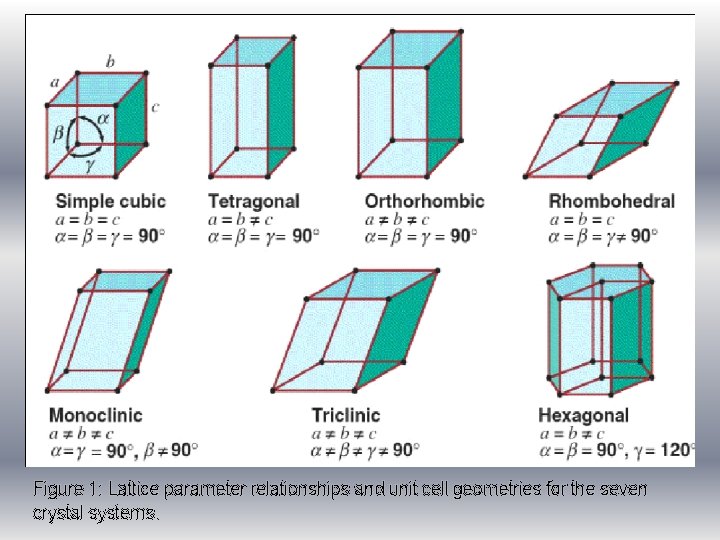
CRYSTAL SYSTEM According to unit cell geometry, crystal structure are groups within the seven crystal system (Figure 1) Unit cell geometry is defined in terms of six parameters: – Three edge lengths a, b and c – Three interaxial angles α, β and γ Lattice parameters • The cubic system has the greatest degree of symmetry, and least symmetry is display by triclinic system. β • FCC and BCC belong to cubic crystal c structure and HCP fall within hexagonal. α γ b x y a

Figure 1: Lattice parameter relationships and unit cell geometries for the seven crystal systems.

CRYSTALLOGRAPHIC DIRECTION AND PLANE It is often necessary to be able to specify certain directions and planes in crystals. Many material properties and processes vary with direction in the crystal. Directions and planes are described using three integers - Miller Indices

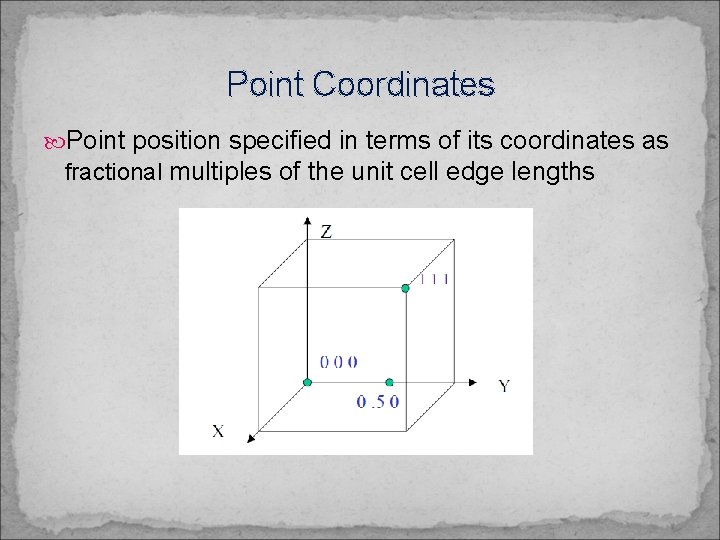
Point Coordinates Point position specified in terms of its coordinates as fractional multiples of the unit cell edge lengths

