Definition of the crystalline state Crystals are solids






![Crystal Lattice directions symbol: [uvw] examples: [100] [010] [001] : z : c a Crystal Lattice directions symbol: [uvw] examples: [100] [010] [001] : z : c a](https://slidetodoc.com/presentation_image_h/ba0d9c550cb0c9af3741453d50d71dae/image-24.jpg)



- Slides: 34

Definition of the crystalline state: • Crystals are solids (but not all solids are crystals!) • Crystals are the most ordered form of the matter • Crystal are 3 -D (2 -D) regular arrays of ions, atoms, molecules; they have triple (double) periodicity • Crystals have long range order. • Each repeating unit (whatever it is) within a crystal has an identical environment

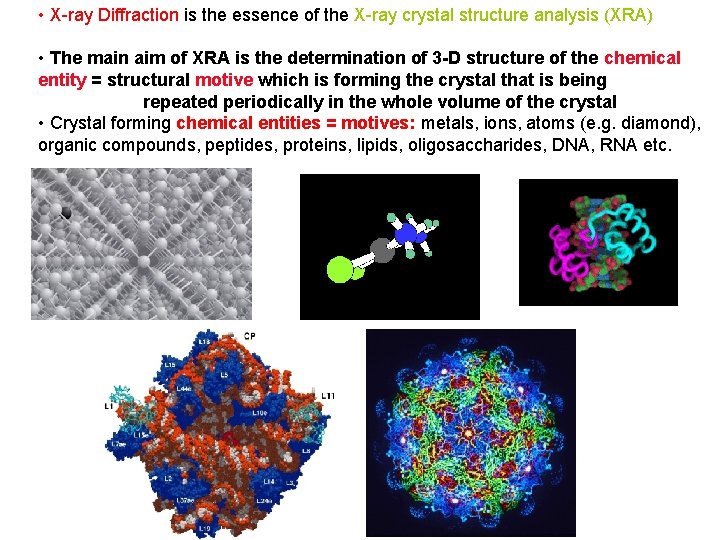
• X-ray Diffraction is the essence of the X-ray crystal structure analysis (XRA) • The main aim of XRA is the determination of 3 -D structure of the chemical entity = structural motive which is forming the crystal that is being repeated periodically in the whole volume of the crystal • Crystal forming chemical entities = motives: metals, ions, atoms (e. g. diamond), organic compounds, peptides, proteins, lipids, oligosaccharides, DNA, RNA etc.

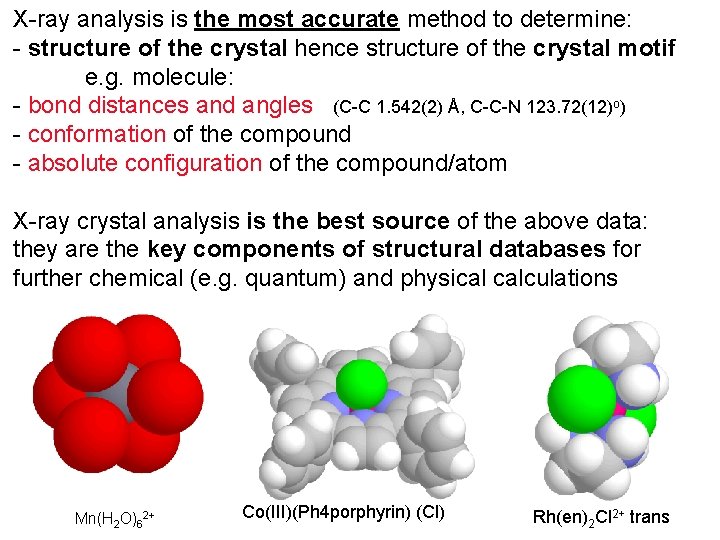
X-ray analysis is the most accurate method to determine: - structure of the crystal hence structure of the crystal motif e. g. molecule: - bond distances and angles (C-C 1. 542(2) Å, C-C-N 123. 72(12)o) - conformation of the compound - absolute configuration of the compound/atom X-ray crystal analysis is the best source of the above data: they are the key components of structural databases for further chemical (e. g. quantum) and physical calculations Mn(H 2 O)62+ Co(III)(Ph 4 porphyrin) (Cl) Rh(en)2 Cl 2+ trans

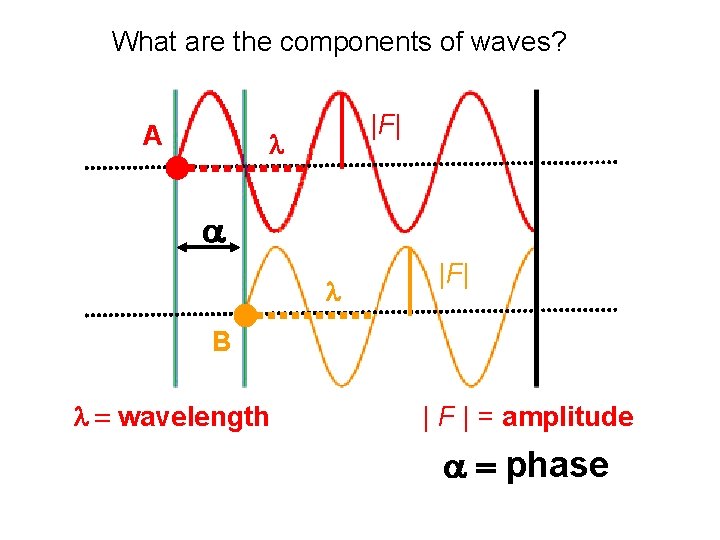
What are the components of waves? A |F| B wavelength | F | = amplitude phase

