CHAPTER 5 Crystal Structure Crystal Structure of Ceramics







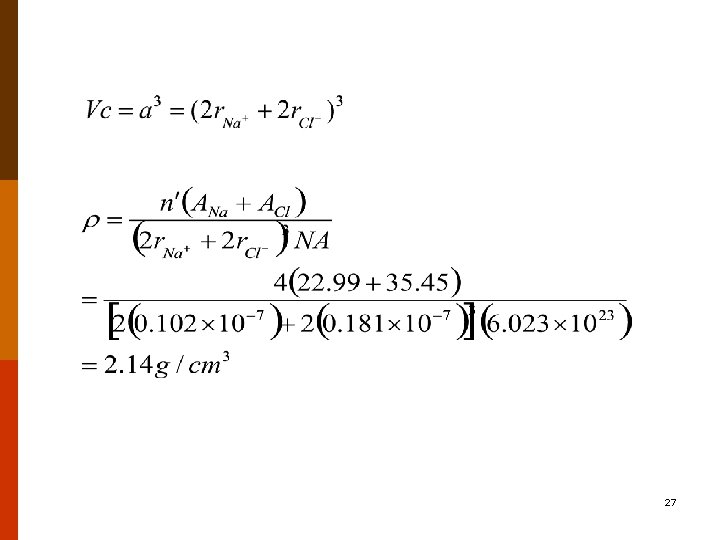








- Slides: 42

CHAPTER 5 Crystal Structure: Crystal Structure of Ceramics 1

I. Introduction to Ceramics u Chemical Composition l mostly are compounds composed of metallic and nonmetallic elements, i. e. , composed of at least two different elements, l usually considering metallic element as cation, and nonmetallic element as anion. l example:Al 2 O 3, Si. O 2, Ti. O 2, Al. N, BN, …… l exceptions:diamond, graphite, …… 2

u Bonding • mostly mixed ionic and covalent bonding. T 12. 1 • % ionic character = ( 1 -e –(0. 25)(XA-XB)2 ) 100 • exception:diamond, silicon, graphite, …… u Coordination number (CN): 4, 6, and 8. • considering the ceramics to be made up of cations and anions • CN relative size of cation and anion u Crystal Structure • considering the ceramics to be made up of cations and anions 3

II. General Features of Ceramic Crystal Structures n The crystal sturctures may be thought of as being composed of cations, and anions. F 12. 2 F 3. 7 -4 n Two characteristics influencing the crystal structure: ˙magnitude of the electrical charge (electrically neutral) ˙relative sizes of the cations and anions ( CN). n The chemical formula of a compound indicates the ratio of cations to anions, for example: Ca. F 2. , Ca+2 : F-1=1: 2. (the crystal must be electrically neutral) 4

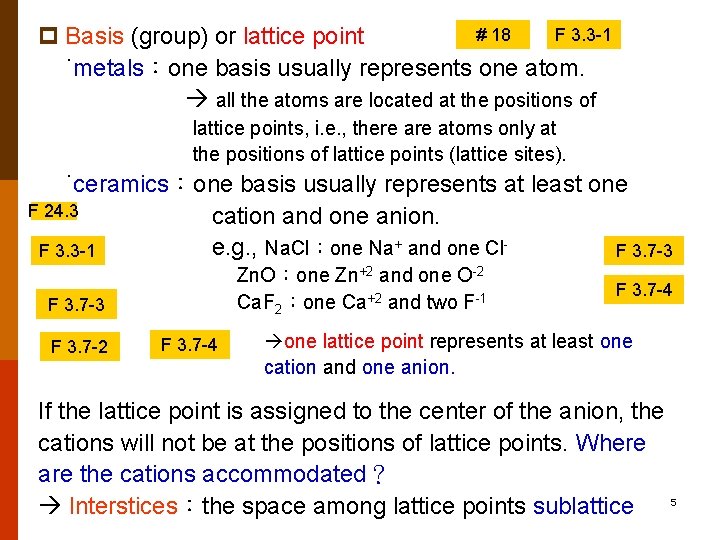
# 18 F 3. 3 -1 p Basis (group) or lattice point ˙metals:one basis usually represents one atom. all the atoms are located at the positions of lattice points, i. e. , there atoms only at the positions of lattice points (lattice sites). ˙ceramics:one basis usually represents at least one F 24. 3 cation and one anion. e. g. , Na. Cl:one Na+ and one Cl. F 3. 3 -1 F 3. 7 -3 Zn. O:one Zn+2 and one O-2 Ca. F 2:one Ca+2 and two F-1 F 3. 7 -3 F 3. 7 -2 F 3. 7 -4 one lattice point represents at least one cation and one anion. If the lattice point is assigned to the center of the anion, the cations will not be at the positions of lattice points. Where are the cations accommodated? 5 Interstices:the space among lattice points sublattice