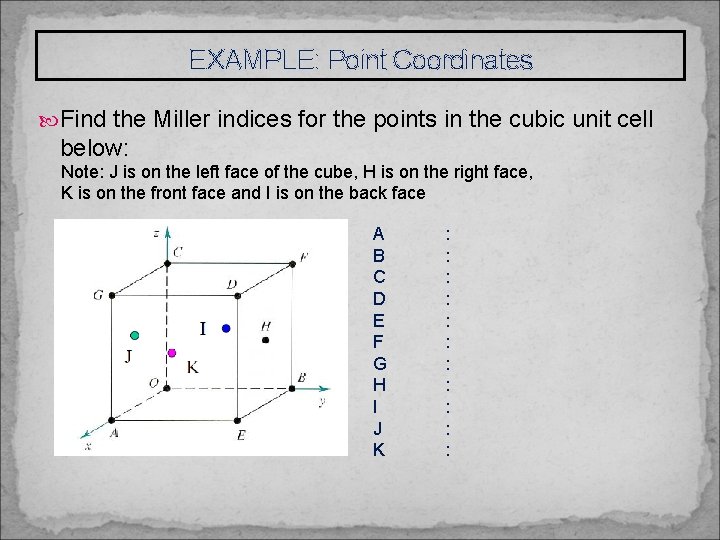
EXAMPLE: Point Coordinates Find the Miller indices for the points in the cubic unit cell below: Note: J is on the left face of the cube, H is on the right face, K is on the front face and I is on the back face A B C D E F G H I J K : : :

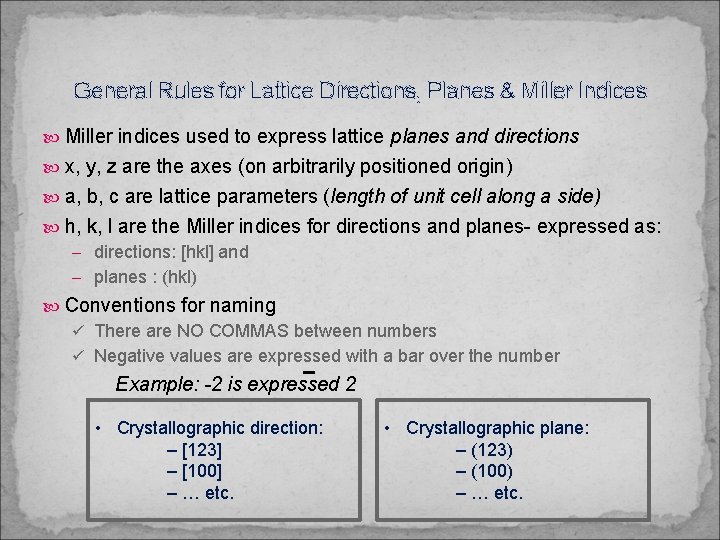
General Rules for Lattice Directions, Planes & Miller Indices Miller indices used to express lattice planes and directions x, y, z are the axes (on arbitrarily positioned origin) a, b, c are lattice parameters (length of unit cell along a side) h, k, l are the Miller indices for directions and planes- expressed as: – directions: [hkl] and – planes : (hkl) Conventions for naming ü There are NO COMMAS between numbers ü Negative values are expressed with a bar over the number Example: -2 is expressed 2 • Crystallographic direction: – [123] – [100] – … etc. • Crystallographic plane: – (123) – (100) – … etc.

CRYSTALLOGRAPHIC DIRECTION: Miller Indices for Direction Defined as a line between two points, or a vector. Methods: 1) Draw vector that pass through the origin of the coordinate system 2) The length of the vector projection on each of the three axes is determined; these are measured in terms of the unit cell dimensions a, b and c. 3) Remove fractions by multiplying or divided by a common factor to reduce them to the smallest integer values 4) Enclose in square brackets [uvw]. The u, v and w integers correspond to the reduced projection along the x, y and z axes, respectively. Negative direction, for example, the [111] direction would have a component in the –y direction.
![EXAMPLE Crystallographic Direction A B C D E F EXAMPLE: Crystallographic Direction [? ? ? ] = A: B: C: D: E: F:](https://slidetodoc.com/presentation_image/21f6152038d1033db126615d2a21e613/image-24.jpg)
EXAMPLE: Crystallographic Direction [? ? ? ] = A: B: C: D: E: F:
![CRYSTALLOGRAPHIC DIRECTION Families of Directions Equivalence of directions 123 Family of directions ü 123 CRYSTALLOGRAPHIC DIRECTION: Families of Directions Equivalence of directions <123> Family of directions ü [123],](https://slidetodoc.com/presentation_image/21f6152038d1033db126615d2a21e613/image-25.jpg)
CRYSTALLOGRAPHIC DIRECTION: Families of Directions Equivalence of directions <123> Family of directions ü [123], [213], [312], [132], [231], [321] ü only in a cubic crystal ü In the cubic system directions having the same indices regardless of order or sign are equivalent.

