Chapter 1 Crystal Structure Chapter 1 Crystal Structure























- Slides: 71

Chapter 1: Crystal Structure

Chapter 1: Crystal Structure The Nobel “Booby” Prize! See the “Ig Nobel” Prize discussed at: http: //improbable. com/ig/

The (Common) Phases of Matter Gases Liquids & Liquid Crystals Solids “Condensed Matter” includes both of these. Our focus is Solids! This doesn’t include Plasmas, or Bose-Einstein condensates but these three are the “common” phases!!

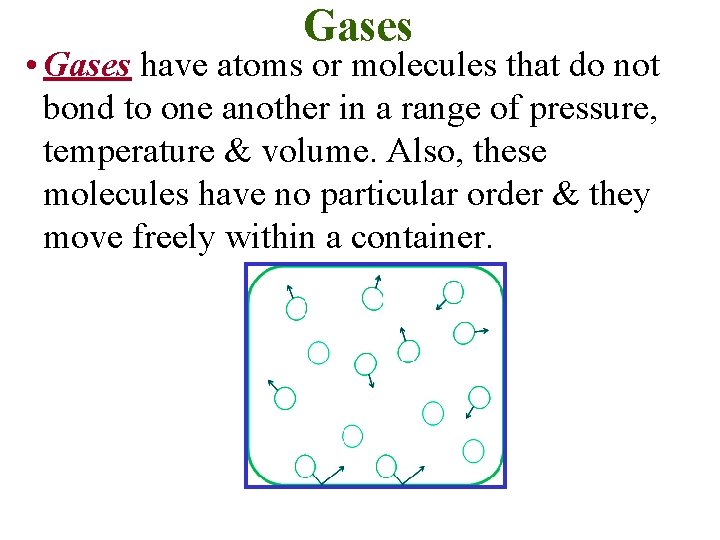
Gases • Gases have atoms or molecules that do not bond to one another in a range of pressure, temperature & volume. Also, these molecules have no particular order & they move freely within a container.

Liquids • Similar to gases, Liquids have no atomic or molecular order & they assume the shape of their containers. • Applying low levels of thermal energy can easily break the existing weak bonds.

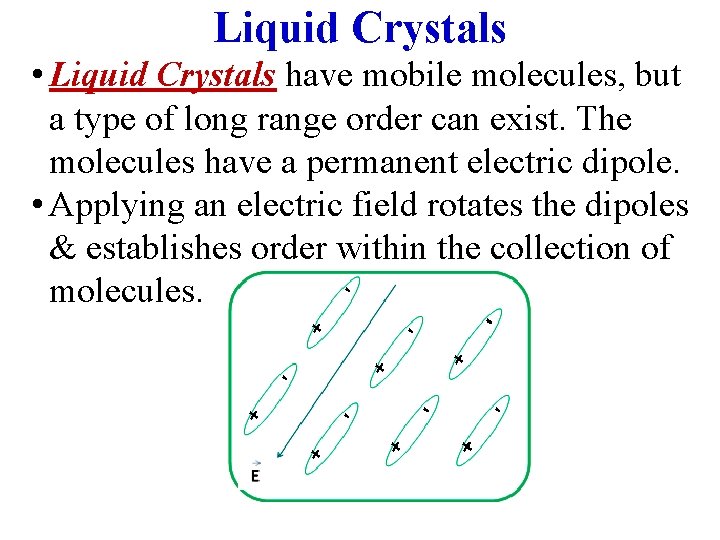
Liquid Crystals • Liquid Crystals have mobile molecules, but a type of long range order can exist. The molecules have a permanent electric dipole. • Applying an electric field rotates the dipoles & establishes order within the collection of molecules.

Solids • Solids consist of atoms or molecules undergoing thermal motion about their equilibrium positions, which are at fixed points in space. • Solids can be crystalline, polycrystalline, or amorphous.

Solids • Solids (at a given temperature, pressure, volume) have stronger interatomic bonds than liquids. • So, Solids require more energy to break the interatomic bonds than liquids.

Crystallography & Crystalline Solids • The following material covers most of the topics in Ch. 1 (Crystal Structure) in the book by Kittel. • In the Supplemental book by Omar, the corresponding material is in Ch. 1, (Crystal Structures & Interatomic Forces). • However, as will be true throughout the course, my discussion will use a large variety of sources other than those books.

Crystal Structure Topics 1. Periodic Arrays of Atoms 2. Fundamental Types of Lattices 3. Index System for Crystal Planes 4. Simple Crystal Structures 5. Direct Imaging of Crystal Structure 6. Non-ideal Crystal Structures 7. Crystal Structure Data

Objectives At the end of this Chapter, you should: 1. Be able to identify a unit cell in a symmetrical pattern. 2. Know that (in 3 dimensions) there are 7 (& ONLY 7!!) Possible unit cell shapes. 3. Be able to define cubic, tetragonal, orthorhombic & hexagonal unit cell shapes

Preliminary Remarks on Crystalline Solids • This material is an overview of the many types of crystalline solids & their crystal structures. • Mostly, this involves some simple mathematics of symmetry. However, it also involves learning some terminology & nomenclature.

• Unfortunately (in my opinion!), the first scientists to be interested in crystals were geologists & crystallographers & not physicists. • So, in order to "speak the language" of crystals, we must use the terminology that they developed. • I sometimes find that inconvenient & abstract.

• Despite this, understanding that language is necessary in order for us to be able discuss the PHYSICS of crystalline solids. • NOTE!! There a huge number of websites that can be used to supplement the textbook discussions & my lectures. • A list of some is posted on the Lecture Page. • We’ll spend 3 or 4 lectures on these topics. Some Lectures on this material are also posted on the Lecture Page. Some of these may undergo some revisions as we proceed. A homework assignment on this material will be given out soon.

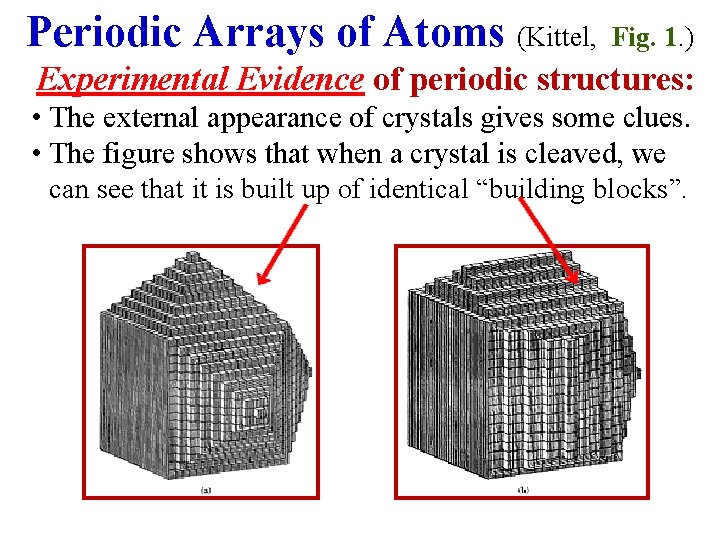
Periodic Arrays of Atoms (Kittel, Fig. 1. ) Experimental Evidence of periodic structures: • The external appearance of crystals gives some clues. • The figure shows that when a crystal is cleaved, we can see that it is built up of identical “building blocks”.

