3 Dimensional Crystal Structure 3 Dimensional Crystal Structure

- Slides: 14

3 -Dimensional Crystal Structure

3 -Dimensional Crystal Structure

3 -D Crystal Structure • General: A crystal structure is defined by primitive lattice vectors a 1, a 2, a 3. • a 1, a 2, a 3: Depend on geometry. Once specified, the primitive lattice structure is specified. • Generate lattice by translating through a direct lattice vector: r = n 1 a 1+n 2 a 2+n 3 a 3. (n 1, n 2, n 3) are integers. r generates the lattice points. Each lattice point corresponds to a set of (n 1, n 2, n 3).

• Basis (or basis set) The set of atoms which, when placed at each lattice point, generates the crystal structure. • Crystal Structure Primitive lattice structure + basis. Translate the basis through all possible lattice vectors r = n 1 a 1+n 2 a 2+n 3 a 3 to get the crystal structure or the DIRECT LATTICE

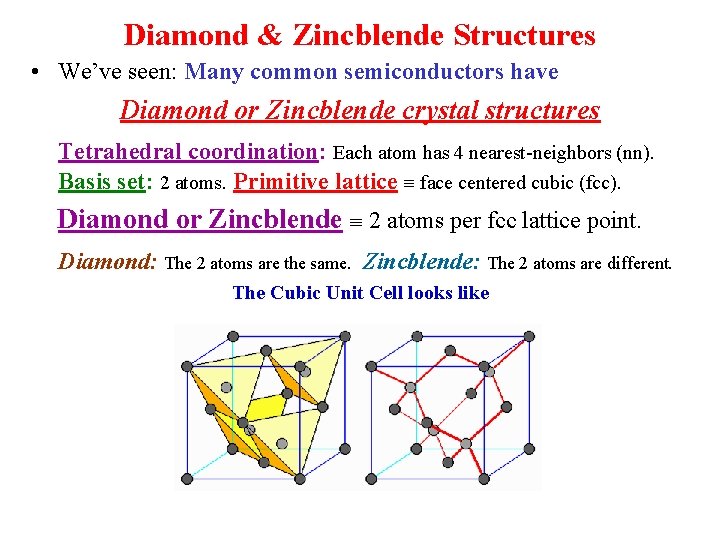
Diamond & Zincblende Structures • We’ve seen: Many common semiconductors have Diamond or Zincblende crystal structures Tetrahedral coordination: Each atom has 4 nearest-neighbors (nn). Basis set: 2 atoms. Primitive lattice face centered cubic (fcc). Diamond or Zincblende 2 atoms per fcc lattice point. Diamond: The 2 atoms are the same. Zincblende: The 2 atoms are different. The Cubic Unit Cell looks like

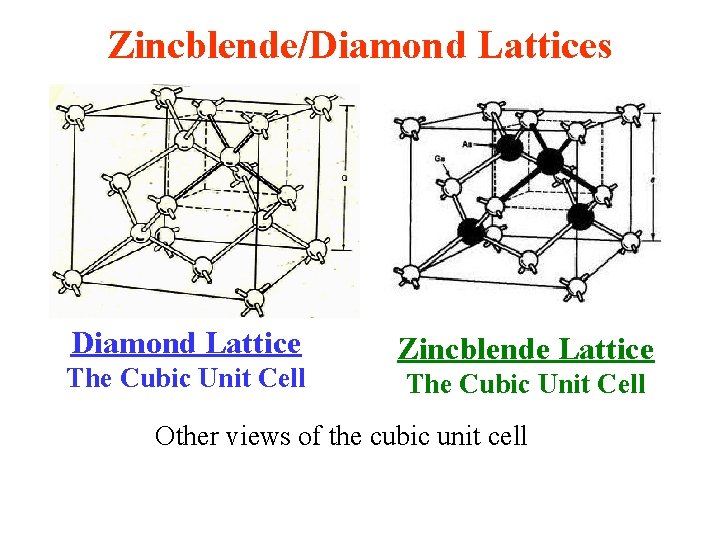
Zincblende/Diamond Lattices Diamond Lattice The Cubic Unit Cell Zincblende Lattice The Cubic Unit Cell Other views of the cubic unit cell