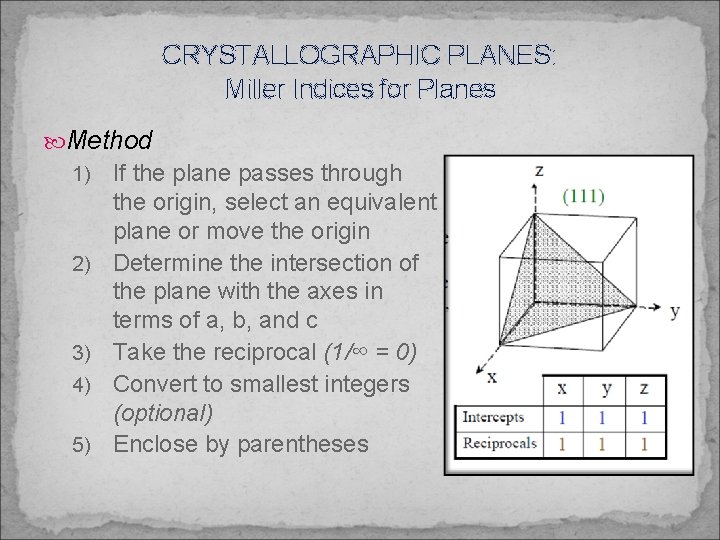
CRYSTALLOGRAPHIC PLANES: Miller Indices for Planes (hkl) Crystallographic plane {hkl} Family of crystallographic planes ü e. g. (hkl), (lhk), (hlk) … etc. In the cubic system planes having the same indices regardless of order or sign are equivalent Hexagonal crystals can be expressed in a four index system (uvtw) Can be converted to a three index system using formulas

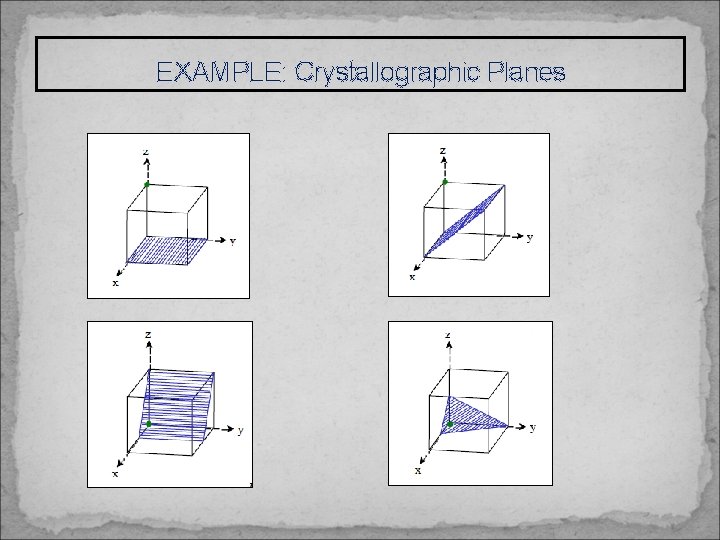
CRYSTALLOGRAPHIC PLANES: Miller Indices for Planes Method 1) 2) 3) 4) 5) If the plane passes through the origin, select an equivalent plane or move the origin Determine the intersection of the plane with the axes in terms of a, b, and c Take the reciprocal (1/∞ = 0) Convert to smallest integers (optional) Enclose by parentheses

