Lecture 2 1 Crystalline Solids Crystalline Solids Polycrystalline








- Slides: 27

Lecture 2. 1 Crystalline Solids

Crystalline Solids Poly-crystalline solids - Grains l Mono-crystalline solids- Whiskers, Wafers l

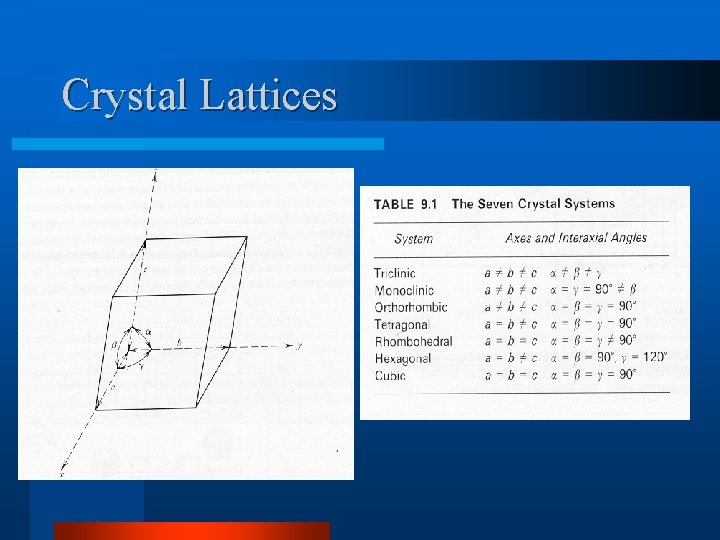
Crystal Lattices

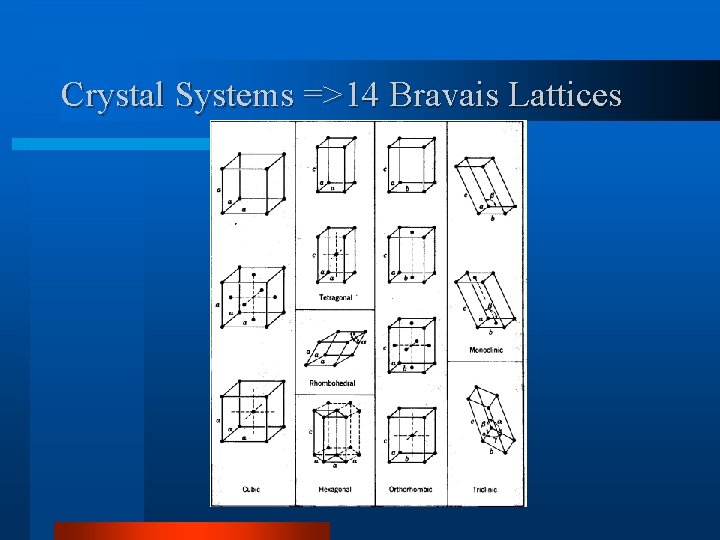
Crystal Systems =>14 Bravais Lattices

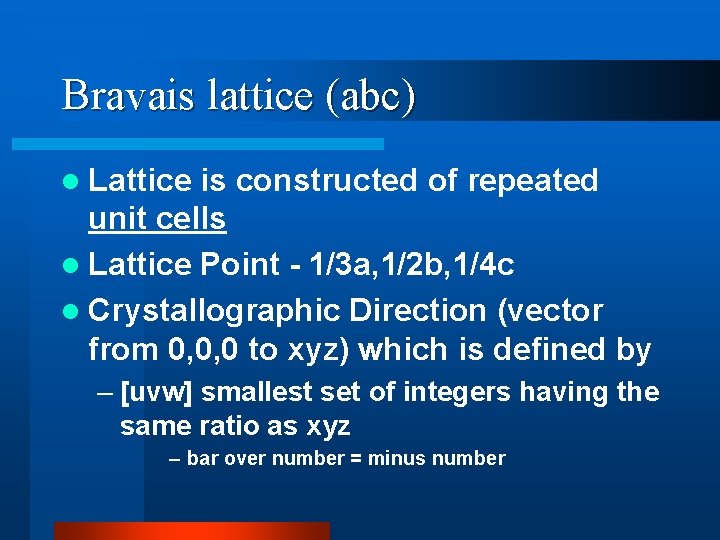
Bravais lattice (abc) l Lattice is constructed of repeated unit cells l Lattice Point - 1/3 a, 1/2 b, 1/4 c l Crystallographic Direction (vector from 0, 0, 0 to xyz) which is defined by – [uvw] smallest set of integers having the same ratio as xyz – bar over number = minus number

