Part of MATERIALS SCIENCE A Learners Guide ENGINEERING



- Slides: 13

Part of MATERIALS SCIENCE & A Learner’s Guide ENGINEERING AN INTRODUCTORY E-BOOK Anandh Subramaniam & Kantesh Balani Materials Science and Engineering (MSE) Indian Institute of Technology, Kanpur- 208016 Email: anandh@iitk. ac. in, URL: home. iitk. ac. in/~anandh http: //home. iitk. ac. in/~anandh/E-book. htm

Mystery of the missing entries in the Bravais List! Few illustrations q Another point of view: There are 7 crystal systems (with 7 preferred unit cells). Each of them can “can have” P (/S), I, F, C type lattice (4 types). Hence the “potential” number is (7 4) = 28. But 14 of these are only distinct. Points to note: q Every lattice that you can construct is present somewhere in the list the issue is where to put them. q Concept of choice of unit cell is also invoked along with the classification of Bravais lattices. q The factors which are taken to into account are (like for the unit cell): Symmetry Size. q The symbol ‘P’ is used for ‘simple’ lattices this is very confusing! This ‘P’ does not mean primitive (primitive is a term associated with unit cells and not lattices). It should have been ‘S’.

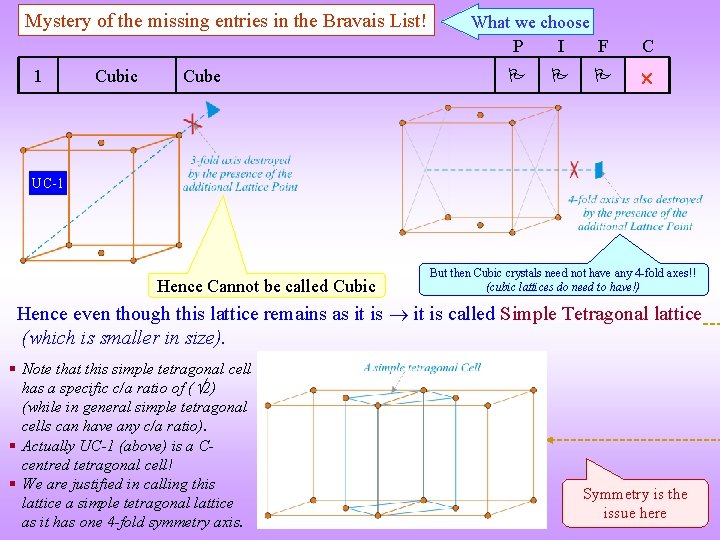
Mystery of the missing entries in the Bravais List! 1 Cubic Cube What we choose P I F C UC-1 Hence Cannot be called Cubic But then Cubic crystals need not have any 4 -fold axes!! (cubic lattices do need to have!) Hence even though this lattice remains as it is called Simple Tetragonal lattice (which is smaller in size). § Note that this simple tetragonal cell has a specific c/a ratio of ( 2) (while in general simple tetragonal cells can have any c/a ratio). § Actually UC-1 (above) is a Ccentred tetragonal cell! § We are justified in calling this lattice a simple tetragonal lattice as it has one 4 -fold symmetry axis. Symmetry is the issue here

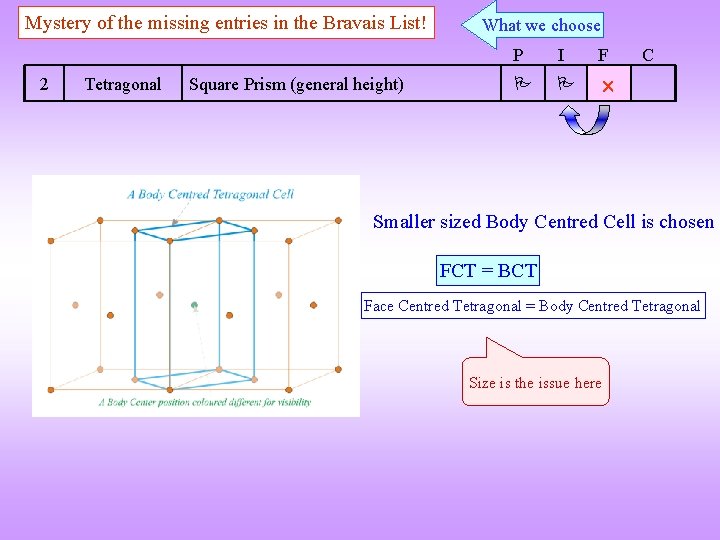
Mystery of the missing entries in the Bravais List! 2 Tetragonal Square Prism (general height) What we choose P I F C Smaller sized Body Centred Cell is chosen FCT = BCT Face Centred Tetragonal = Body Centred Tetragonal Size is the issue here

