Motifs Part of MATERIALS SCIENCE A Learners Guide





- Slides: 16

Motifs Part of MATERIALS SCIENCE & A Learner’s Guide ENGINEERING AN INTRODUCTORY E-BOOK Anandh Subramaniam & Kantesh Balani Materials Science and Engineering (MSE) Indian Institute of Technology, Kanpur- 208016 Email: anandh@iitk. ac. in, URL: home. iitk. ac. in/~anandh http: //home. iitk. ac. in/~anandh/E-book. htm

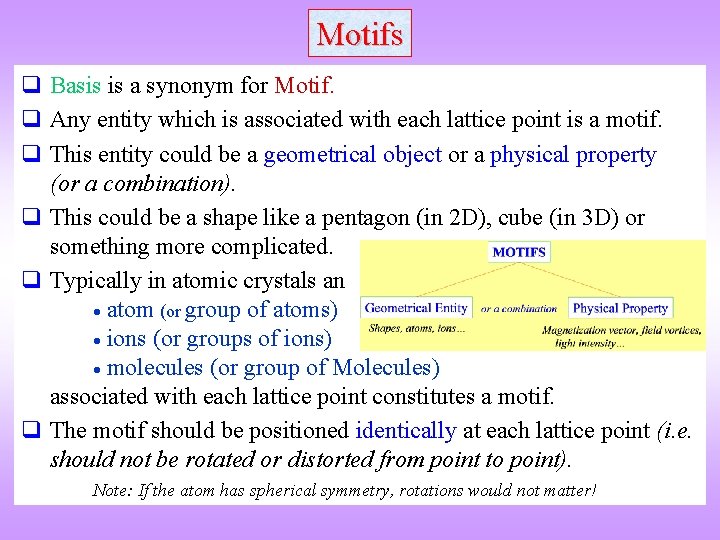
Motifs q Basis is a synonym for Motif. q Any entity which is associated with each lattice point is a motif. q This entity could be a geometrical object or a physical property (or a combination). q This could be a shape like a pentagon (in 2 D), cube (in 3 D) or something more complicated. q Typically in atomic crystals an atom (or group of atoms) ions (or groups of ions) molecules (or group of Molecules) associated with each lattice point constitutes a motif. q The motif should be positioned identically at each lattice point (i. e. should not be rotated or distorted from point to point). Note: If the atom has spherical symmetry, rotations would not matter!

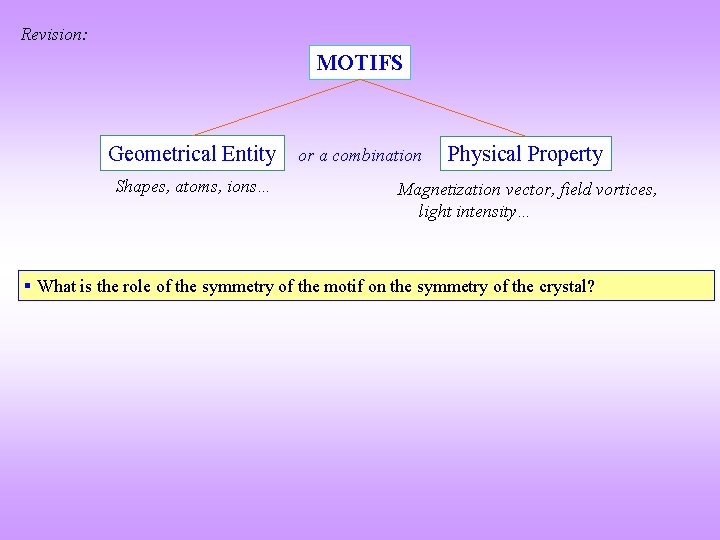
Revision: MOTIFS Geometrical Entity or a combination Shapes, atoms, ions… Physical Property Magnetization vector, field vortices, light intensity… § What is the role of the symmetry of the motif on the symmetry of the crystal?