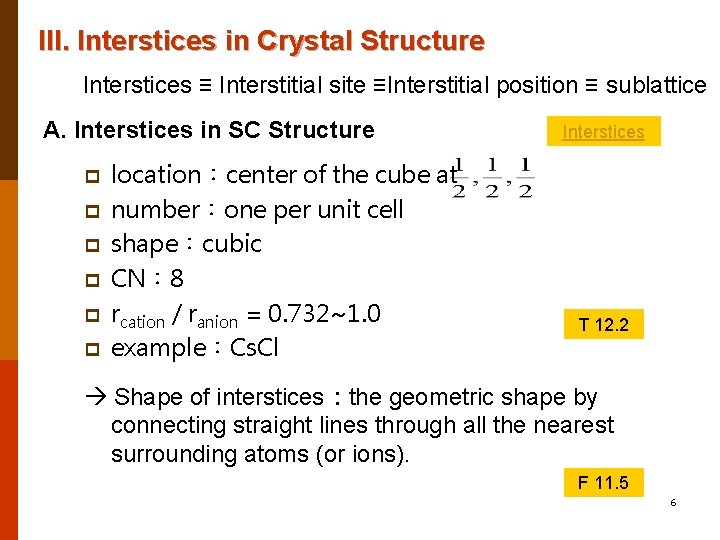
III. Interstices in Crystal Structure Interstices ≡ Interstitial site ≡Interstitial position ≡ sublattice A. Interstices in SC Structure p p p location:center of the cube at number:one per unit cell shape:cubic CN: 8 rcation / ranion = 0. 732~1. 0 example:Cs. Cl Interstices T 12. 2 Shape of interstices:the geometric shape by connecting straight lines through all the nearest surrounding atoms (or ions). F 11. 5 6

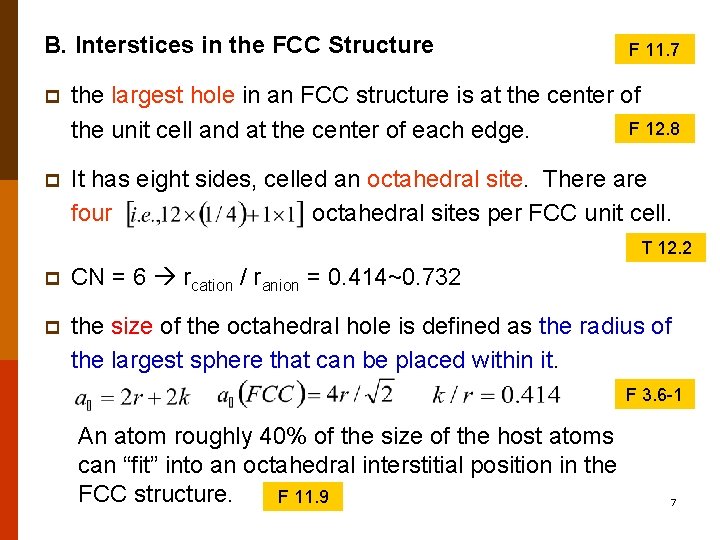
B. Interstices in the FCC Structure F 11. 7 p the largest hole in an FCC structure is at the center of F 12. 8 the unit cell and at the center of each edge. p It has eight sides, celled an octahedral site. There are four octahedral sites per FCC unit cell. T 12. 2 p CN = 6 rcation / ranion = 0. 414~0. 732 p the size of the octahedral hole is defined as the radius of the largest sphere that can be placed within it. F 3. 6 -1 An atom roughly 40% of the size of the host atoms can “fit” into an octahedral interstitial position in the FCC structure. F 11. 9 7