Close Packed Structures l Cubic, BCC, FCC, HCC are the focus of this course l Determining distances and volume fractions in Cubic Structures

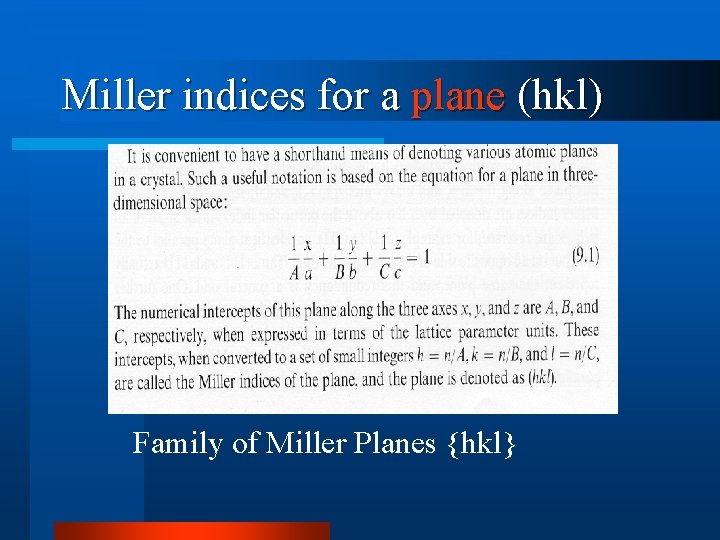
Miller indices for a plane (hkl) Family of Miller Planes {hkl}

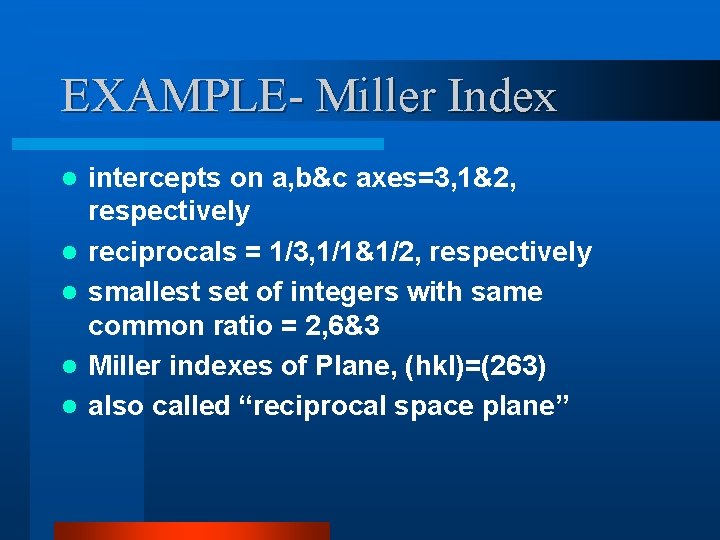
EXAMPLE- Miller Index l l l intercepts on a, b&c axes=3, 1&2, respectively reciprocals = 1/3, 1/1&1/2, respectively smallest set of integers with same common ratio = 2, 6&3 Miller indexes of Plane, (hkl)=(263) also called “reciprocal space plane”