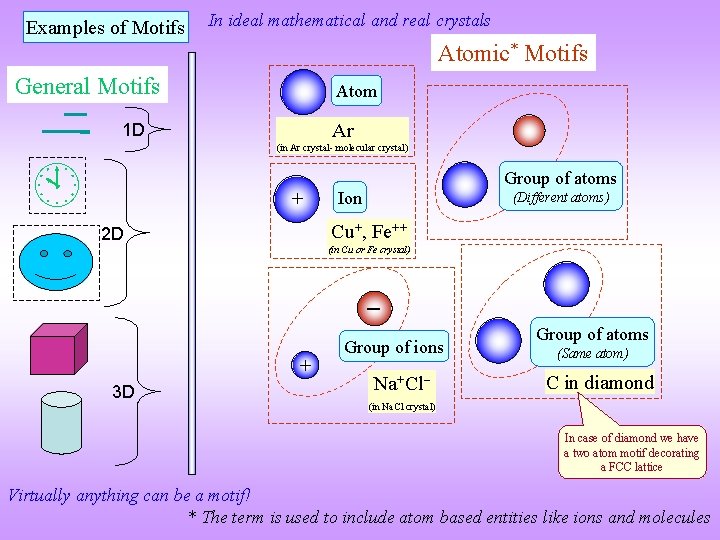
Examples of Motifs In ideal mathematical and real crystals Atomic* Motifs General Motifs Atom Ar 1 D (in Ar crystal- molecular crystal) + Group of atoms Ion (Different atoms) Cu+, Fe++ 2 D (in Cu or Fe crystal) + 3 D Group of ions Na+Cl Group of atoms (Same atom) C in diamond (in Na. Cl crystal) In case of diamond we have a two atom motif decorating a FCC lattice Virtually anything can be a motif! * The term is used to include atom based entities like ions and molecules

q Viruses can be crystallized and the motif now is an individual virus (a entity much larger than the usual atomic motifs) A complete virus is sitting as a motif on each lattice position (instead of atoms or ions!) We get a crystal of ‘virus’ Crystal of Tobacco Mosaic Virus [1] Crystal Physics, G. S. Zhdanov, Oliver & Boyd, Ediburgh, 1965

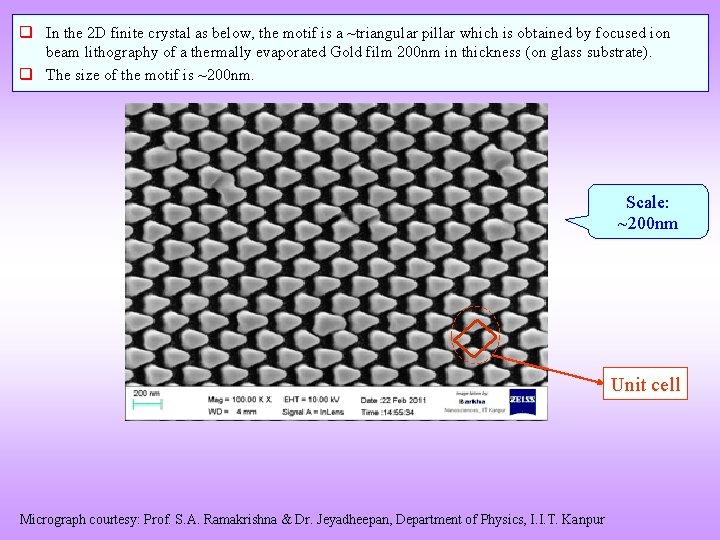
q In the 2 D finite crystal as below, the motif is a ~triangular pillar which is obtained by focused ion beam lithography of a thermally evaporated Gold film 200 nm in thickness (on glass substrate). q The size of the motif is ~200 nm. Scale: ~200 nm Unit cell Micrograph courtesy: Prof. S. A. Ramakrishna & Dr. Jeyadheepan, Department of Physics, I. I. T. Kanpur

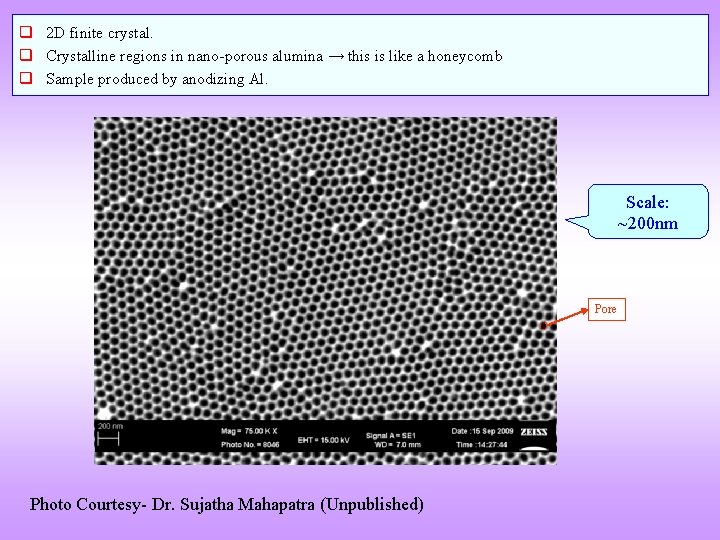
q 2 D finite crystal. q Crystalline regions in nano-porous alumina → this is like a honeycomb q Sample produced by anodizing Al. Scale: ~200 nm Pore Photo Courtesy- Dr. Sujatha Mahapatra (Unpublished)