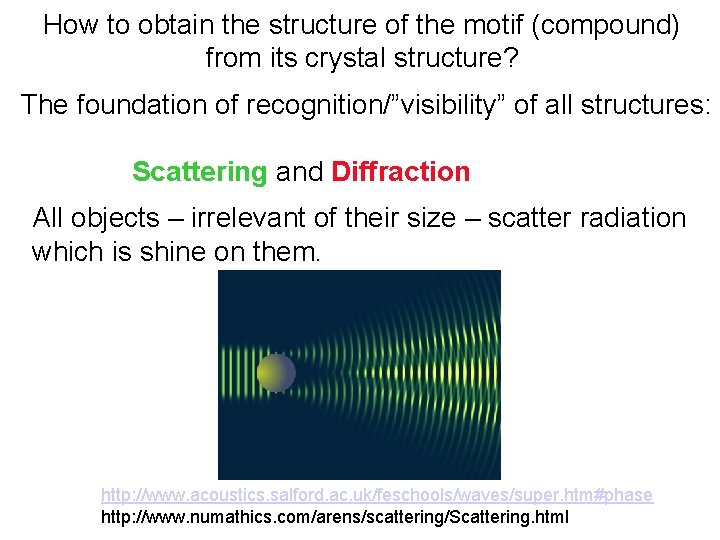
How to obtain the structure of the motif (compound) from its crystal structure? The foundation of recognition/”visibility” of all structures: Scattering and Diffraction All objects – irrelevant of their size – scatter radiation which is shine on them. http: //www. acoustics. salford. ac. uk/feschools/waves/super. htm#phase http: //www. numathics. com/arens/scattering/Scattering. html

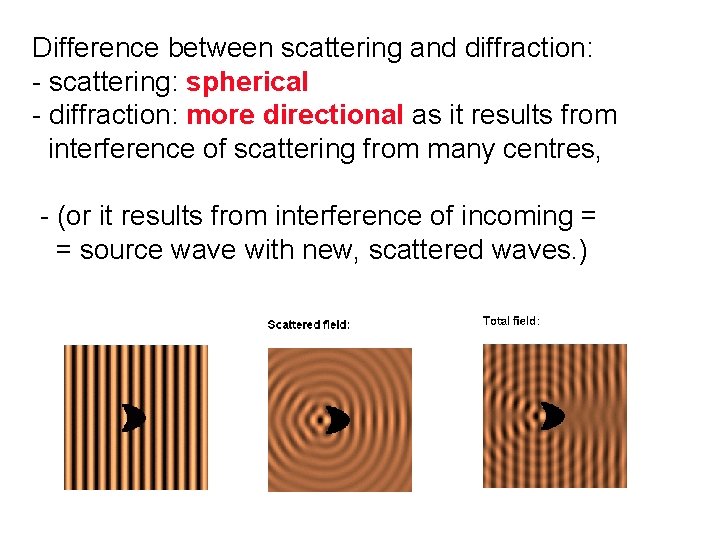
Difference between scattering and diffraction: - scattering: spherical - diffraction: more directional as it results from interference of scattering from many centres, - (or it results from interference of incoming = = source wave with new, scattered waves. )

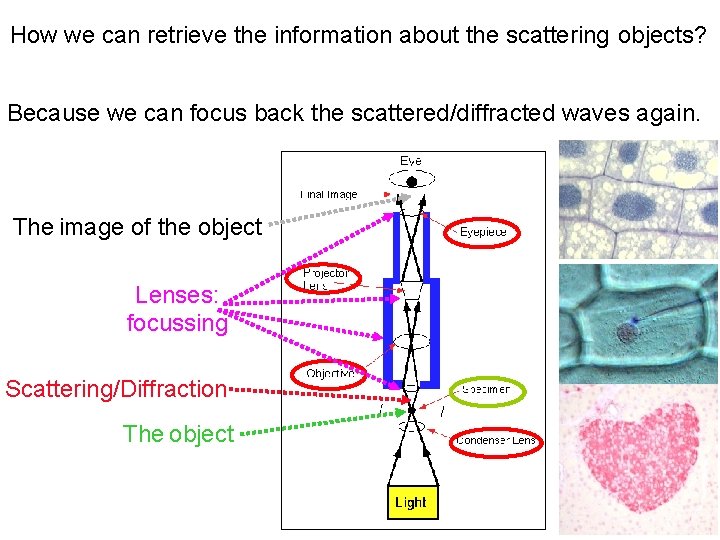
How we can retrieve the information about the scattering objects? Because we can focus back the scattered/diffracted waves again. The image of the object Lenses: focussing Scattering/Diffraction The object