Experimental Evidence of periodic structures. • The early crystallographers noted that the index numbers that define plane orientations are exact integers. Cleaving a Crystal

Elementary Crystallography Solid Material Types Crystalline Polycrystalline Single Crystals Amorphous

Crystals are Everywhere!

More Crystals

Still More Crystals

Still More Crystals

Early ideas • All crystals are solids, but all solids are not crystalline! Crystals have symmetry (As first reported by Kepler who studied snowflakes!!) & long range order • Spheres & small shapes can be packed to produce regular shapes.

What are Crystals? • A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly, repeating pattern extending in all three spatial dimensions.

Crystallography The Study of Crystals. • Scientists who specialize in the study of crystals are called crystallographers. • Early studies of crystals were carried out by mineralogists & geologists who studied the symmetries and shapes (morphology) of naturallyoccurring mineral specimens.

Crystallography The Study of Crystals. • If you would like to see some beautiful crystals, just look around this floor of the building, where our Geosciences colleagues have displayed numerous rock crystal samples, with labels to tell us what they are. Note that these are naturally occurring crystals!

Crystallography • These early studies led to the correct idea that crystals are regular three-dimensional arrays (Bravais lattices) of atoms and molecules. • A single unit cell is repeated indefinitely along three principal directions that are not necessarily perpendicular.

Crystallography • Crystallography ≡ A branch of science dealing with the geometric description of crystals & their internal arrangements. • It is also the science of crystals & the math used to describe them.

Crystallography • Crystallography is a VERY OLD field which pre-dates Solid State Physics by about a century! So (unfortunately, in some ways!) much of the terminology (& theory notation) of Solid State Physics originated in crystallography.

Crystallography • The purpose of Ch. 1 of Kittel’s book is mainly to introduce this crystallography terminology & notation to you.

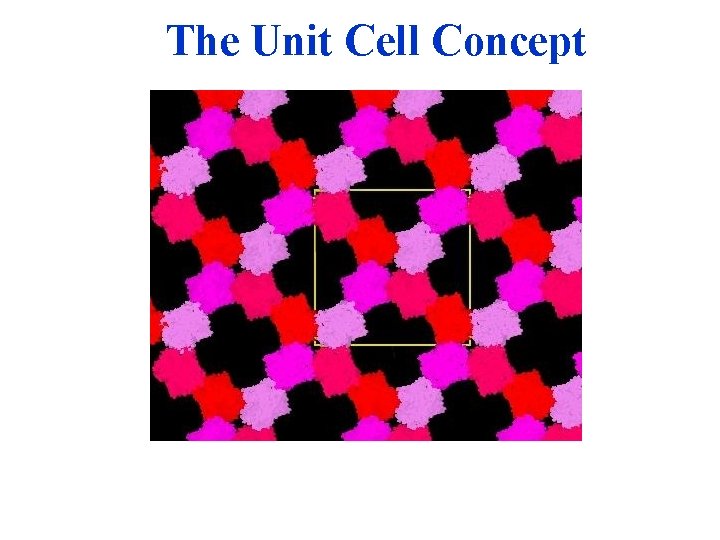
The Unit Cell Concept

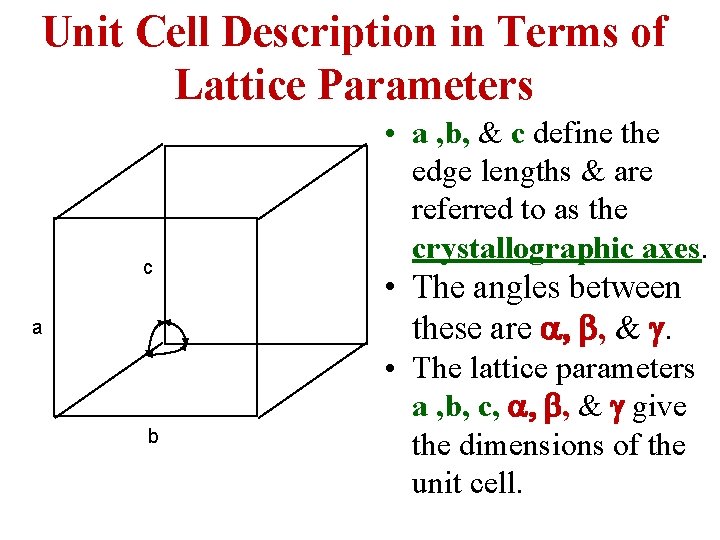
Unit Cell Description in Terms of Lattice Parameters c a b • a , b, & c define the edge lengths & are referred to as the crystallographic axes. • The angles between these are a, b, & g. • The lattice parameters a , b, c, a, b, & g give the dimensions of the unit cell.

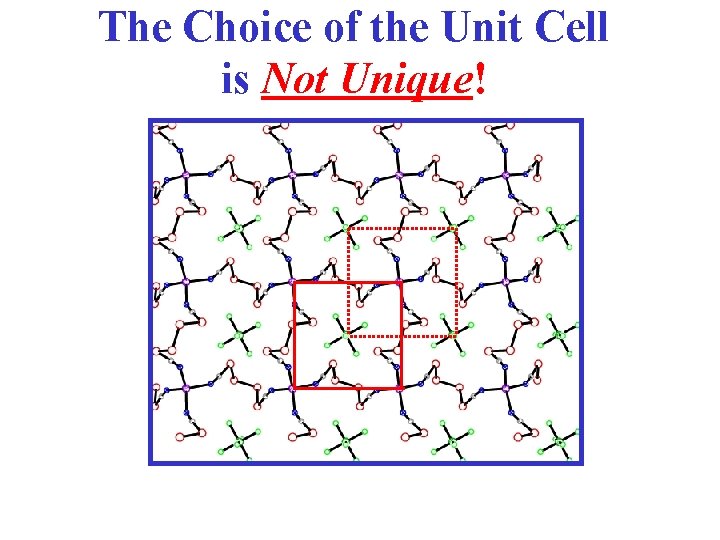
The Choice of the Unit Cell is Not Unique!

The Three General Types of Solids Single Crystal, Polycrystalline, Amorphous • Each type is characterized by the size of the ordered region within the material. An ordered region is a spatial volume in which atoms or molecules have a regular geometric arrangement or periodicity.