Chip of the LED light sensing assembly of a mouse

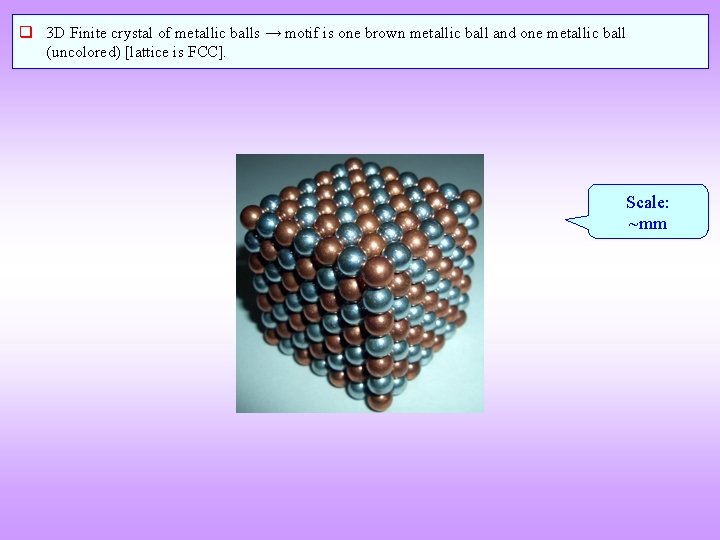
q 3 D Finite crystal of metallic balls → motif is one brown metallic ball and one metallic ball (uncolored) [lattice is FCC]. Scale: ~mm

q Crystals have been synthesized with silver nanocrystals as the motif in an FCC lattice. Each lattice point is occupied by a silver nanocrystal having the shape of a truncated octahedron- a tetrakaidecahedron (with orientational and positional order). q The orientation relation between the particles and the lattice is as follows: [110] lattice || [110]Ag, [001]lattice || [1 10]Ag Ag nanocrystal as the motif

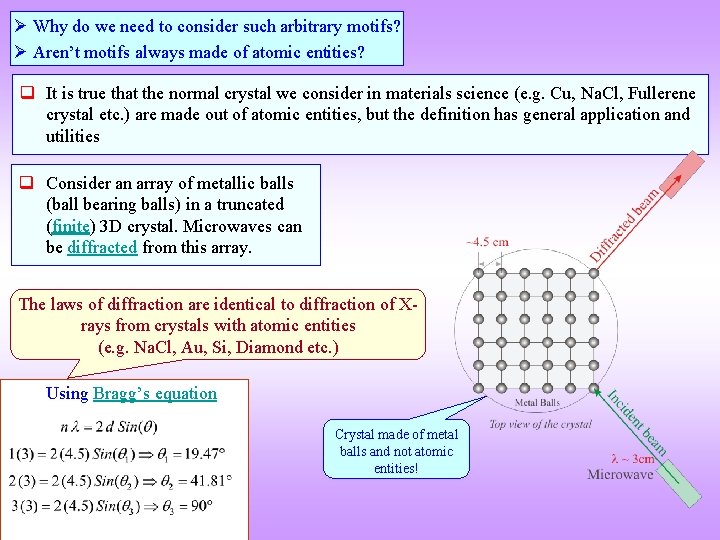
Why do we need to consider such arbitrary motifs? Aren’t motifs always made of atomic entities? q It is true that the normal crystal we consider in materials science (e. g. Cu, Na. Cl, Fullerene crystal etc. ) are made out of atomic entities, but the definition has general application and utilities q Consider an array of metallic balls (ball bearing balls) in a truncated (finite) 3 D crystal. Microwaves can be diffracted from this array. The laws of diffraction are identical to diffraction of Xrays from crystals with atomic entities (e. g. Na. Cl, Au, Si, Diamond etc. ) Using Bragg’s equation Crystal made of metal balls and not atomic entities!

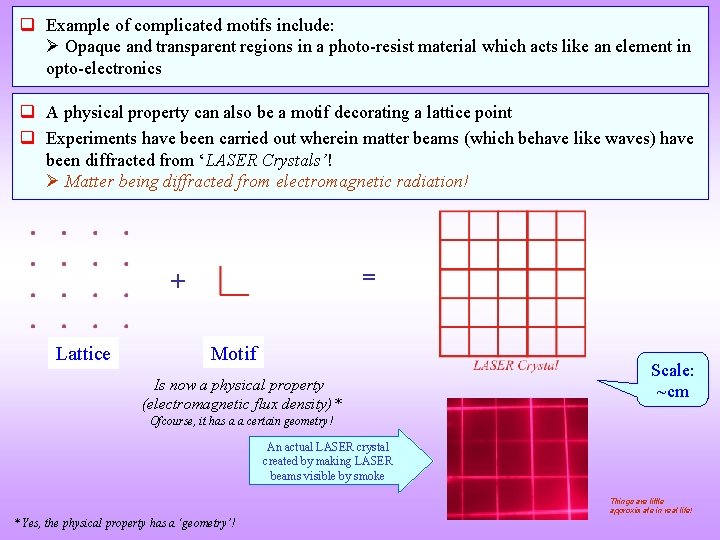
q Example of complicated motifs include: Opaque and transparent regions in a photo-resist material which acts like an element in opto-electronics q A physical property can also be a motif decorating a lattice point q Experiments have been carried out wherein matter beams (which behave like waves) have been diffracted from ‘LASER Crystals’! Matter being diffracted from electromagnetic radiation! = + Lattice Motif Is now a physical property (electromagnetic flux density)* Scale: ~cm Ofcourse, it has a a certain geometry! An actual LASER crystal created by making LASER beams visible by smoke Things are little approximate in real life! * Yes, the physical property has a ‘geometry’!