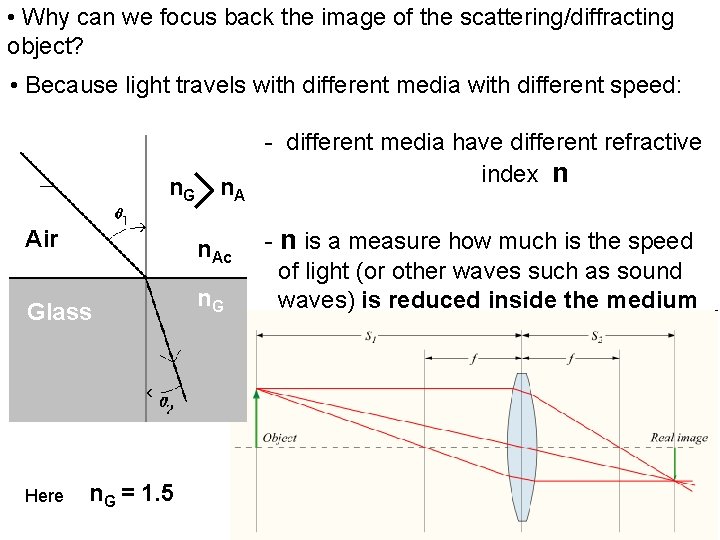
• Why can we focus back the image of the scattering/diffracting object? • Because light travels with different media with different speed: n. G > n > A Air n. Ac Glass n. G Here n. G = 1. 5 - different media have different refractive index n - n is a measure how much is the speed of light (or other waves such as sound waves) is reduced inside the medium

What should be the relationship between effective scattering and the size of the object ? Web examples http: //www. acoustics. salford. ac. uk/feschools/waves/super. htm#phase The power of scattering/diffraction by an object is: - directly proportional to the similarity between the wavelength of the incident radiation and the size of the scattering object - Larger object – larger waves needed for an effective scattering - Smaller objects – smaller wave needed for an effective scattering

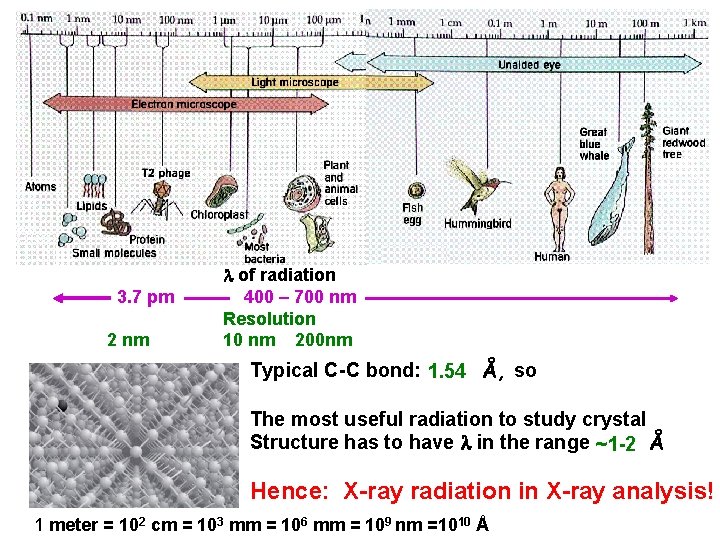
3. 7 pm 2 nm of radiation 400 – 700 nm Resolution 10 nm 200 nm Typical C-C bond: 1. 54 Å, so The most useful radiation to study crystal Structure has to have in the range ~1 -2 Å Hence: X-ray radiation in X-ray analysis! 1 meter = 102 cm = 103 mm = 106 mm = 109 nm =1010 Å

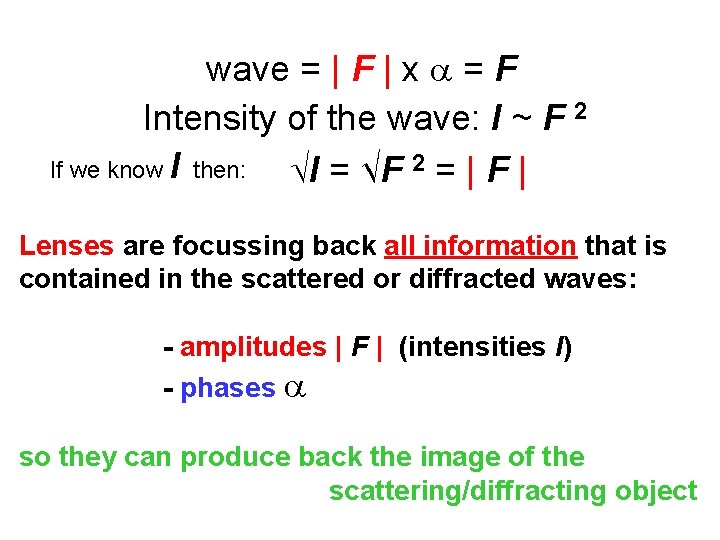
wave = | F | x = F Intensity of the wave: I ~ F 2 If we know I then: I = √F 2 = | F | Lenses are focussing back all information that is contained in the scattered or diffracted waves: - amplitudes | F | (intensities I) - phases so they can produce back the image of the scattering/diffracting object