All Solids! • All solids have “resistance” to changes in both shape and volume. • Solids can be Crystalline or Amorphous • Crystals are solids that consist of a periodic array of atoms, ions, or molecules – If this periodicity is preserved over “large” (macroscopic) distances, the solid has “Long-range Order” • Amorphous solids do not have Long-Range Order, but they often have Short Range Order

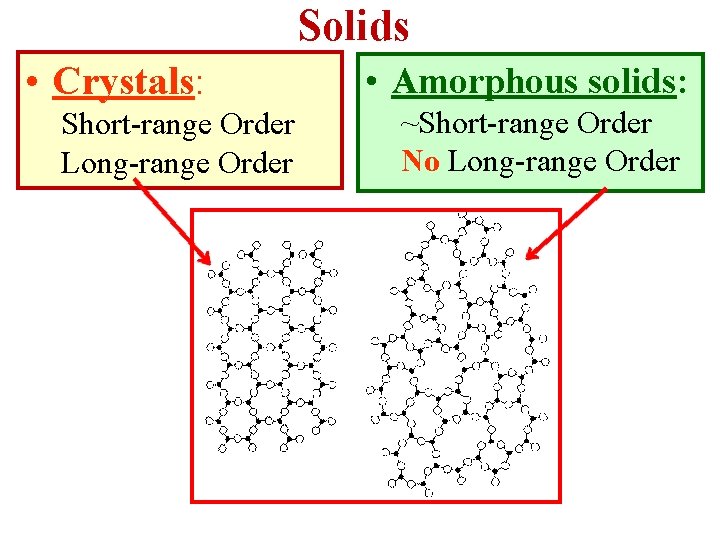
Solids • Crystals: Short-range Order Long-range Order • Amorphous solids: ~Short-range Order No Long-range Order

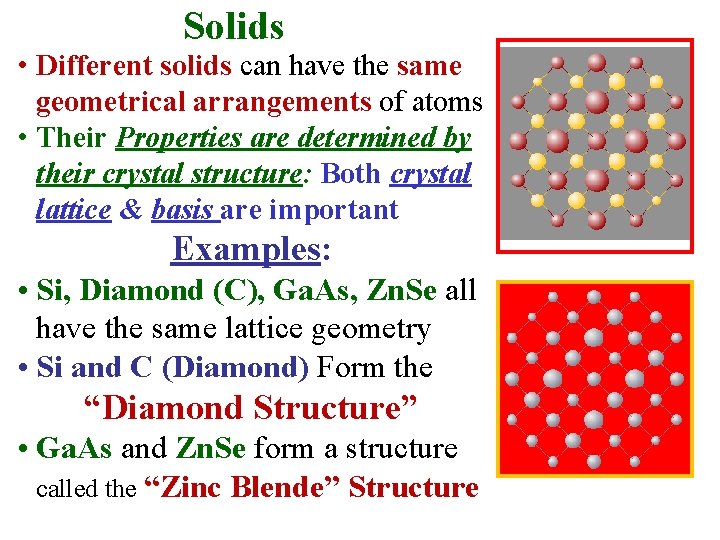
Solids • Different solids can have the same geometrical arrangements of atoms • Their Properties are determined by their crystal structure: Both crystal lattice & basis are important Examples: • Si, Diamond (C), Ga. As, Zn. Se all have the same lattice geometry • Si and C (Diamond) Form the “Diamond Structure” • Ga. As and Zn. Se form a structure called the “Zinc Blende” Structure

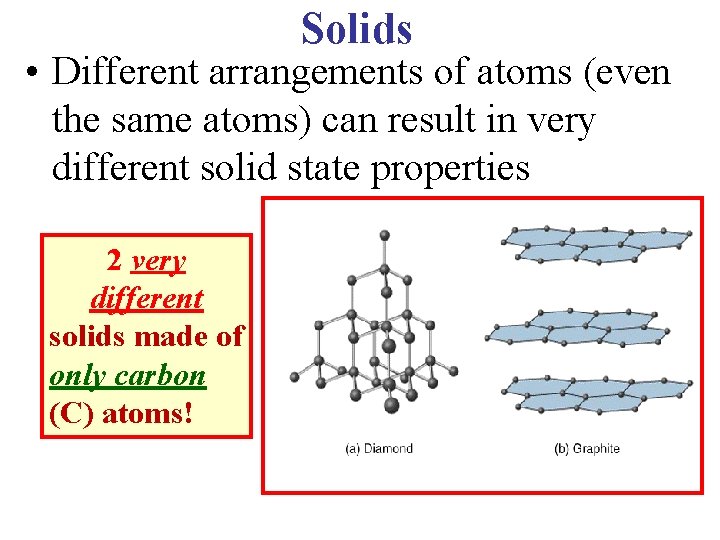
Solids • Different arrangements of atoms (even the same atoms) can result in very different solid state properties 2 very different solids made of only carbon (C) atoms!

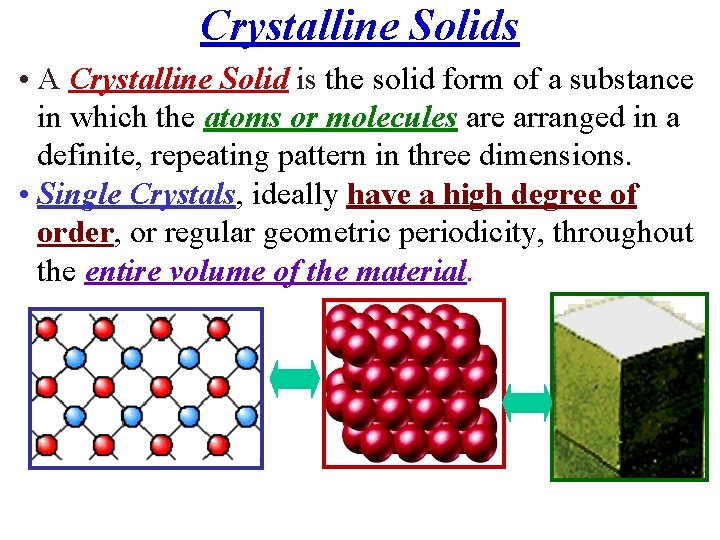
Crystalline Solids • A Crystalline Solid is the solid form of a substance in which the atoms or molecules are arranged in a definite, repeating pattern in three dimensions. • Single Crystals, ideally have a high degree of order, or regular geometric periodicity, throughout the entire volume of the material.

