Chapter 8 Gravimetric Methods of Analysis Gravimetric Methods










- Slides: 14

Chapter 8 Gravimetric Methods of Analysis

Gravimetric Methods of Analysis • • • -Gravimetric methods of analysis are based on the measurement of mass -Two major types of gravimetric methods -Precipitation Methods -Analyte is converted into sparingly soluble precipitate • -Precipitate is then filtered, washed free of impurities, and converted to a product of known composition by suitable heat treatment, and the product is weighed -Volatilization Methods • -The analyte or its decomposition products are volatilized at a suitable temperature • -The volatile product is then collected and weighed

8 A—Properties of precipitates and Precipitating Agents • • • -Ideally, a gravimetric precipitating agent should react specifically or at least selectively with the analyte -Specific reagents(rare) react only with a single chemical species -Selective reagents(more common) react with only a limited number of species -Characteristics of Ideal Precipitating Reagents 1 -Readily filtered and washed free of contaminants • 2 -Of sufficiently low solubility so that no significant loss of the solid occurs during filtration and washing • 3 -Unreactive with constituents in the atmosphere • 4 -Of known composition after it is dried or ignited

-8 A-1 Particle Size and Filterability of Precipitates • -Precipitates made up of large particles are generally desirable in gravimetric analysis because large particles are easier to filter and wash free of impurities. Also, they are usually purer than precipitates made up of finer particles • -What Factors Determine Particle Size? • -Colloidal suspensions 1 e-7 to 1 e-4 cm in diameter • -No tendency to settle from solution, not easily filtered • -Crystalline suspension > 1/10 mm in diameter • -Settle spontaneously and readily filtered – -Particle size is influenced by precipitate solubility, temperature, reactant concentrations, and the rate at which reactants are mixed

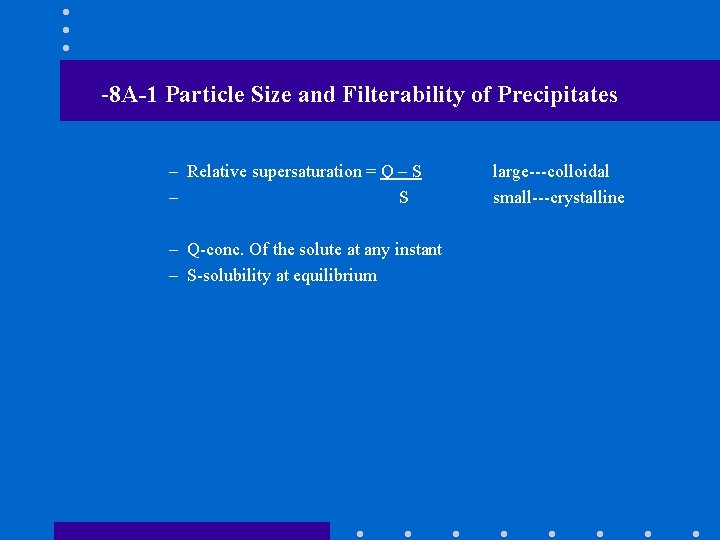
-8 A-1 Particle Size and Filterability of Precipitates – Relative supersaturation = Q – S – Q-conc. Of the solute at any instant – S-solubility at equilibrium large---colloidal small---crystalline

-8 A-1 Particle Size and Filterability of Precipitates • • -How Do Precipitates Form? • -Nucleation is a process in which a minimum number of atoms, ions, or molecules join together to form a stable solid • -Precipitates form by nucleation and particle growth • -If nucleation predominates, a large number of small particles result • -If particle growth predominates, a smaller number of large particles result -Controlling Particle Size -Temperature elevation can increase solubility of the precipitate -Larger particles can be obtained by p. H control

-8 A-2 Colloidal Precipitates • -Individual colloidal particles are so small that they are not retained by ordinary filters and Brownian motion prevents settling • -Coagulation of Colloids – -Coagulation can be hastened by heating, stirring, and adding an electrolyte to the medium. – -Colloidal suspensions are stable because all the particles present are either positively or negatively charged – -Adsorption is a process in which a substance is held on the surface of a solid – -Absorption involves retention of a substance within the pores of a solid – -The charge on a colloidal particle formed in a gravimetric analysis is determined by the charge of the lattice ion that is in excess when the precipitation is complete