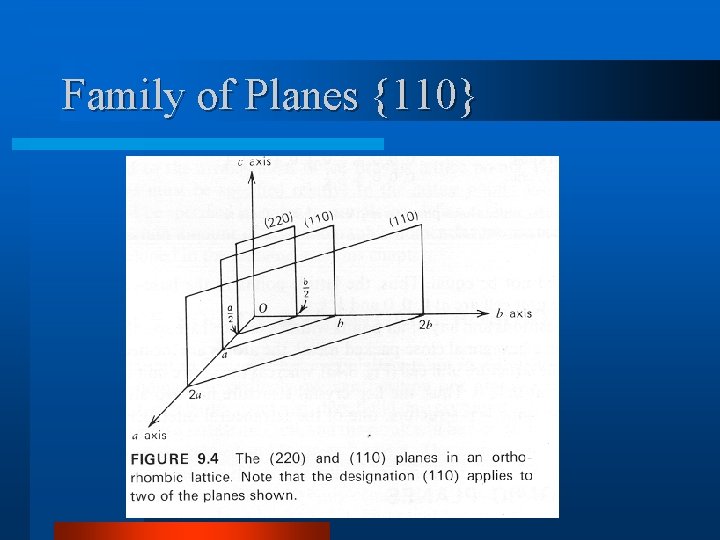
Family of Planes {110}

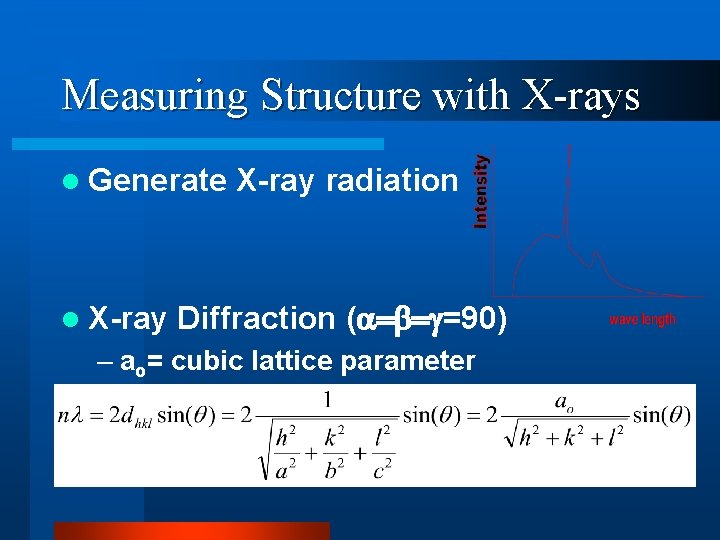
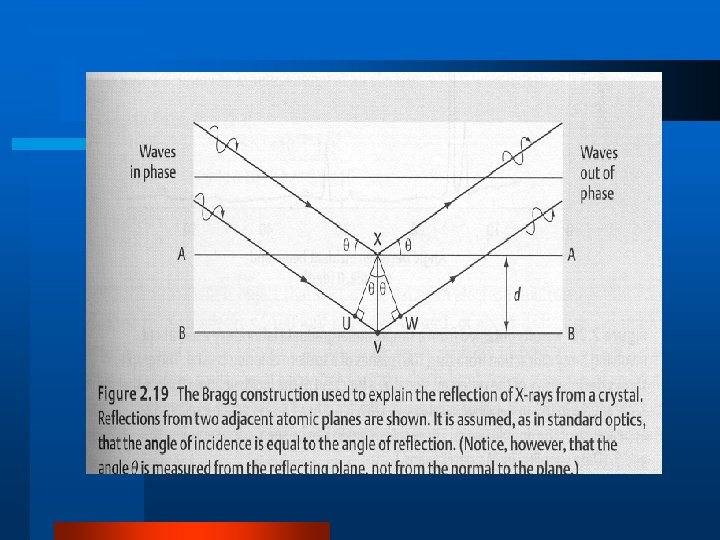
Measuring Structure with X-rays l Generate l X-ray radiation Diffraction ( =90) – ao= cubic lattice parameter