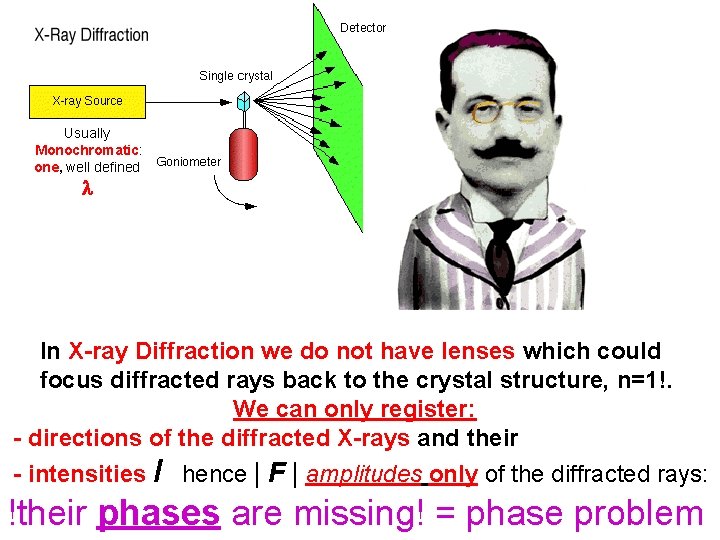
What? !! Usually Monochromatic: one, well defined NO LENSES!!!! In X-ray Diffraction we do not have lenses which could focus diffracted rays back to the crystal structure, n=1!. We can only register: - directions of the diffracted X-rays and their - intensities I hence | F | amplitudes only of the diffracted rays: !their phases are missing! = phase problem

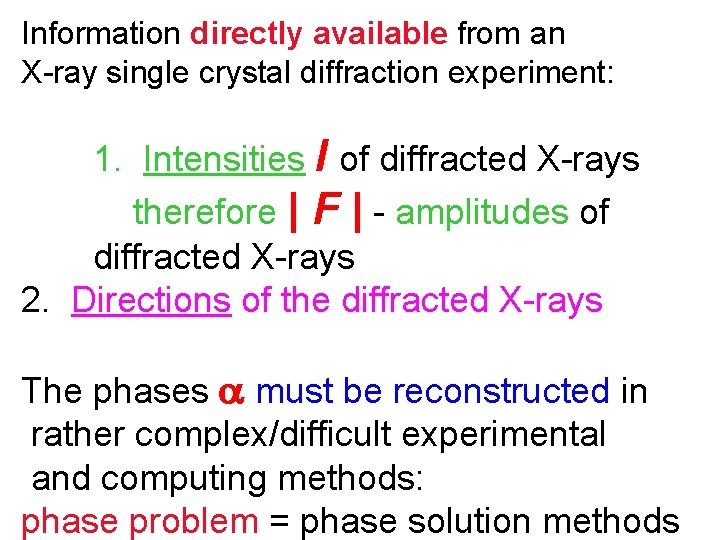
Information directly available from an X-ray single crystal diffraction experiment: 1. Intensities I of diffracted X-rays therefore | F | - amplitudes of diffracted X-rays 2. Directions of the diffracted X-rays The phases must be reconstructed in rather complex/difficult experimental and computing methods: phase problem = phase solution methods

The key-feature of XRD and XRA is the interaction between the crystal and the incoming X-ray radiation (l in range off 0. 8 – 2 Å). X-rays in the crystal are: • scattered by the electrons: - Thomson scattering: the electron oscillates in the electric field of the incoming X-ray beam and an oscillating electric charge radiates electromagnetic waves - this is elastic and coherent scattering: frequencies and wavelengths of the incoming X-rays and scattered-diffracted X-rays are the same/unchanged • this scattering is becoming very discreet in terms of directions • some scattered X-ray waves are reinforced, some weakened • as we are dealing here with the diffraction - REFLECTIONS • which is amplified by millions copies of the same atoms (electrons!) in the same positions in the crystal space due to crystal periodic, repetitive (in 3 -D) unique character But why not to measure scattering from one molecule and determine its structure this way? …. .

• We cannot measure (yet) the X-ray scattering produced by single chemical entity (organic molecule): it is too weak. We use crystals as 3 -D amplifiers of scattering coming from single crystal motif. X-ray Diffraction is well welcomed “side effect” of this process due to amplifying or cancelling effect of scattered radiation emitted by electrons There also other types of interactions of X-rays with electrons: e. g. excitations. These type of high energy phenomena would damage the single molecule almost immediately. In crystal there are thousands of molecules – some of them survive long enough to give a measurable radiation. .

v

v

= Crystal structure = Crystal Lattice + + motif The 3 -D periodicity of the crystal can be simplified and represented by an abstract crystal lattice.