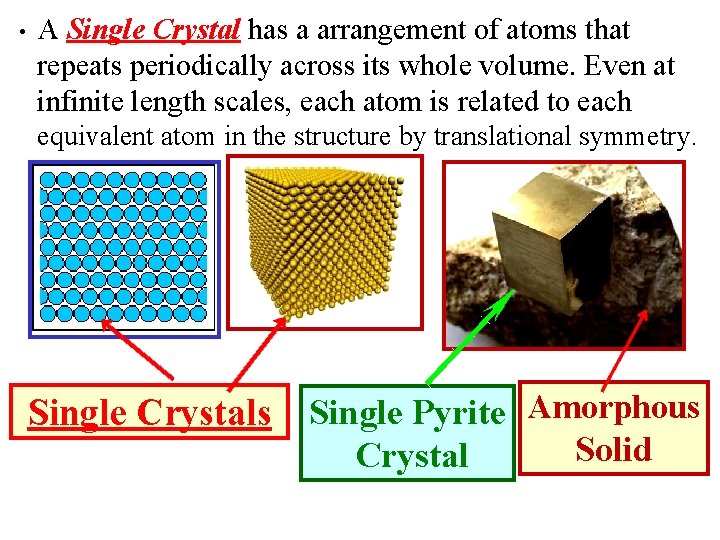
• A Single Crystal has a arrangement of atoms that repeats periodically across its whole volume. Even at infinite length scales, each atom is related to each equivalent atom in the structure by translational symmetry. Single Crystals Single Pyrite Amorphous Crystal Solid

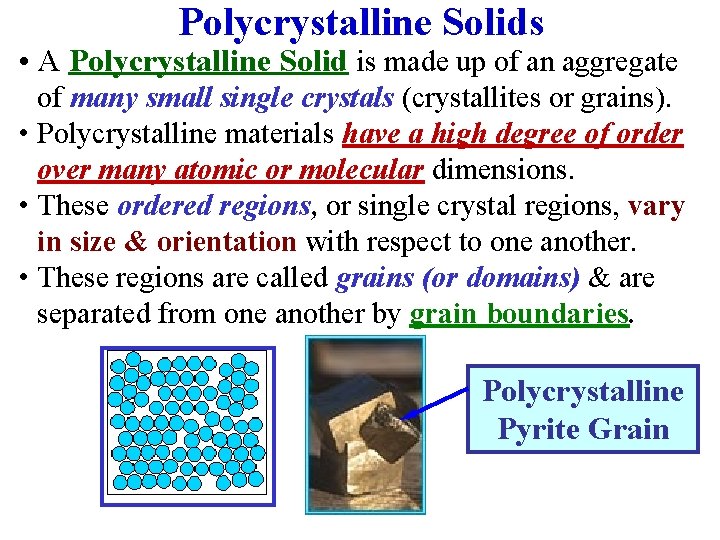
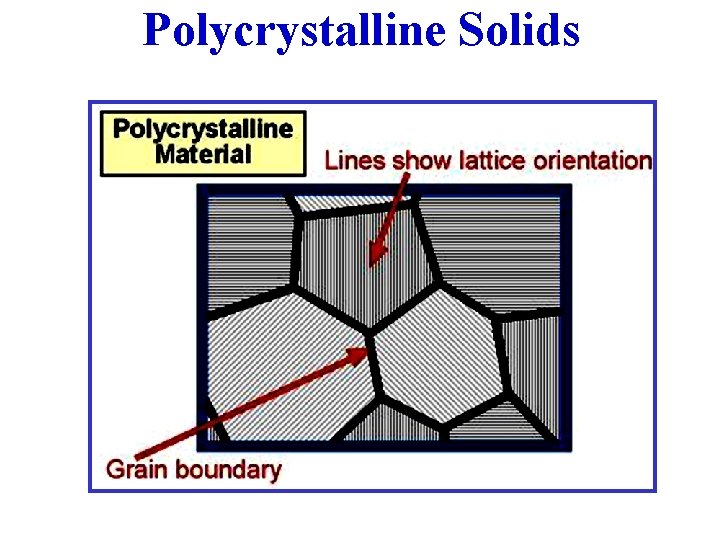
Polycrystalline Solids • A Polycrystalline Solid is made up of an aggregate of many small single crystals (crystallites or grains). • Polycrystalline materials have a high degree of order over many atomic or molecular dimensions. • These ordered regions, or single crystal regions, vary in size & orientation with respect to one another. • These regions are called grains (or domains) & are separated from one another by grain boundaries. Polycrystalline Pyrite Grain

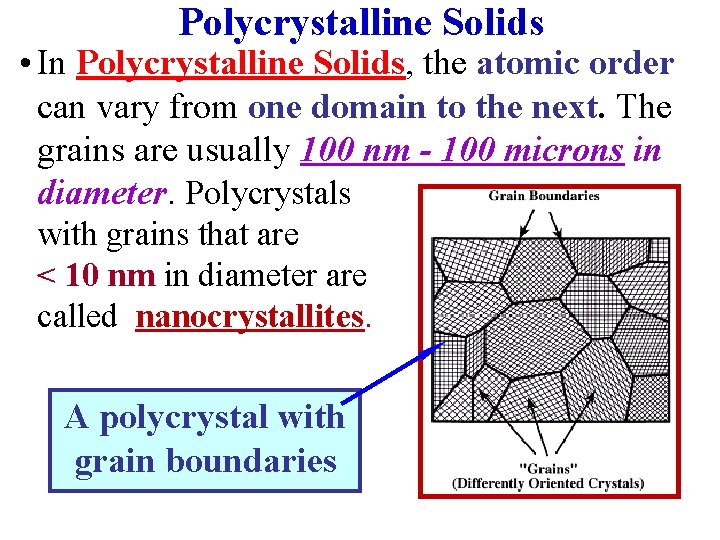
Polycrystalline Solids • In Polycrystalline Solids, the atomic order can vary from one domain to the next. The grains are usually 100 nm - 100 microns in diameter. Polycrystals with grains that are < 10 nm in diameter are called nanocrystallites. A polycrystal with grain boundaries

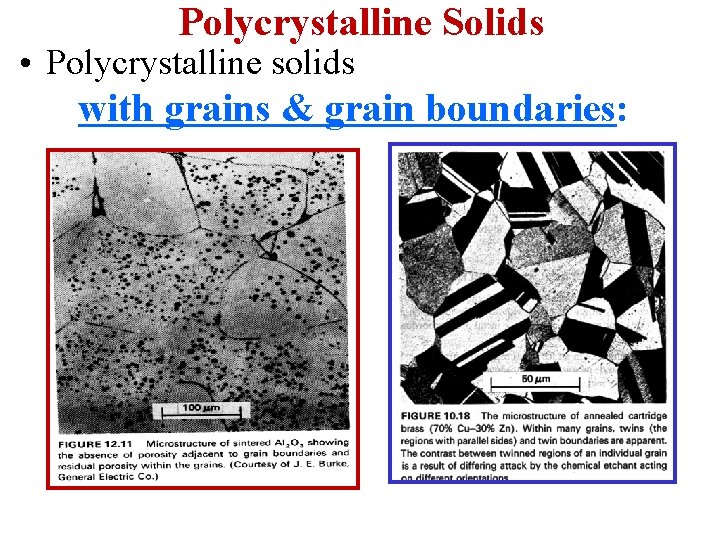
Polycrystalline Solids • Polycrystalline solids with grains & grain boundaries:

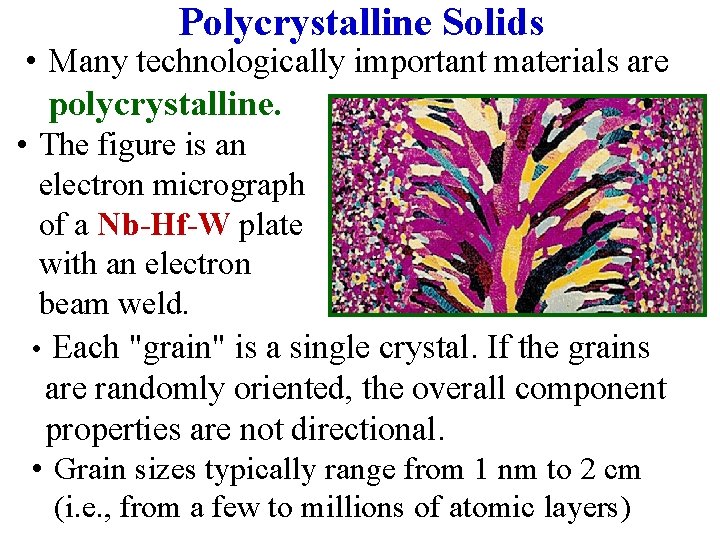
Polycrystalline Solids • Many technologically important materials are polycrystalline. • The figure is an electron micrograph of a Nb-Hf-W plate with an electron beam weld. • Each "grain" is a single crystal. If the grains are randomly oriented, the overall component properties are not directional. • Grain sizes typically range from 1 nm to 2 cm (i. e. , from a few to millions of atomic layers)

Polycrystalline Solids

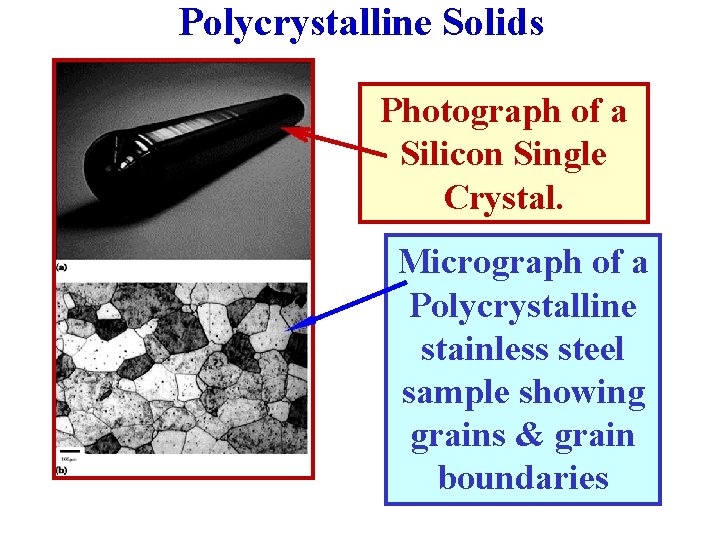
Polycrystalline Solids Photograph of a Silicon Single Crystal. Micrograph of a Polycrystalline stainless steel sample showing grains & grain boundaries

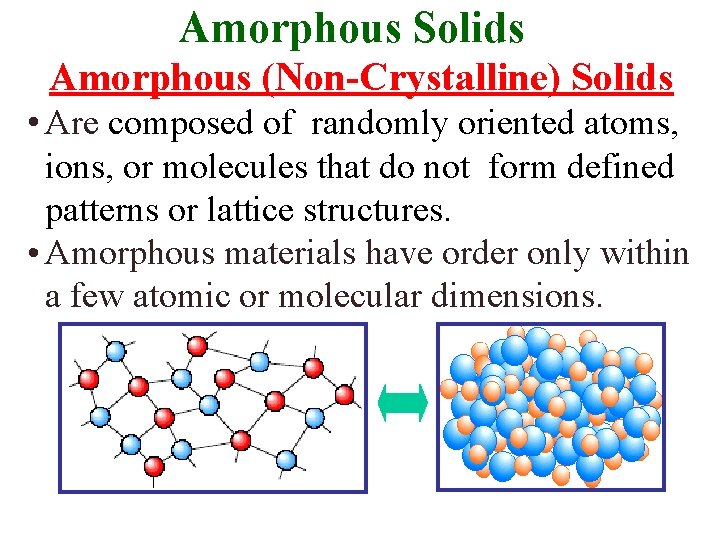
Amorphous Solids Amorphous (Non-Crystalline) Solids • Are composed of randomly oriented atoms, ions, or molecules that do not form defined patterns or lattice structures. • Amorphous materials have order only within a few atomic or molecular dimensions.

Amorphous Solids Amorphous (Non-crystalline) Solids • Have order only within a few atomic or molecular dimensions. They do not have any long-range order, but they have varying degrees of shortrange order. Examples of amorphous materials include amorphous silicon, plastics, & glasses.

Amorphous Solids Amorphous (Non-crystalline) Solids • Have no regular, long range order of arrangement of atoms. Some examples from everyday life: 1. Polymers, 2. Ceramics, 3. Window Glass, 4. “Cotton Candy”! • The two sub-states of amorphous solids are the Rubbery and Glassy states

Amorphous Solids Amorphous (Non-crystalline) Solids • Have no regular, long range order of arrangement of atoms. • Can be prepared by rapidly cooling molten material. Rapid cooling minimizes time for the atoms to pack into a more thermodynamically favorable crystalline state.

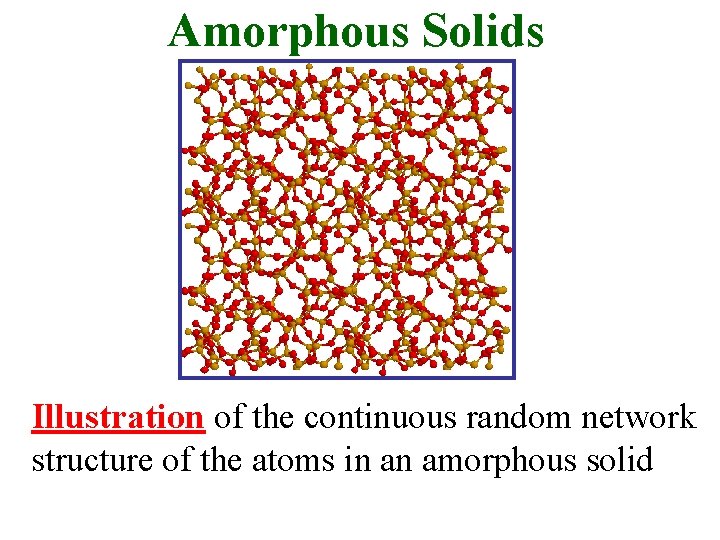
Amorphous Solids Illustration of the continuous random network structure of the atoms in an amorphous solid