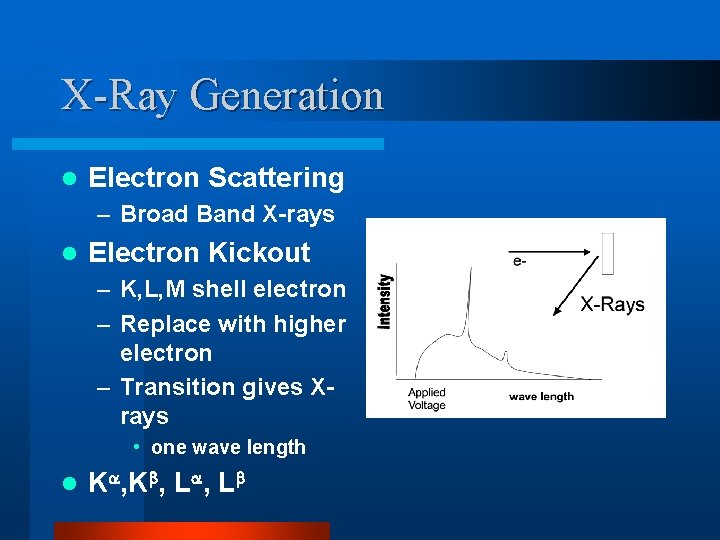
X-Ray Generation l Electron Scattering – Broad Band X-rays l Electron Kickout – K, L, M shell electron – Replace with higher electron – Transition gives Xrays • one wave length l K , L , L K K

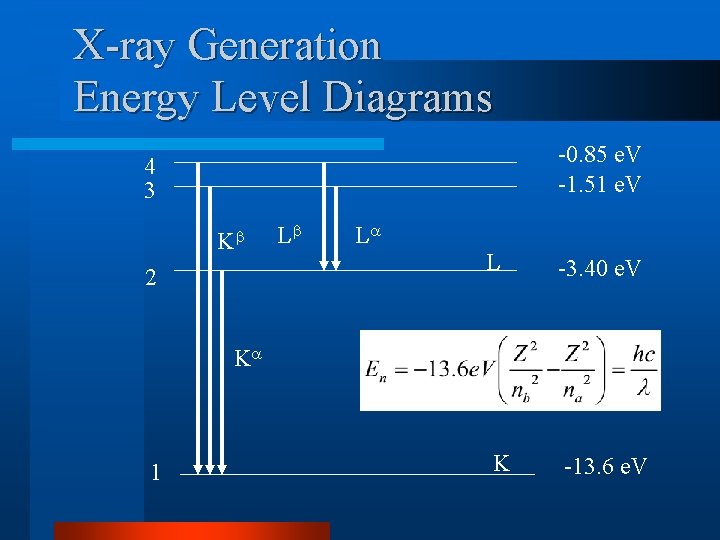
X-ray Generation Energy Level Diagrams -0. 85 e. V -1. 51 e. V 4 3 K 2 L L L -3. 40 e. V K 1 K -13. 6 e. V


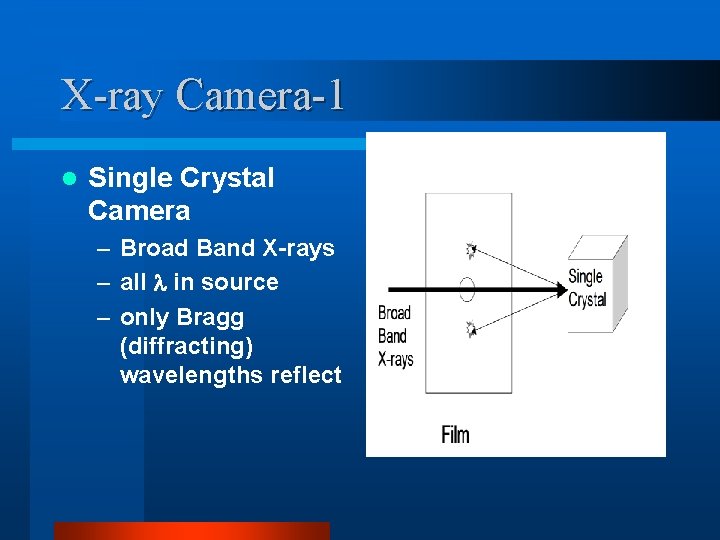
X-ray Camera-1 l Single Crystal Camera – Broad Band X-rays – all in source – only Bragg (diffracting) wavelengths reflect