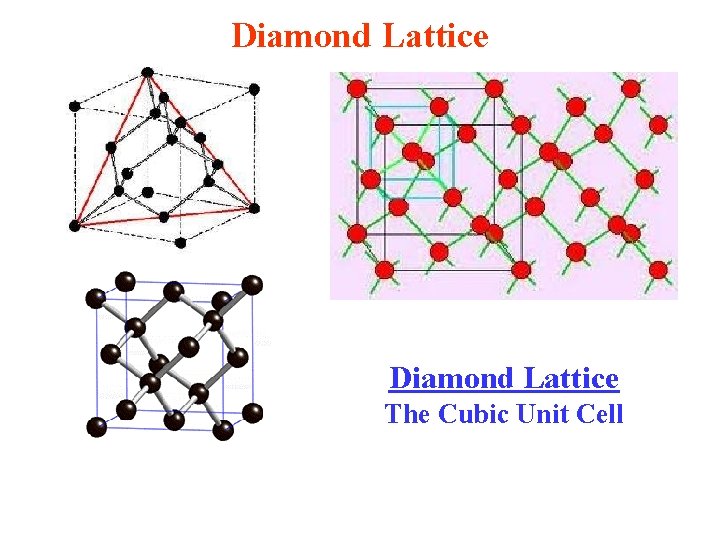
Diamond Lattice The Cubic Unit Cell

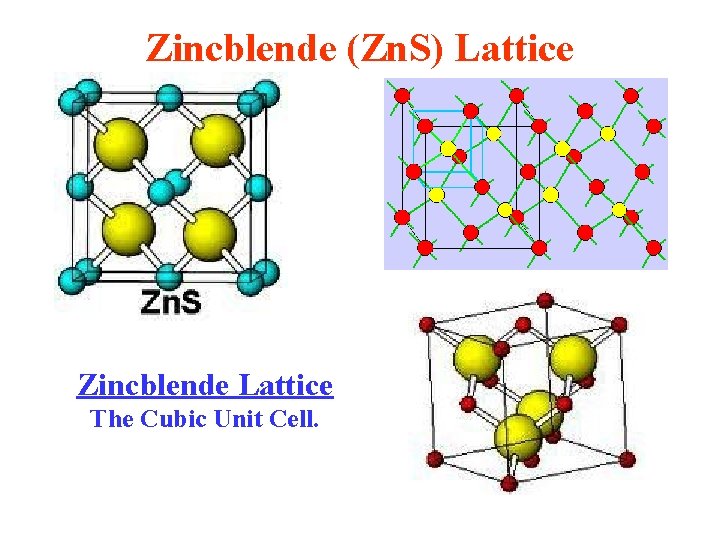
Zincblende (Zn. S) Lattice Zincblende Lattice The Cubic Unit Cell.

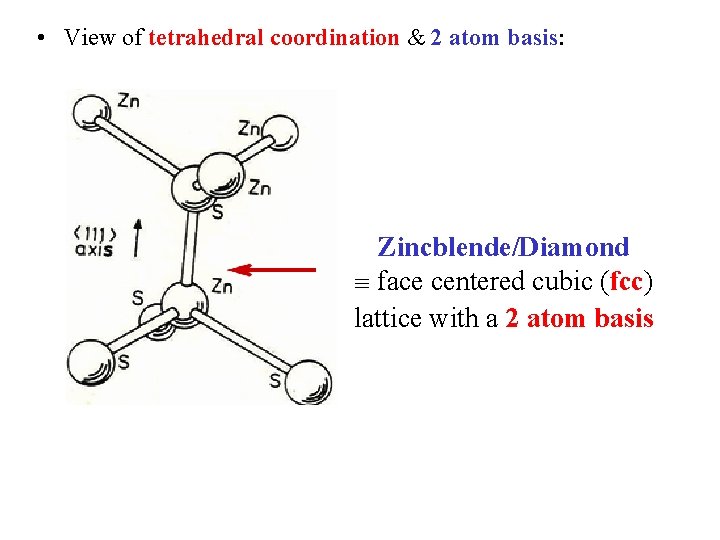
• View of tetrahedral coordination & 2 atom basis: Zincblende/Diamond face centered cubic (fcc) lattice with a 2 atom basis

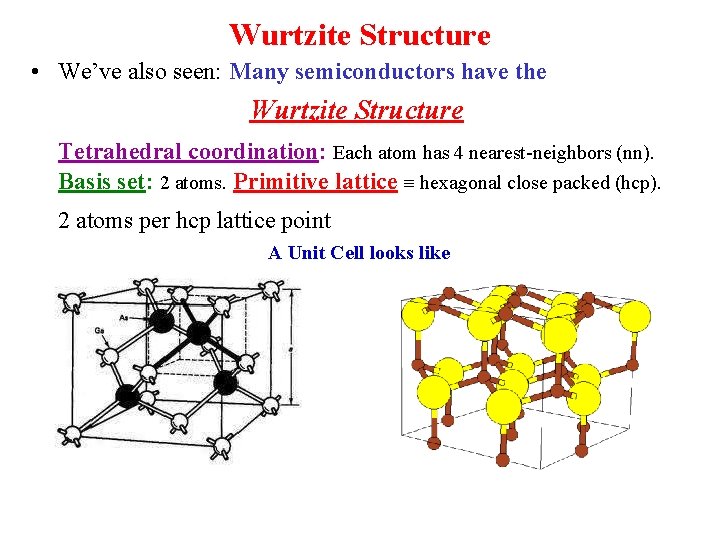
Wurtzite Structure • We’ve also seen: Many semiconductors have the Wurtzite Structure Tetrahedral coordination: Each atom has 4 nearest-neighbors (nn). Basis set: 2 atoms. Primitive lattice hexagonal close packed (hcp). 2 atoms per hcp lattice point A Unit Cell looks like