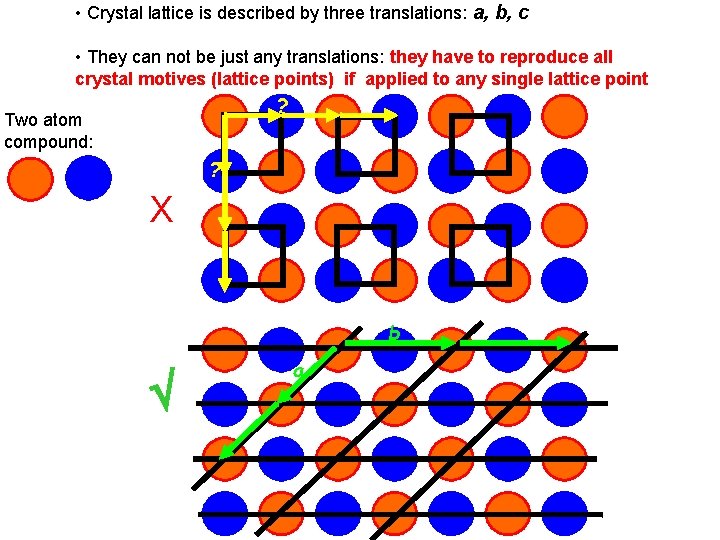
• Crystal lattice is described by three translations: a, b, c • They can not be just any translations: they have to reproduce all crystal motives (lattice points) if applied to any single lattice point Two atom compound: ? It is NOT the unit cell ? X b It is the right unit cell a

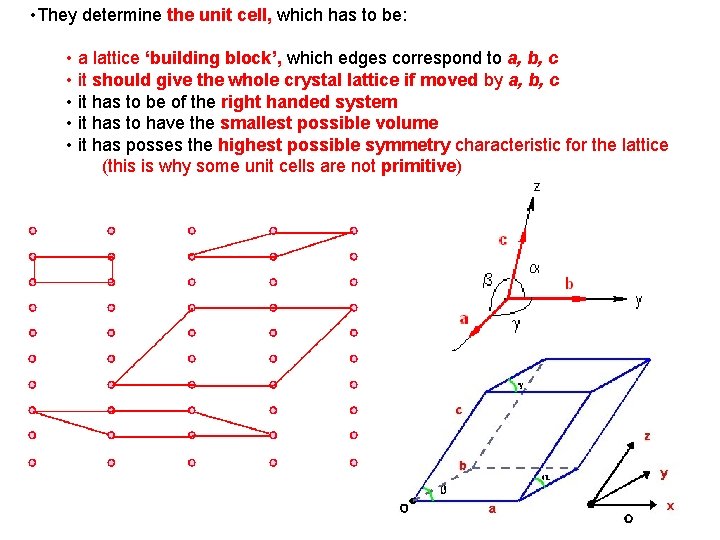
• They determine the unit cell, which has to be: • a lattice ‘building block’, which edges correspond to a, b, c • it should give the whole crystal lattice if moved by a, b, c • it has to be of the right handed system • it has to have the smallest possible volume • it has posses the highest possible symmetry characteristic for the lattice (this is why some unit cells are not primitive)

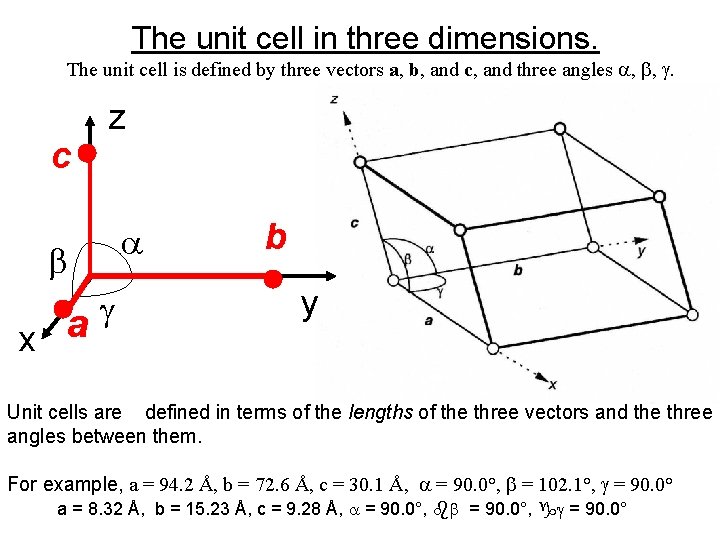
The unit cell in three dimensions. The unit cell is defined by three vectors a, b, and c, and three angles , , . c z x a b y Unit cells are defined in terms of the lengths of the three vectors and the three angles between them. For example, a = 94. 2 Å, b = 72. 6 Å, c = 30. 1 Å, = 90. 0°, = 102. 1°, = 90. 0° a = 8. 32 Å, b = 15. 23 Å, c = 9. 28 Å, = 90. 0°, = 90. 0°