Si. Ge Crystal

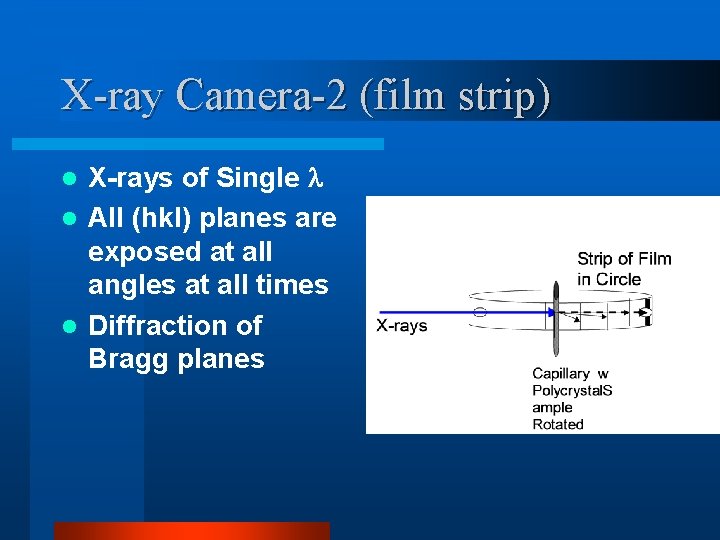
X-ray Camera-2 (film strip) X-rays of Single l All (hkl) planes are exposed at all angles at all times l Diffraction of Bragg planes l

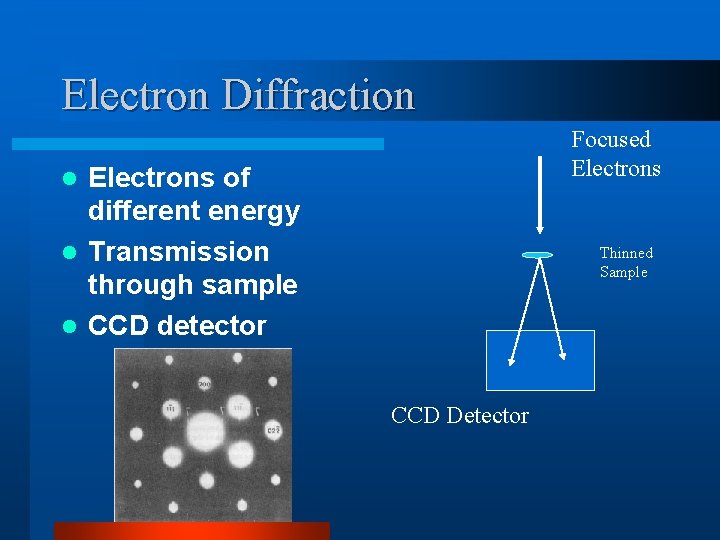
Electron Diffraction Focused Electrons of different energy l Transmission through sample l CCD detector l Thinned Sample CCD Detector

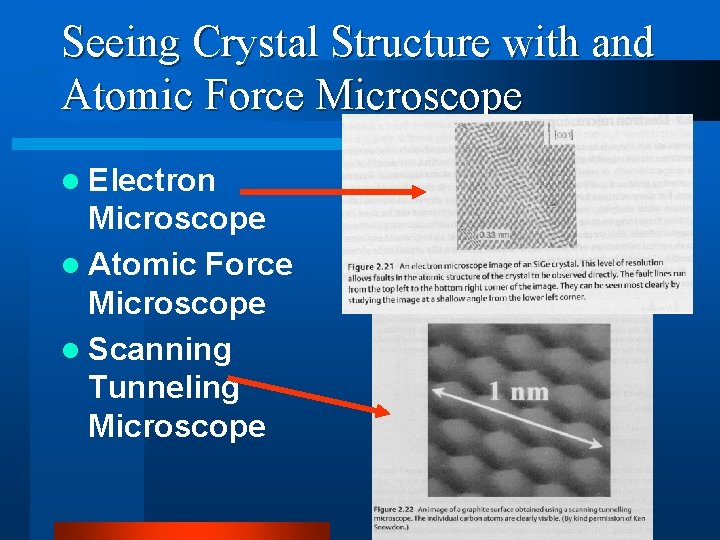
Seeing Crystal Structure with and Atomic Force Microscope l Electron Microscope l Atomic Force Microscope l Scanning Tunneling Microscope