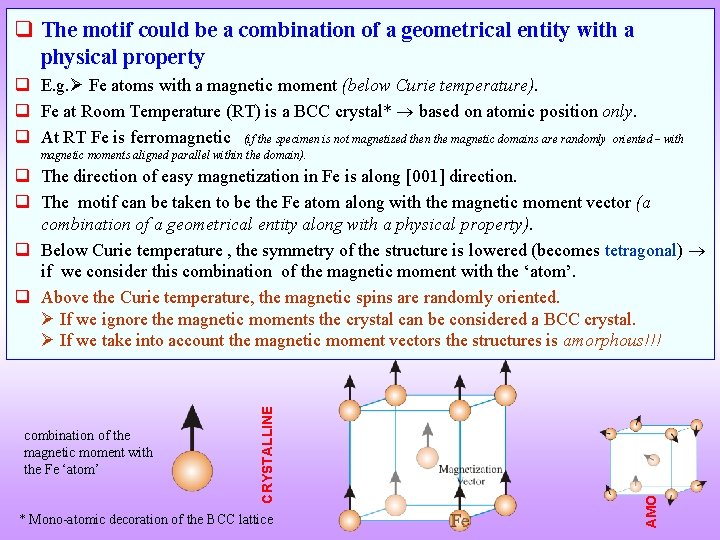
q The motif could be a combination of a geometrical entity with a physical property q E. g. Fe atoms with a magnetic moment (below Curie temperature). q Fe at Room Temperature (RT) is a BCC crystal* based on atomic position only. q At RT Fe is ferromagnetic (if the specimen is not magnetized then the magnetic domains are randomly oriented with magnetic moments aligned parallel within the domain). * Mono-atomic decoration of the BCC lattice AMORPHOUS combination of the magnetic moment with the Fe ‘atom’ CRYSTALLINE q The direction of easy magnetization in Fe is along [001] direction. q The motif can be taken to be the Fe atom along with the magnetic moment vector (a combination of a geometrical entity along with a physical property). q Below Curie temperature , the symmetry of the structure is lowered (becomes tetragonal) if we consider this combination of the magnetic moment with the ‘atom’. q Above the Curie temperature, the magnetic spins are randomly oriented. If we ignore the magnetic moments the crystal can be considered a BCC crystal. If we take into account the magnetic moment vectors the structures is amorphous!!!

q Wigner crystal q Electrons repel each other and can get ordered to this repulsive interaction. This is a Wigner crystal! (here, we ignore the atomic entitles). q We can also visualize other purely repulsively ordered crystals.

Ordering of Nuclear spins q We had seen that electron spin (magnetic moment arising from the spin) can get ordered (e. g. ferromagnetic ordering of spins in solid Fe at room temperature). q Similarly, nuclear spin can also get ordered.

The case of the vortices in type-II superconductors q In type-II superconductors the transition to non-superconducting state occurs gradually via penetration of flux lines via magnetic vortices. q These vortices can ‘crystallize’ in a hexagonal Abrikosov lattice (lattice decorated with a physical property). This is a repulsively ordered crystal. Causing disorder in this perfect crystal cost ‘elastic energy’. q On heating or increasing the magnetic field strength the crystal may ‘melt’ (i. e. melting of a physical property lattice). Before melting the flux tubes become ‘wiggly’.
 Remedial teaching conclusion
Remedial teaching conclusion English science and blank are my favorite subject
English science and blank are my favorite subject Natural materials and man made materials
Natural materials and man made materials Household materials useful or harmful
Household materials useful or harmful Natural man made
Natural man made What is adopting materials
What is adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Global vs analytical learners
Global vs analytical learners How to teach grammar to young learners
How to teach grammar to young learners Heather nichole atkins
Heather nichole atkins Global vs analytical learners
Global vs analytical learners Kinesthetic learners characteristics
Kinesthetic learners characteristics Eager classification versus lazy classification
Eager classification versus lazy classification Kinesthetic learners learn best by
Kinesthetic learners learn best by When is cognitivism beneficial for learners
When is cognitivism beneficial for learners Global analytic continuum
Global analytic continuum Article 4 the teacher and the profession
Article 4 the teacher and the profession