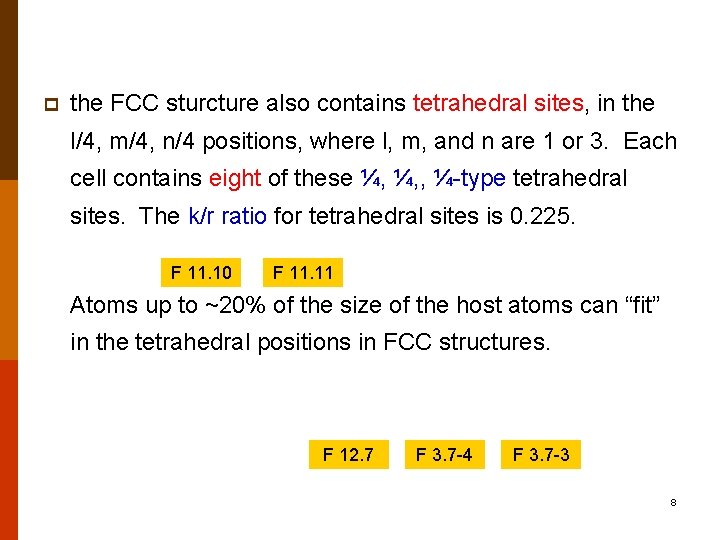
p the FCC sturcture also contains tetrahedral sites, in the l/4, m/4, n/4 positions, where l, m, and n are 1 or 3. Each cell contains eight of these ¼, ¼, , ¼-type tetrahedral sites. The k/r ratio for tetrahedral sites is 0. 225. F 11. 10 F 11. 11 Atoms up to ~20% of the size of the host atoms can “fit” in the tetrahedral positions in FCC structures. F 12. 7 F 3. 7 -4 F 3. 7 -3 8

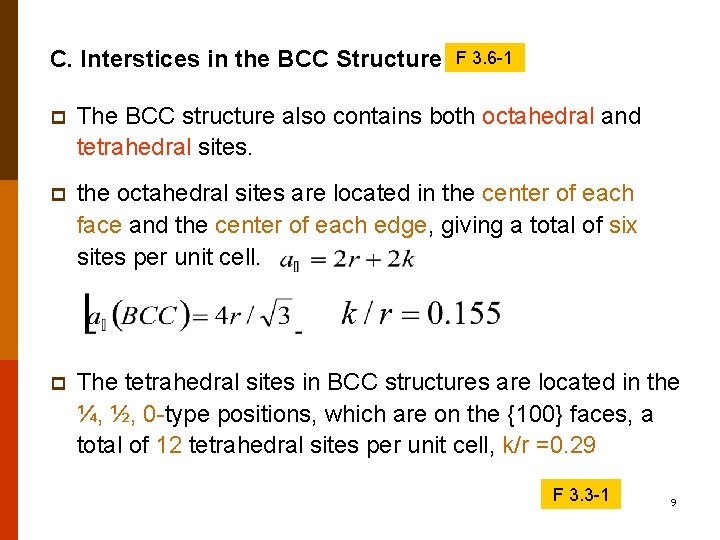
C. Interstices in the BCC Structure F 3. 6 -1 p The BCC structure also contains both octahedral and tetrahedral sites. p the octahedral sites are located in the center of each face and the center of each edge, giving a total of six sites per unit cell. p The tetrahedral sites in BCC structures are located in the ¼, ½, 0 -type positions, which are on the {100} faces, a total of 12 tetrahedral sites per unit cell, k/r =0. 29 F 3. 3 -1 9