Diffracting Planes

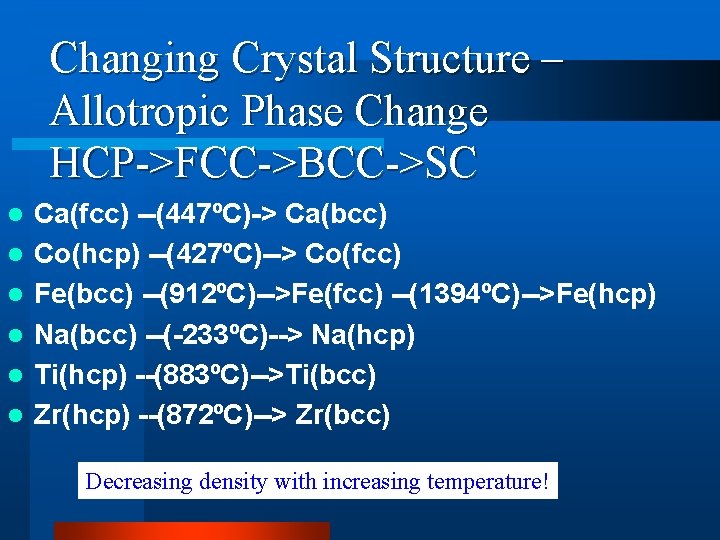
Changing Crystal Structure – Allotropic Phase Change HCP->FCC->BCC->SC l l l Ca(fcc) --(447ºC)-> Ca(bcc) Co(hcp) --(427ºC)--> Co(fcc) Fe(bcc) --(912ºC)-->Fe(fcc) --(1394ºC)-->Fe(hcp) Na(bcc) --(-233ºC)--> Na(hcp) Ti(hcp) --(883ºC)-->Ti(bcc) Zr(hcp) --(872ºC)--> Zr(bcc) Decreasing density with increasing temperature!