-8 A-2 Colloidal Precipitates • • • -Peptization of Colloids • -Peptization is a process by which a coagulated colloid returns to its dispersed state -Occurs when a coagulated colloid is washed -Treatment of Colloidal Precipitates • -Colloids are best precipitated from hot, stirred solutions containing sufficient electrolyte to ensure coagulation • -Digestion is a process in which a precipitate is heated for an hour or more in the solution from which it is formed

-8 A-3 Crystalline Precipitates • -Crystalline precipitates are generally more easily filtered and purified than coagulated colloids • -Improving Particle Size and Filterability – -The particle size and filterability of crystalline solids can be improved by minimizing Q and/or maximizing S – -Digestion improves the purity and filterability of crystalline solids

-8 A-4 Coprecipitation • • • -Coprecipitation is a process in which normally soluble compounds are carried out of solution by a precipitate • 1 -Surface Adsorption – -Major source of contamination in coagulated colloids, but is of no significance in crystalline precipitates 2 -Mixed-Crystal Contamination – -Type of coprecipitation in which a contaminant ion replaces an ion in the lattice of a crystal 3 -Occlusion and Mechanical Entrapment -Compound is trapped within a pocket formed during rapid crystal growth 4 -Coprecipitation Errors -Coprecipitation cause either positive or negative errors

-8 A-5 Precipitation from Homogeneous Solution • -Homogeneous precipitation is a process in which a precipitate is formed by slow generation of a precipitating reagent homogeneously throughout a solution • -Solids formed by homogeneous precipitation are generally purer and more easily filtered than precipitates generated by direct addition of a reagent to the analyte solution

8 B—Drying and Ignition of Precipitates • -After filtration, a gravimetric precipitate is heated until its mass is constant(weighing form) • -The temperature required to dehydrate a precipitate may be as low as 100°C or as high as 1000°C • -Recording thermal decomposition curves is often called thermogravimetry or thermal gravimetric analysis, and the mass vs. temp. curves generated are called thermograms

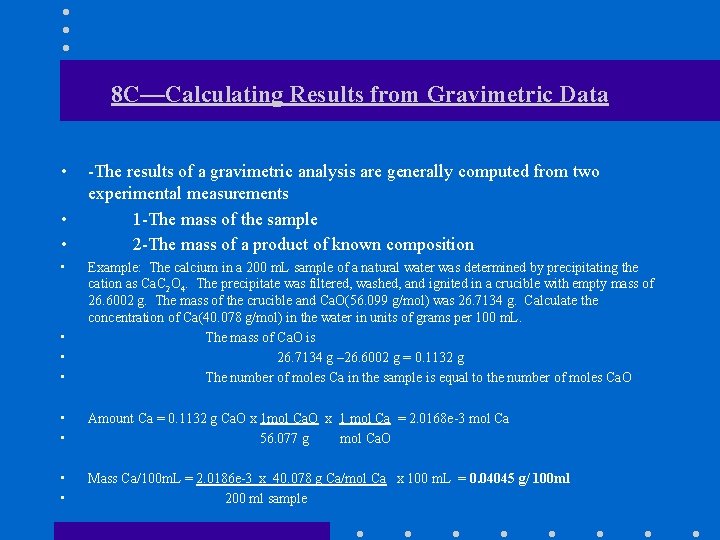
8 C—Calculating Results from Gravimetric Data • • -The results of a gravimetric analysis are generally computed from two experimental measurements 1 -The mass of the sample 2 -The mass of a product of known composition • • • Example: The calcium in a 200 m. L sample of a natural water was determined by precipitating the cation as Ca. C 2 O 4. The precipitate was filtered, washed, and ignited in a crucible with empty mass of 26. 6002 g. The mass of the crucible and Ca. O(56. 099 g/mol) was 26. 7134 g. Calculate the concentration of Ca(40. 078 g/mol) in the water in units of grams per 100 m. L. The mass of Ca. O is 26. 7134 g – 26. 6002 g = 0. 1132 g The number of moles Ca in the sample is equal to the number of moles Ca. O • • Amount Ca = 0. 1132 g Ca. O x 1 mol Ca. O x 1 mol Ca = 2. 0168 e-3 mol Ca 56. 077 g mol Ca. O • • Mass Ca/100 m. L = 2. 0186 e-3 x 40. 078 g Ca/mol Ca x 100 m. L = 0. 04045 g/ 100 ml 200 ml sample

8 D—Applications of Gravimetric Methods • • • 1 -Inorganic Precipitating Agents 2 -Reducing Agents 3 -Organic Precipitating Agents 4 -Organic Functional Group Analysis 5 -Volatilization Methods