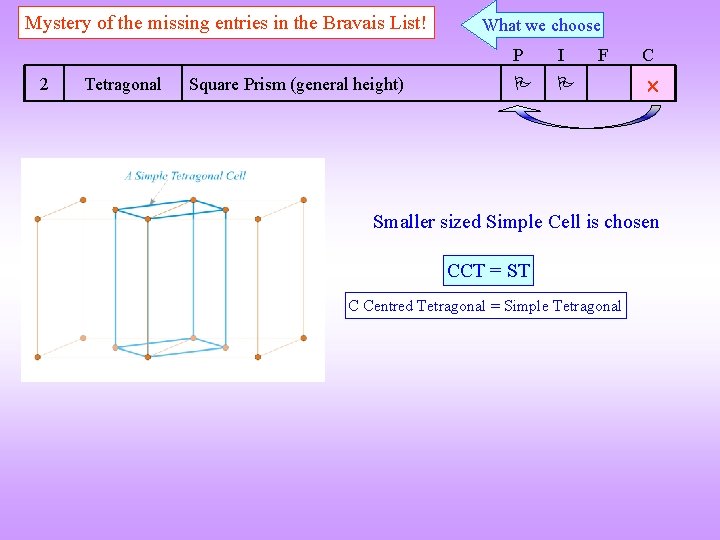
Mystery of the missing entries in the Bravais List! 2 Tetragonal Square Prism (general height) What we choose P I F C Smaller sized Simple Cell is chosen CCT = ST C Centred Tetragonal = Simple Tetragonal

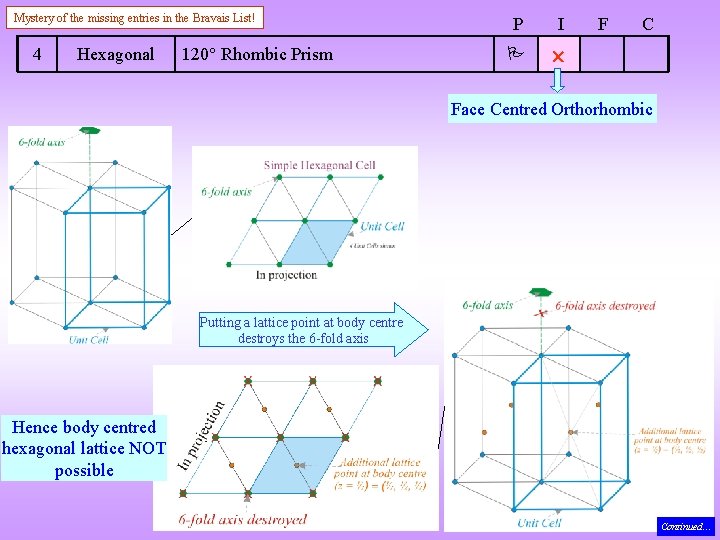
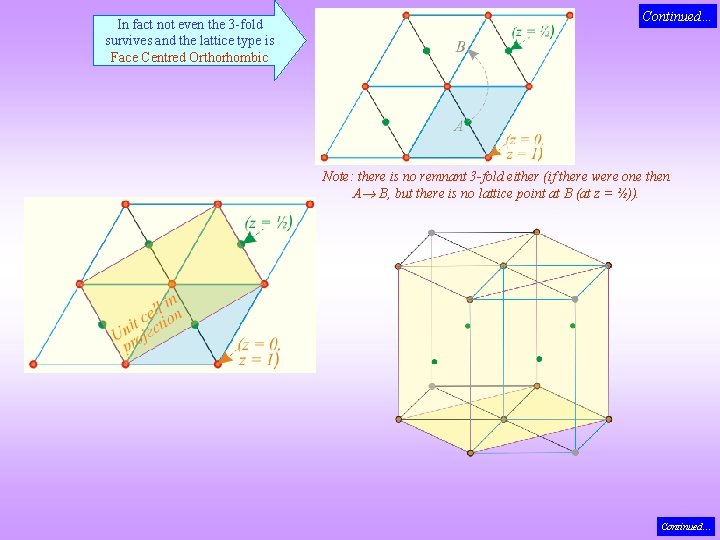
Mystery of the missing entries in the Bravais List! 4 Hexagonal 120 Rhombic Prism P I F C Face Centred Orthorhombic Putting a lattice point at body centre destroys the 6 -fold axis Hence body centred hexagonal lattice NOT possible Continued…