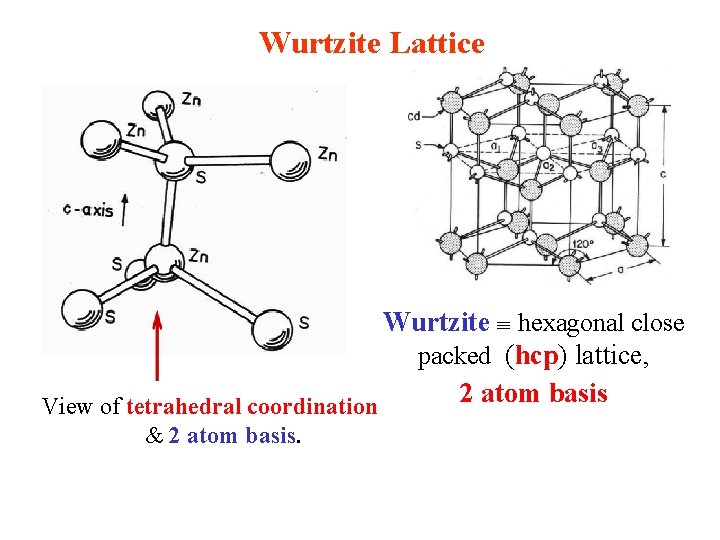
Wurtzite Lattice Wurtzite hexagonal close packed (hcp) lattice, 2 atom basis View of tetrahedral coordination & 2 atom basis.

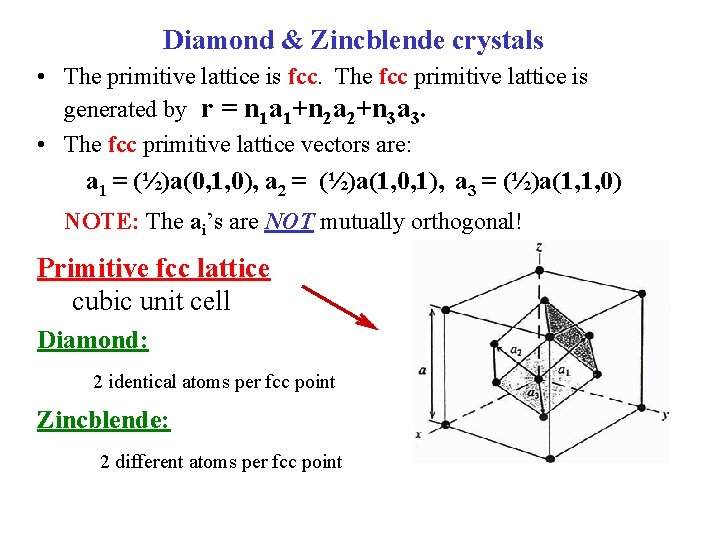
Diamond & Zincblende crystals • The primitive lattice is fcc. The fcc primitive lattice is generated by r = n 1 a 1+n 2 a 2+n 3 a 3. • The fcc primitive lattice vectors are: a 1 = (½)a(0, 1, 0), a 2 = (½)a(1, 0, 1), a 3 = (½)a(1, 1, 0) NOTE: The ai’s are NOT mutually orthogonal! Primitive fcc lattice cubic unit cell Diamond: 2 identical atoms per fcc point Zincblende: 2 different atoms per fcc point

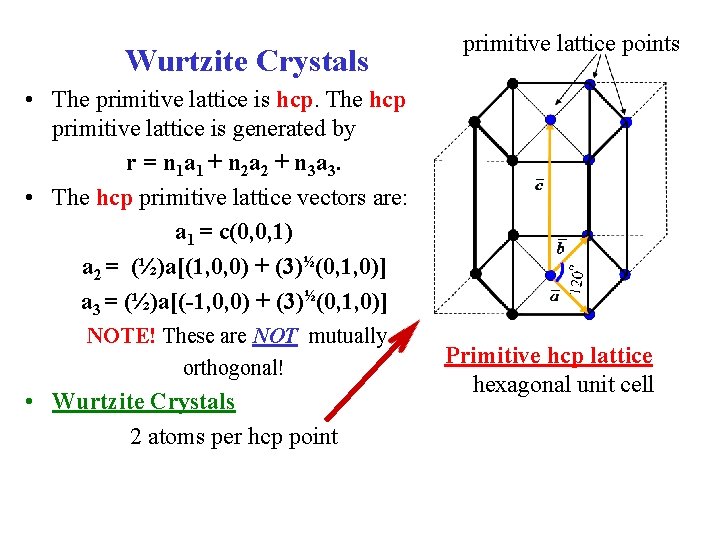
Wurtzite Crystals primitive lattice points • The primitive lattice is hcp. The hcp primitive lattice is generated by r = n 1 a 1 + n 2 a 2 + n 3 a 3. • The hcp primitive lattice vectors are: a 1 = c(0, 0, 1) a 2 = (½)a[(1, 0, 0) + (3)½(0, 1, 0)] a 3 = (½)a[(-1, 0, 0) + (3)½(0, 1, 0)] NOTE! These are NOT mutually orthogonal! • Wurtzite Crystals 2 atoms per hcp point Primitive hcp lattice hexagonal unit cell

Group Theory • Applications: It is used to simplify the computational effort necessary in the highly computational electronic bandstructure calculations.