Content of the Unit cell c Motif = molecule, atoms Size and the arrangement (symmetry) of the unit cell Crystal structure To get the structure of the motive we have to: get the information about the unit cell size and its arrangement

In the crystal lattice we can distinguish: - lattice points - lattice directions - lattice planes Co-ordinates of the lattice points are given in the fractions u, v, w of the a, b, c lattice translations z y c a x b (u, v, w)
![Crystal Lattice directions symbol uvw examples 100 010 001 z c a Crystal Lattice directions symbol: [uvw] examples: [100] [010] [001] : z : c a](https://slidetodoc.com/presentation_image_h/ba0d9c550cb0c9af3741453d50d71dae/image-24.jpg)
Crystal Lattice directions symbol: [uvw] examples: [100] [010] [001] : z : c a b c y x a b

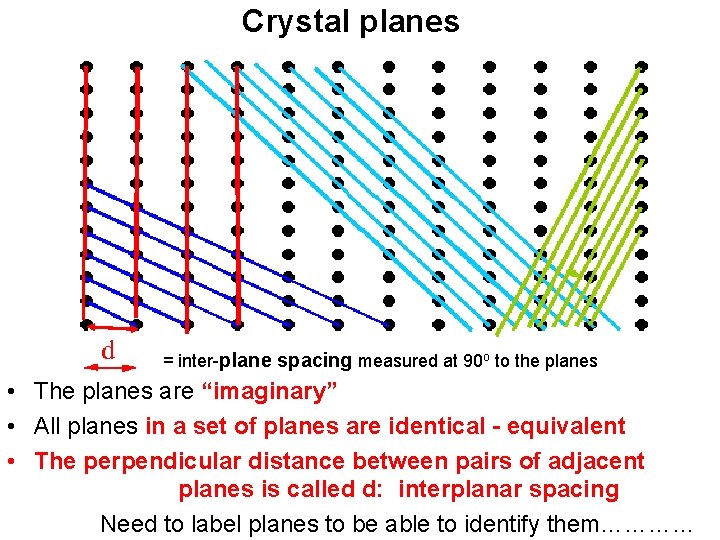
Crystal planes = inter-plane spacing measured at 90 o to the planes • The planes are “imaginary” • All planes in a set of planes are identical - equivalent • The perpendicular distance between pairs of adjacent planes is called d: interplanar spacing Need to label planes to be able to identify them…………

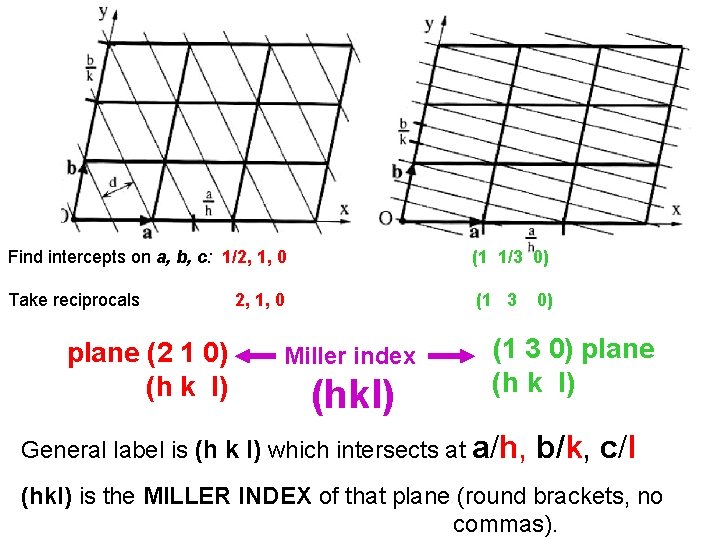
Find intercepts on a, b, c: 1/2, 1, 0 (1 1/3 0) Take reciprocals (1 3 plane (2 1 0) (h k l) 2, 1, 0 Miller index (hkl) 0) (1 3 0) plane (h k l) General label is (h k l) which intersects at a/h, b/k, c/l (hkl) is the MILLER INDEX of that plane (round brackets, no commas).

z Standard triangle 1 c O x 1 a z (111) 1 c y 1 b O x 1 a (222) y 1 b

Plane perpendicular to x cuts at 1, , (1 0 0) plane c c b b a a c (0 1 0) plane (0 0 1) plane a b NB an index 0 means that the plane is parallel to that axis