In fact not even the 3 -fold survives and the lattice type is Face Centred Orthorhombic Continued… Note: there is no remnant 3 -fold either (if there were one then A B, but there is no lattice point at B (at z = ½)). Continued…

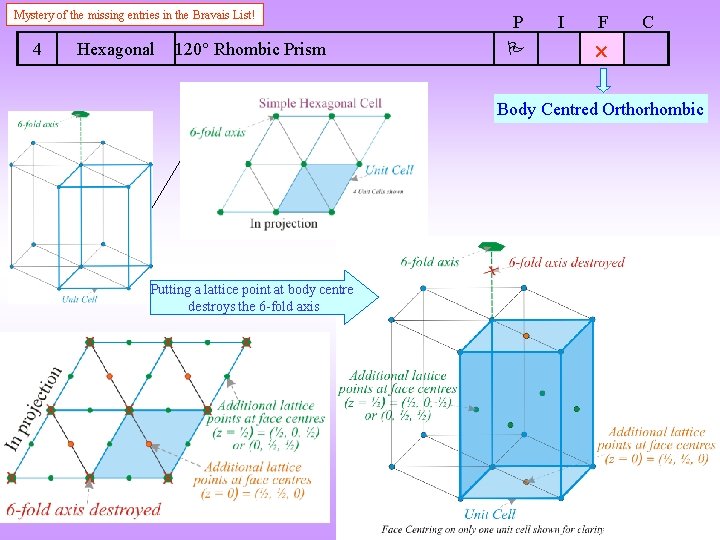
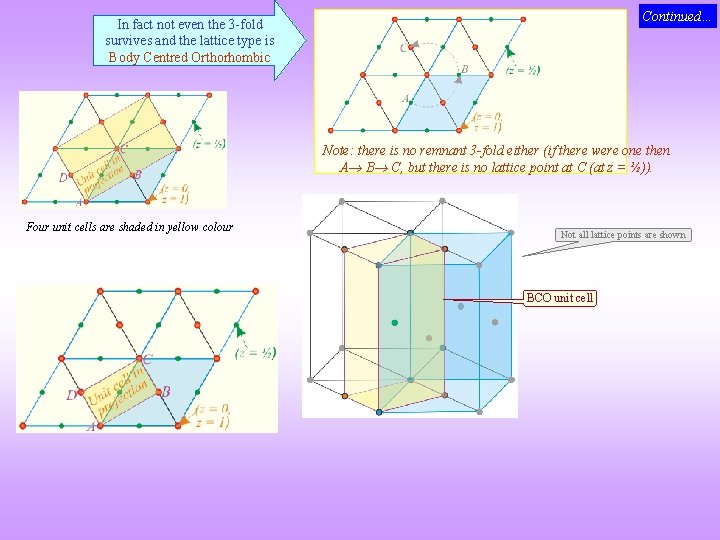
Mystery of the missing entries in the Bravais List! 4 Hexagonal 120 Rhombic Prism P I F C Body Centred Orthorhombic Putting a lattice point at body centre destroys the 6 -fold axis

Continued… In fact not even the 3 -fold survives and the lattice type is Body Centred Orthorhombic Note: there is no remnant 3 -fold either (if there were one then A B C, but there is no lattice point at C (at z = ½)). Four unit cells are shaded in yellow colour Not all lattice points are shown BCO unit cell