EXAMPLE: Crystallographic Planes

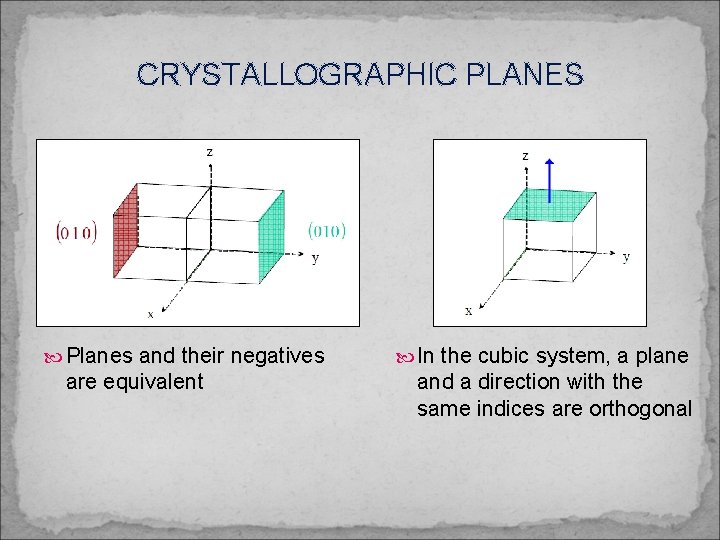
CRYSTALLOGRAPHIC PLANES Planes and their negatives are equivalent In the cubic system, a plane and a direction with the same indices are orthogonal

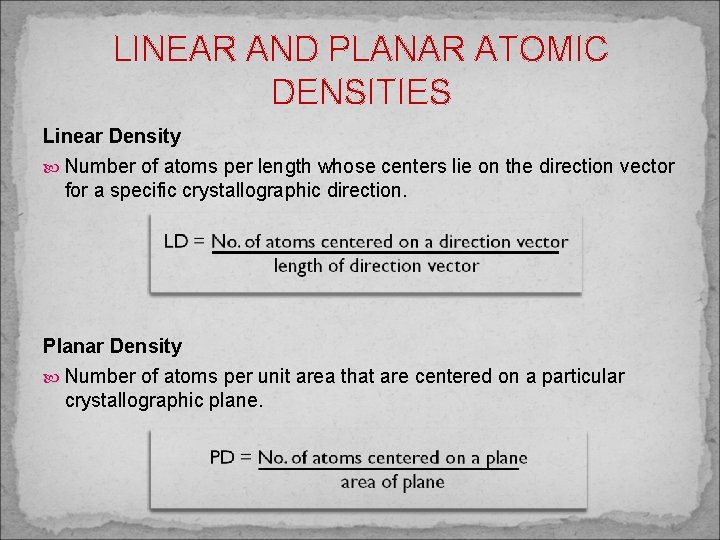
LINEAR AND PLANAR ATOMIC DENSITIES Linear Density Number of atoms per length whose centers lie on the direction vector for a specific crystallographic direction. Planar Density Number of atoms per unit area that are centered on a particular crystallographic plane.
![EXAMPLE 1 Calculate the linear density of the 100 direction for BCC 2 Calculate EXAMPLE 1. Calculate the linear density of the [100] direction for BCC 2. Calculate](https://slidetodoc.com/presentation_image/21f6152038d1033db126615d2a21e613/image-31.jpg)
EXAMPLE 1. Calculate the linear density of the [100] direction for BCC 2. Calculate the planar density of the (100) plane for FCC

LINEAR AND PLANAR ATOMIC DENSITIES Why do we care? Properties, in general, depend on linear and planar density. Examples: ü Speed of sound along directions ü Slip (deformation in metals) depends on linear and planar density ü Slip occurs on planes that have the greatest density of atoms in direction with highest density (we would say along closest packed directions on the closest packed planes)