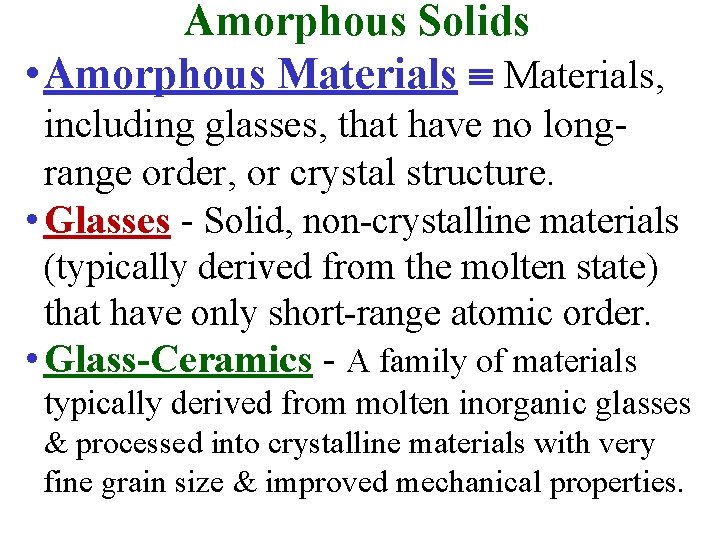
Amorphous Solids • Amorphous Materials, including glasses, that have no longrange order, or crystal structure. • Glasses - Solid, non-crystalline materials (typically derived from the molten state) that have only short-range atomic order. • Glass-Ceramics - A family of materials typically derived from molten inorganic glasses & processed into crystalline materials with very fine grain size & improved mechanical properties.

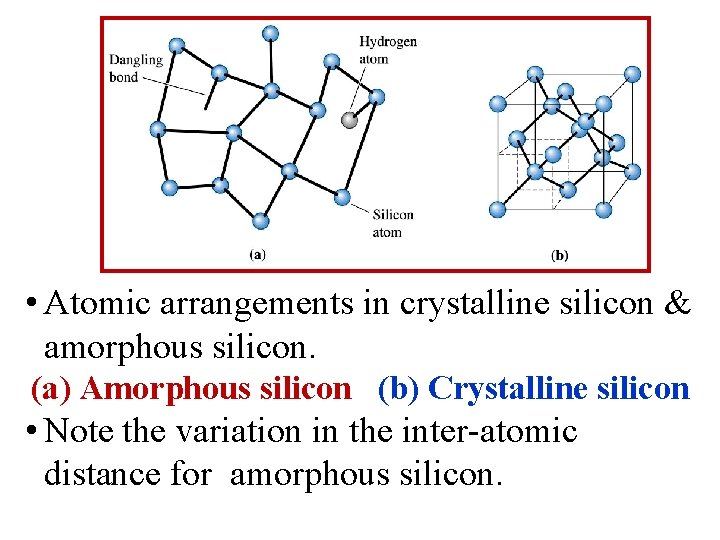
• Atomic arrangements in crystalline silicon & amorphous silicon. (a) Amorphous silicon (b) Crystalline silicon • Note the variation in the inter-atomic distance for amorphous silicon.

Crystals • The periodic array of atoms, ions, or molecules that form the solid is called the Crystal Structure Space (Crystal) Lattice + Basis • The Space (Crystal) Lattice is a regular periodic arrangement of POINTS in space, & is purely a mathematical abstraction. • A Crystal Structure is formed by “putting” the identical atoms (or group of atoms) on the points of the space lattice This group of atoms is called the Basis

Departures from the “Perfect Crystal” • A “Perfect Crystal” is an idealization that does not exist in nature. In some ways, even a crystal surface is an imperfection, because the periodicity is interrupted there. • We also know that each atom undergoes thermal vibrations around their equilibrium positions for temperatures T > 0 K. • These can be viewed as “imperfections”.

Departures from the “Perfect Crystal” • Real Crystals always have foreign atoms (impurities), missing atoms (vacancies), & atoms between lattice sites (interstitials) where they should not be. Each of these spoils the perfect crystal structure.

Solid State Physics • Started in the early 20 th Century when the fact that Crystals Can Diffract X-rays was discovered. • Around that same time, the new theory of Quantum Mechanics was being accepted & applied to various problems. Some of the early problems it was applied to were the explanation of observed X-ray diffraction patterns for various crystals & (later) the behavior of electrons in a crystalline solid.

Crystallography A Basic Knowledge of Elementary Crystallography is Essential for Solid State Physicists!!! • A crystal’s symmetry has a profound influence on many (most!) of its properties. • A crystal structure should be specified completely, concisely & unambiguously. • Structures are classified into different types according to the symmetries they possess. • In this course, we only consider solids with “simple” structures.

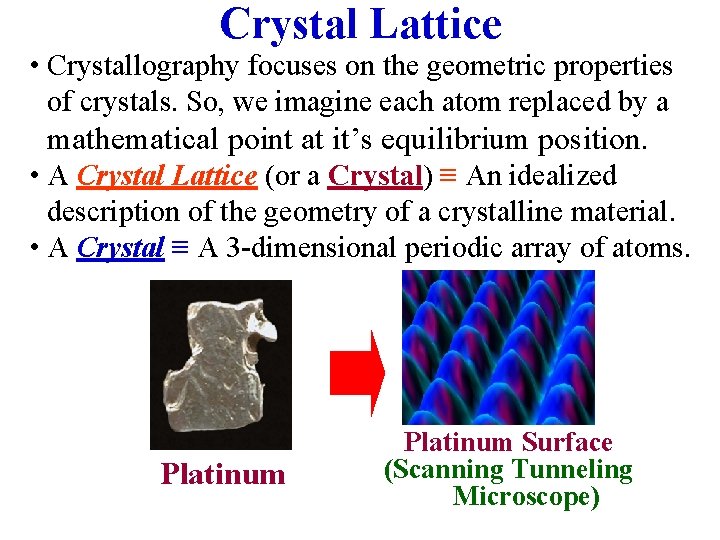
Crystal Lattice • Crystallography focuses on the geometric properties of crystals. So, we imagine each atom replaced by a mathematical point at it’s equilibrium position. • A Crystal Lattice (or a Crystal) ≡ An idealized description of the geometry of a crystalline material. • A Crystal ≡ A 3 -dimensional periodic array of atoms. Platinum Surface (Scanning Tunneling Microscope)