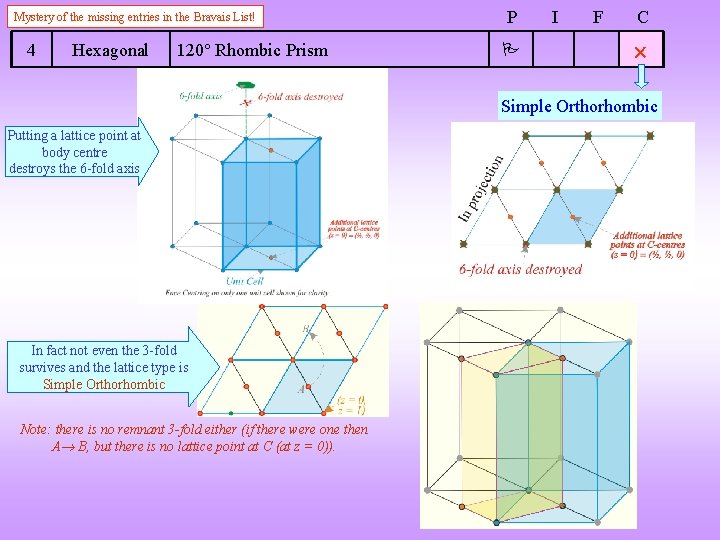
Mystery of the missing entries in the Bravais List! 4 Hexagonal 120 Rhombic Prism P I F C Simple Orthorhombic Putting a lattice point at body centre destroys the 6 -fold axis In fact not even the 3 -fold survives and the lattice type is Simple Orthorhombic Note: there is no remnant 3 -fold either (if there were one then A B, but there is no lattice point at C (at z = 0)).

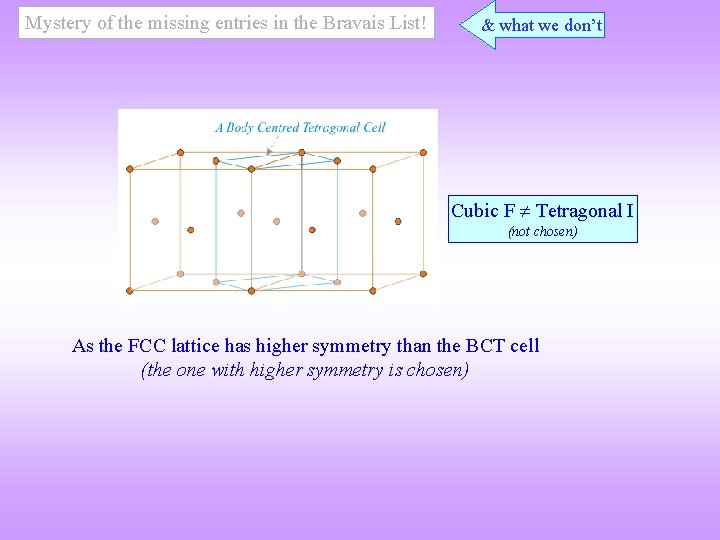
Mystery of the missing entries in the Bravais List! & what we don’t Cubic F Tetragonal I (not chosen) As the FCC lattice has higher symmetry than the BCT cell (the one with higher symmetry is chosen)

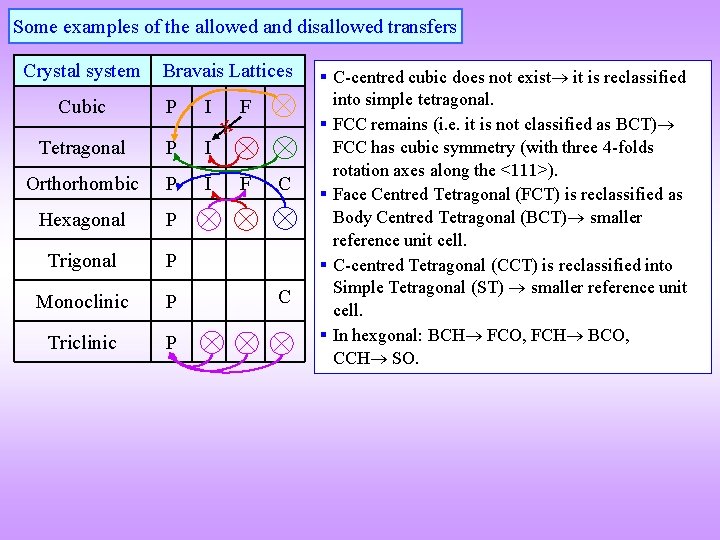
Some examples of the allowed and disallowed transfers Crystal system Bravais Lattices Cubic P I Tetragonal P I Orthorhombic P I Hexagonal P Trigonal P Monoclinic P Triclinic P x F F C C § C-centred cubic does not exist it is reclassified into simple tetragonal. § FCC remains (i. e. it is not classified as BCT) FCC has cubic symmetry (with three 4 -folds rotation axes along the <111>). § Face Centred Tetragonal (FCT) is reclassified as Body Centred Tetragonal (BCT) smaller reference unit cell. § C-centred Tetragonal (CCT) is reclassified into Simple Tetragonal (ST) smaller reference unit cell. § In hexgonal: BCH FCO, FCH BCO, CCH SO.