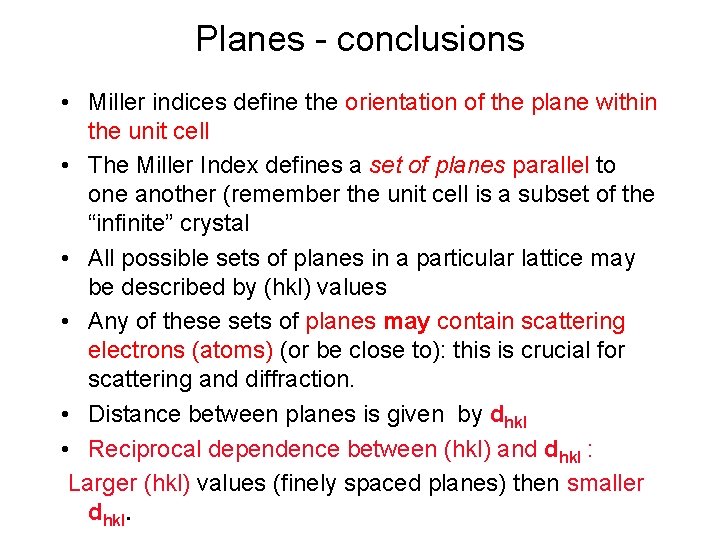
Planes - conclusions • Miller indices define the orientation of the plane within the unit cell • The Miller Index defines a set of planes parallel to one another (remember the unit cell is a subset of the “infinite” crystal • All possible sets of planes in a particular lattice may be described by (hkl) values • Any of these sets of planes may contain scattering electrons (atoms) (or be close to): this is crucial for scattering and diffraction. • Distance between planes is given by dhkl • Reciprocal dependence between (hkl) and dhkl : Larger (hkl) values (finely spaced planes) then smaller dhkl.

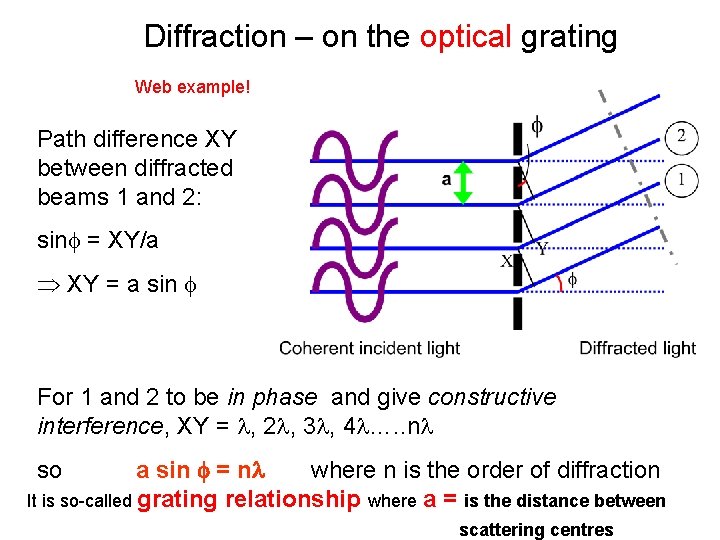
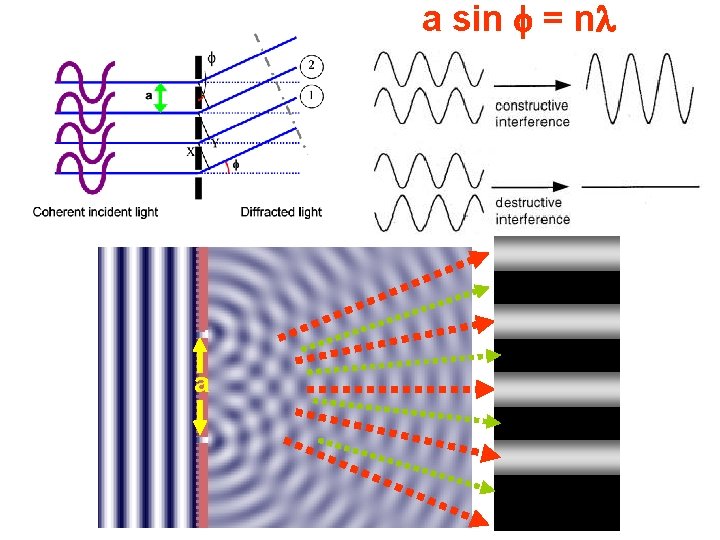
Diffraction – on the optical grating Web example! Path difference XY between diffracted beams 1 and 2: sin = XY/a XY = a sin For 1 and 2 to be in phase and give constructive interference, XY = , 2 , 3 , 4 …. . n a sin = n where n is the order of diffraction It is so-called grating relationship where a = is the distance between so scattering centres

a sin = n a

Principles of BRAGG X-ray diffraction experiment: diffracted X-rays Crystal Incoming X-ray Non-diffracted X-rays (97%) detector