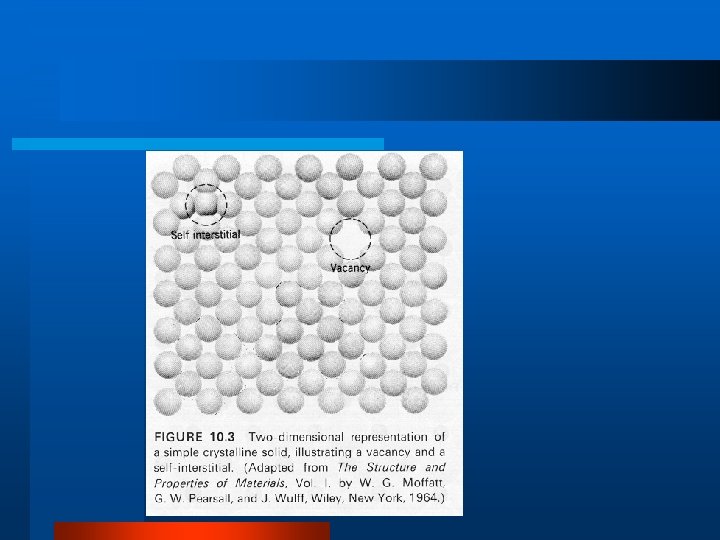
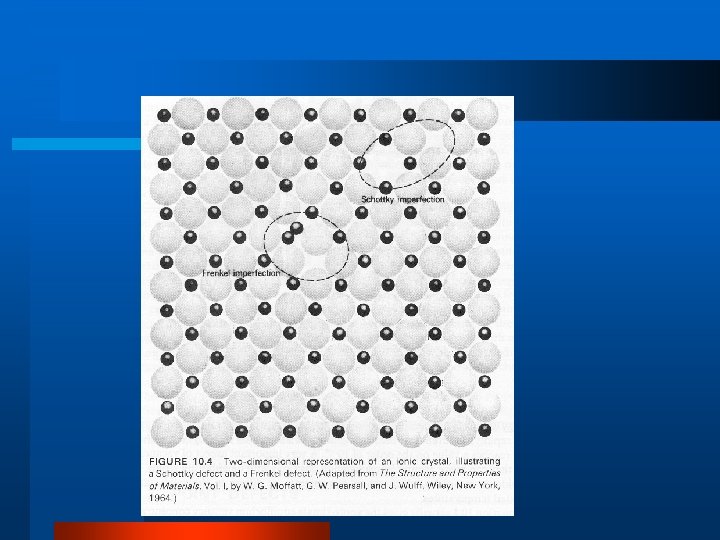
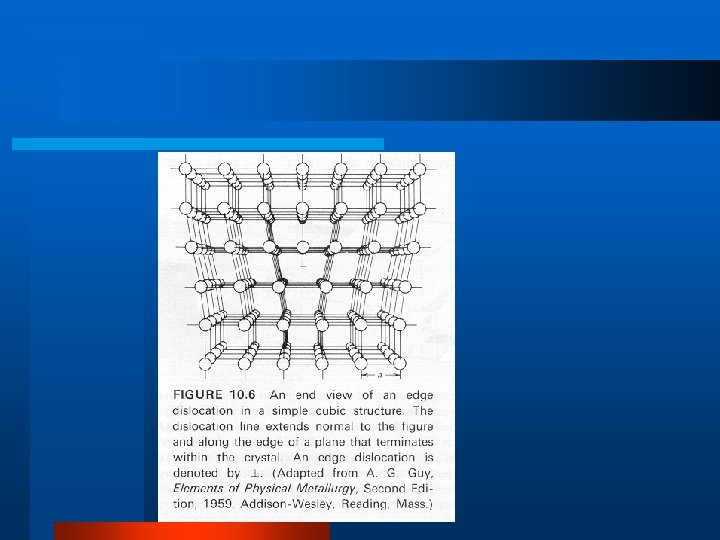
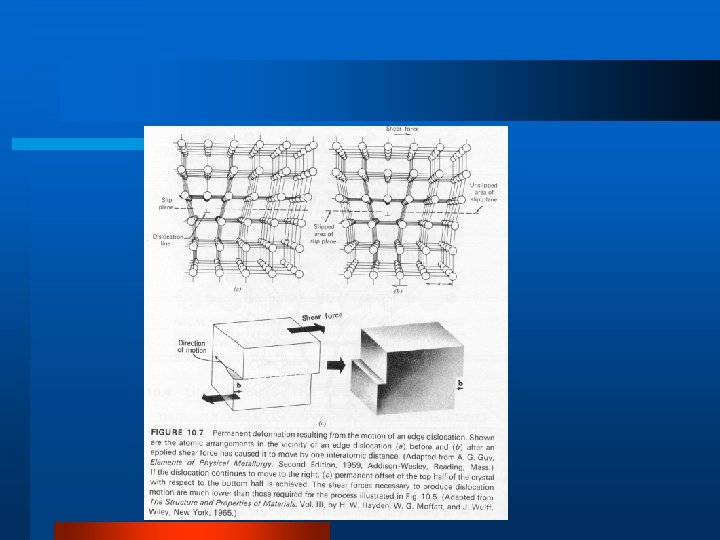
Defects in Crystal Structure l Vacancy, Interstitial, Impurity l Schottky Defect l Frenkel Defect l Dislocations – edge dislocation, line, screw l Grain Boundary






Effect of Structure on Mechanical Properties l Elasticity l Plastic Deformation