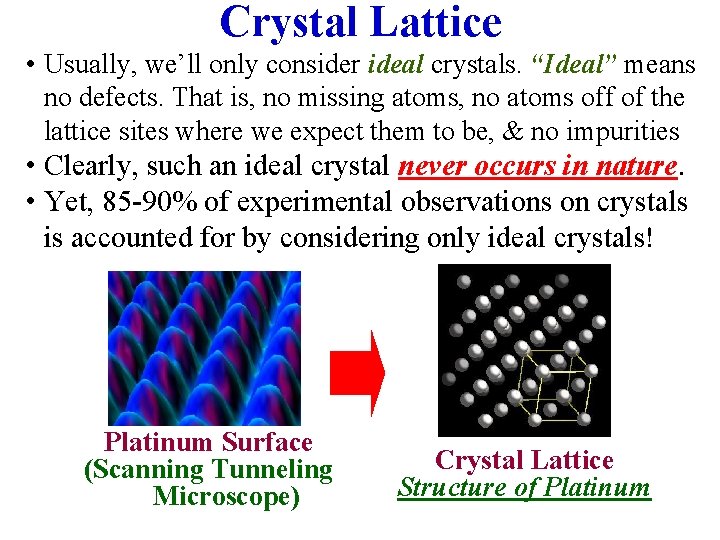
Crystal Lattice • Usually, we’ll only consider ideal crystals. “Ideal” means no defects. That is, no missing atoms, no atoms off of the lattice sites where we expect them to be, & no impurities • Clearly, such an ideal crystal never occurs in nature. • Yet, 85 -90% of experimental observations on crystals is accounted for by considering only ideal crystals! Platinum Surface (Scanning Tunneling Microscope) Crystal Lattice Structure of Platinum

Crystal Lattice Mathematically A Lattice is Defined as an Infinite Array of Points in Space in which each point has identical surroundings to all others. The points are arranged exactly in a periodic manner. A 2 Dimensional Example y B α b O C a D A x E

Ideal Crystal ≡ An infinite periodic repetition of identical structural units in space. • The simplest structural unit we can imagine is a Single Atom. This corresponds to a solid made up of only one kind of atom ≡ An Elemental Solid. • However, this structural unit could also be a group of several atoms or even molecules. The simplest structural unit for a given solid is called the BASIS

• The structure of an Ideal Crystal can be described in terms of a pure mathematical abstraction called a Lattice. • Mathematicians have studied the mathematics of lattices starting several thousand years ago! A Lattice ≡ A 3 -dimensional periodic array of points in space.

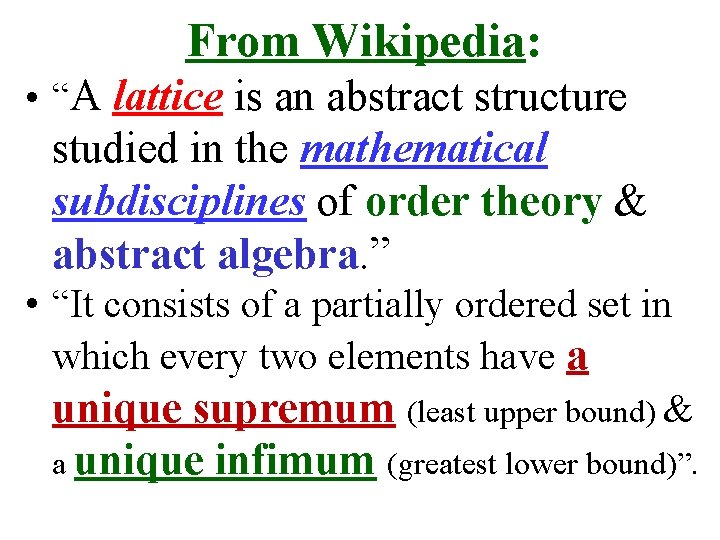
From Wikipedia: • “A lattice is an abstract structure studied in the mathematical subdisciplines of order theory & abstract algebra. ” • “It consists of a partially ordered set in which every two elements have a unique supremum (least upper bound) & a unique infimum (greatest lower bound)”.

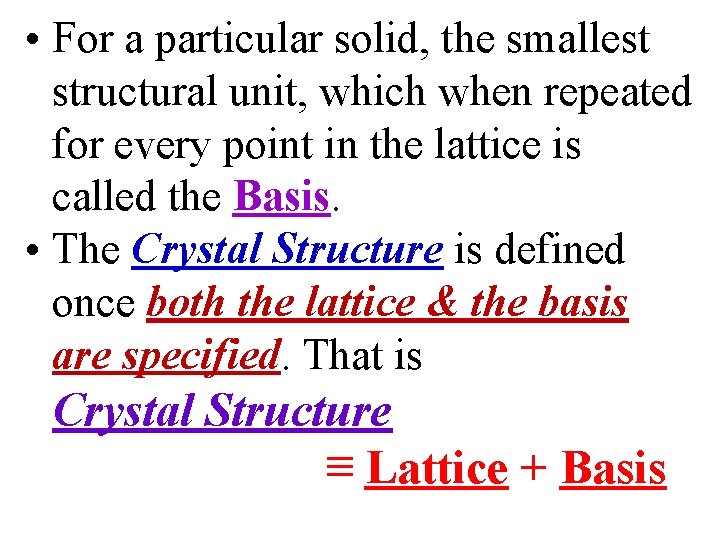
• For a particular solid, the smallest structural unit, which when repeated for every point in the lattice is called the Basis. • The Crystal Structure is defined once both the lattice & the basis are specified. That is Crystal Structure ≡ Lattice + Basis

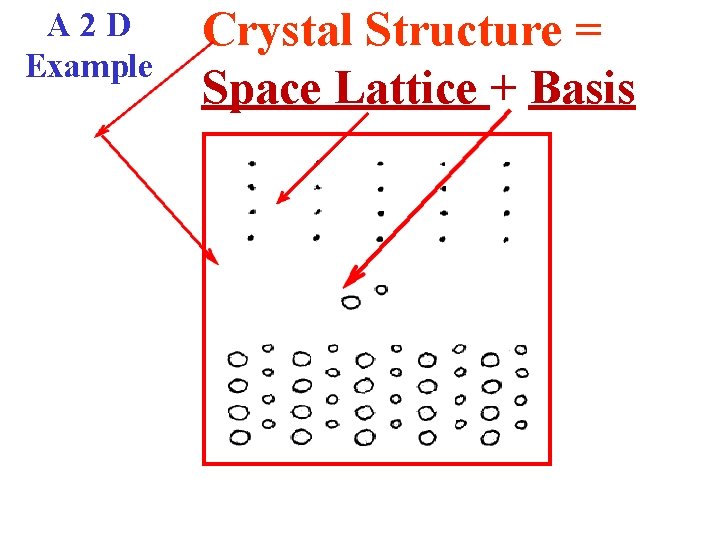
A 2 D Example Crystal Structure = Space Lattice + Basis