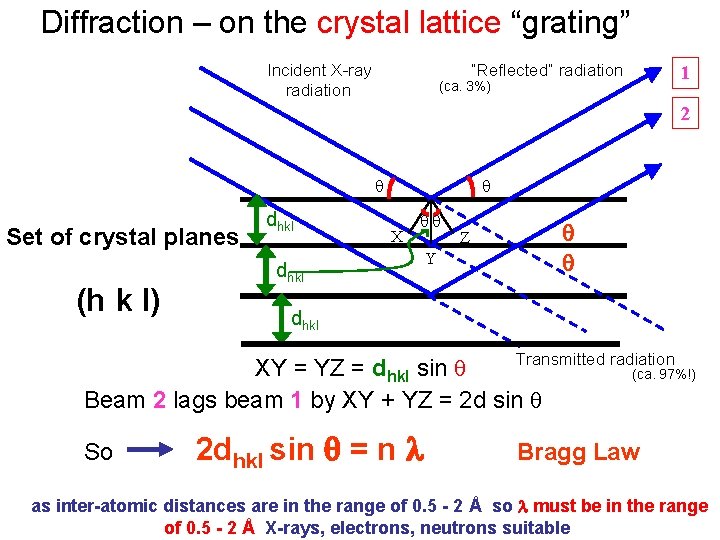
Diffraction – on the crystal lattice “grating” Incident X-ray radiation “Reflected” radiation (ca. 3%) 1 2 Set of crystal planes (h k l) dhkl X dhkl Y Z dhkl Transmitted radiation XY = YZ = dhkl sin (ca. 97%!) Beam 2 lags beam 1 by XY + YZ = 2 d sin So 2 dhkl sin = n Bragg Law as inter-atomic distances are in the range of 0. 5 - 2 Å so must be in the range of 0. 5 - 2 Å X-rays, electrons, neutrons suitable

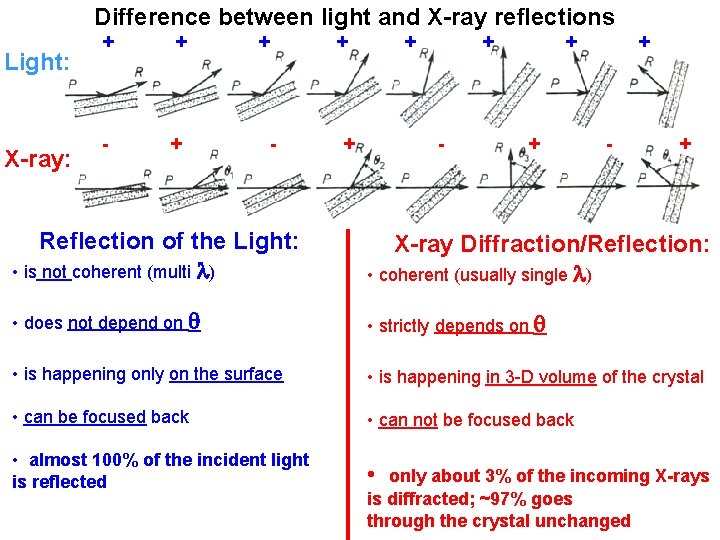
Difference between light and X-ray reflections Light: X-ray: + + - + Reflection of the Light: • is not coherent (multi ) X-ray Diffraction/Reflection: • coherent (usually single ) • does not depend on • strictly depends on • is happening only on the surface • is happening in 3 -D volume of the crystal • can be focused back • can not be focused back • almost 100% of the incident light is reflected • only about 3% of the incoming X-rays is diffracted; ~97% goes through the crystal unchanged
 Crystalline vs non crystalline
Crystalline vs non crystalline Atom and its structure
Atom and its structure Insidan region jh
Insidan region jh Crystal solid and amorphous solid
Crystal solid and amorphous solid Definition of crystalline
Definition of crystalline Chem
Chem Anisotropic
Anisotropic Crystalline substances
Crystalline substances What is crystalline candy
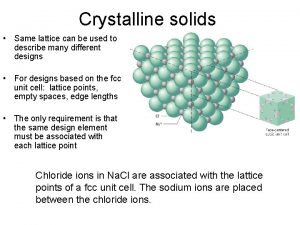
What is crystalline candy Crystalline solid
Crystalline solid Cengage
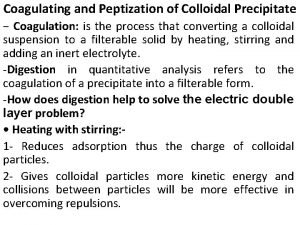
Cengage Difference between colloidal and crystalline precipitate
Difference between colloidal and crystalline precipitate Difference between colloidal and crystalline precipitate
Difference between colloidal and crystalline precipitate Gravimetric methods of analysis
Gravimetric methods of analysis Poser un rivet pop
Poser un rivet pop Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Crystalline silicate clays
Crystalline silicate clays Crystalline solid
Crystalline solid Destiny 2 crystalline formations
Destiny 2 crystalline formations Ccl
Ccl Largest crystals
Largest crystals Diluvent
Diluvent How to grow sugar crystals
How to grow sugar crystals Are crystals pure substances
Are crystals pure substances Kato-katz and kato thick procedure
Kato-katz and kato thick procedure Why do ionic bonds form
Why do ionic bonds form Copper ii sulphate crystals
Copper ii sulphate crystals Hcp crystallographic directions
Hcp crystallographic directions Negative birefringent crystals meaning
Negative birefringent crystals meaning Lattice imperfection
Lattice imperfection Point defects in crystals
Point defects in crystals Identification test for tragacanth
Identification test for tragacanth Phonon momentum
Phonon momentum What is ideal crystal
What is ideal crystal Sugar crystals experiment conclusion
Sugar crystals experiment conclusion