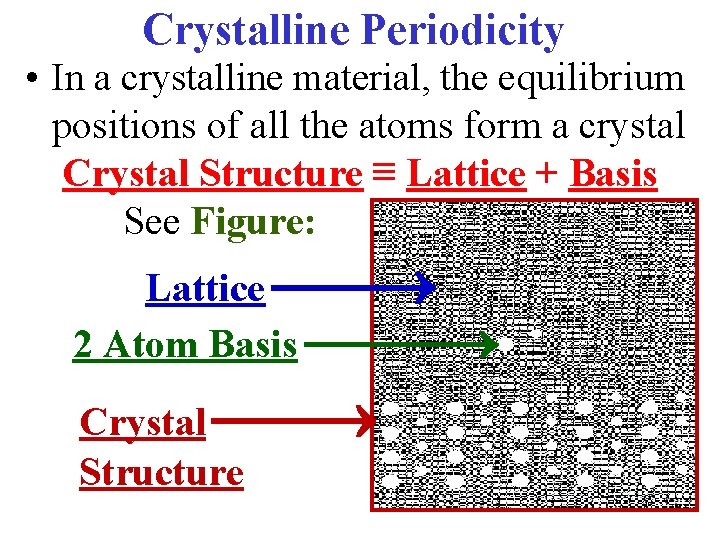
Crystalline Periodicity • In a crystalline material, the equilibrium positions of all the atoms form a crystal Crystal Structure ≡ Lattice + Basis See Figure: Lattice 2 Atom Basis Crystal Structure

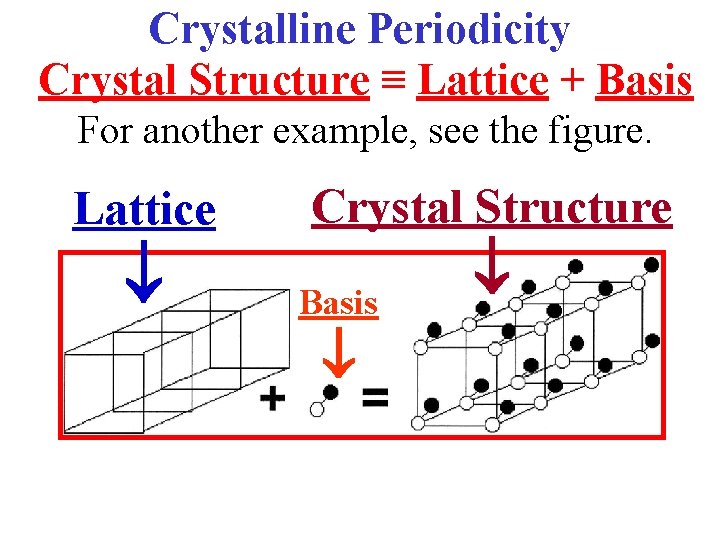
Crystalline Periodicity Crystal Structure ≡ Lattice + Basis For another example, see the figure. Lattice Crystal Structure Basis

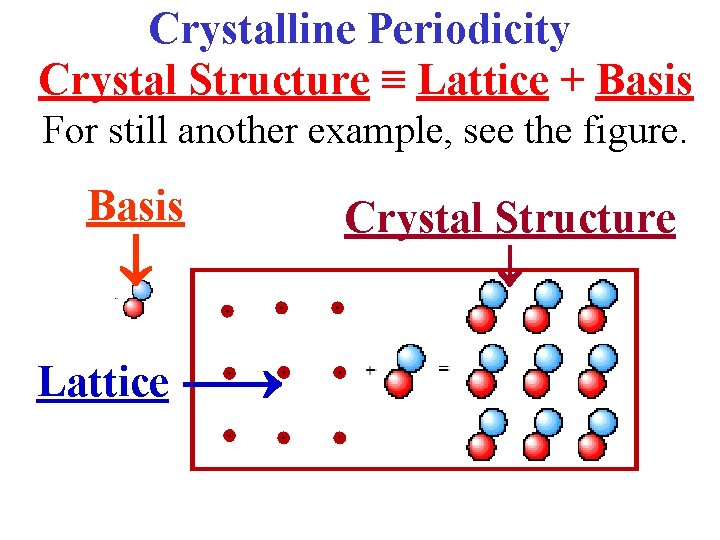
Crystalline Periodicity Crystal Structure ≡ Lattice + Basis For still another example, see the figure. Basis Lattice Crystal Structure

A Two-Dimensional (Bravais) Lattice with Different Choices for the Basis

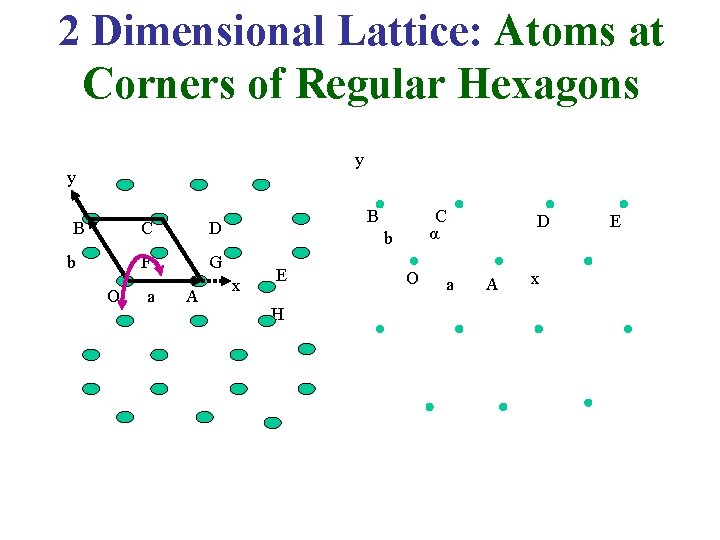
2 Dimensional Lattice: Atoms at Corners of Regular Hexagons y y B b O C D F G a A B C α b x E H O a D A x E

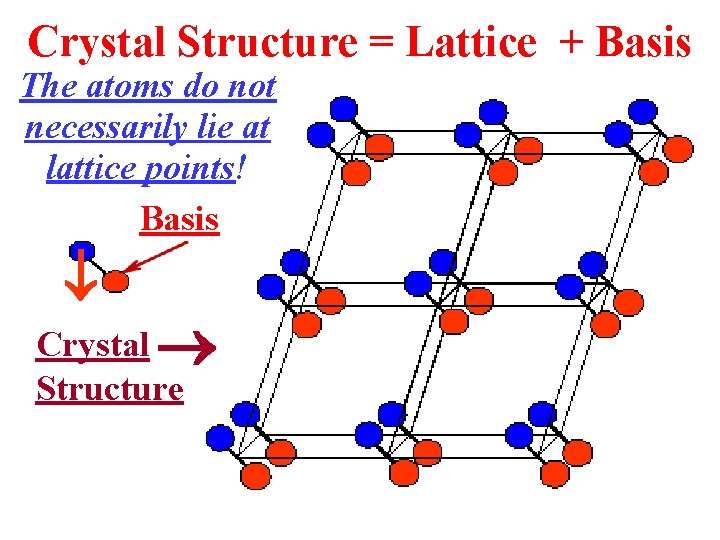
Crystal Structure = Lattice + Basis The atoms do not necessarily lie at lattice points! Basis Crystal Structure