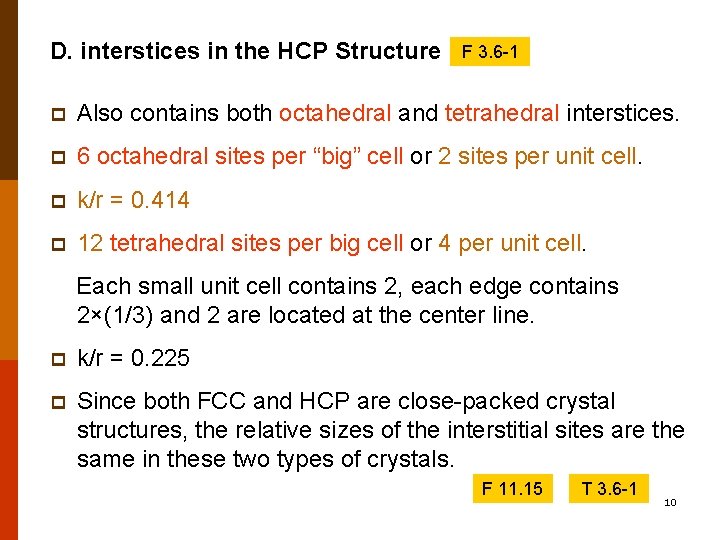
D. interstices in the HCP Structure F 3. 6 -1 p Also contains both octahedral and tetrahedral interstices. p 6 octahedral sites per “big” cell or 2 sites per unit cell. p k/r = 0. 414 p 12 tetrahedral sites per big cell or 4 per unit cell. Each small unit cell contains 2, each edge contains 2×(1/3) and 2 are located at the center line. p k/r = 0. 225 p Since both FCC and HCP are close-packed crystal structures, the relative sizes of the interstitial sites are the same in these two types of crystals. F 11. 15 T 3. 6 -1 10

IV. Crystal Structures based on Number of Atoms (Ions) per Lattice Site p One atom per lattice site metals p #8 #9 Multiple atoms per lattice site ceramics F 3. 7 -3 11

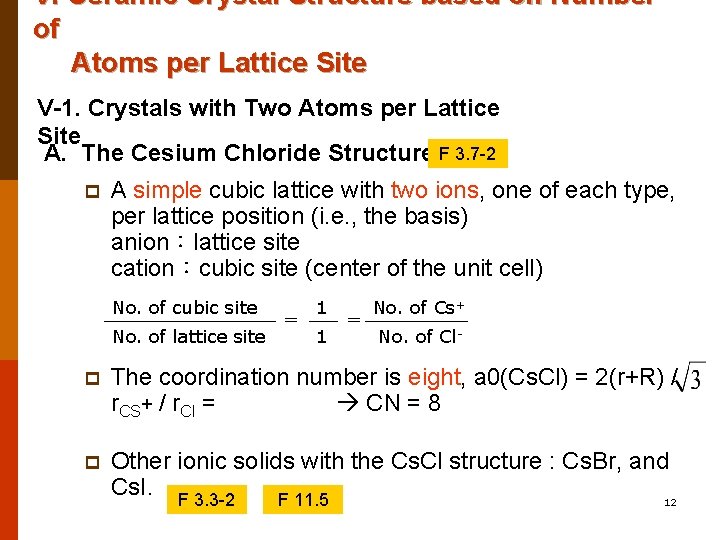
V. Ceramic Crystal Structure based on Number of Atoms per Lattice Site V-1. Crystals with Two Atoms per Lattice Site A. The Cesium Chloride Structure F 3. 7 -2 p A simple cubic lattice with two ions, one of each type, per lattice position (i. e. , the basis) anion:lattice site cation:cubic site (center of the unit cell) No. of cubic site No. of lattice site = 1 1 = No. of Cs+ No. of Cl- p The coordination number is eight, a 0(Cs. Cl) = 2(r+R) / r. CS+ / r. Cl = CN = 8 p Other ionic solids with the Cs. Cl structure : Cs. Br, and Cs. I. F 3. 3 -2 12 F 11. 5

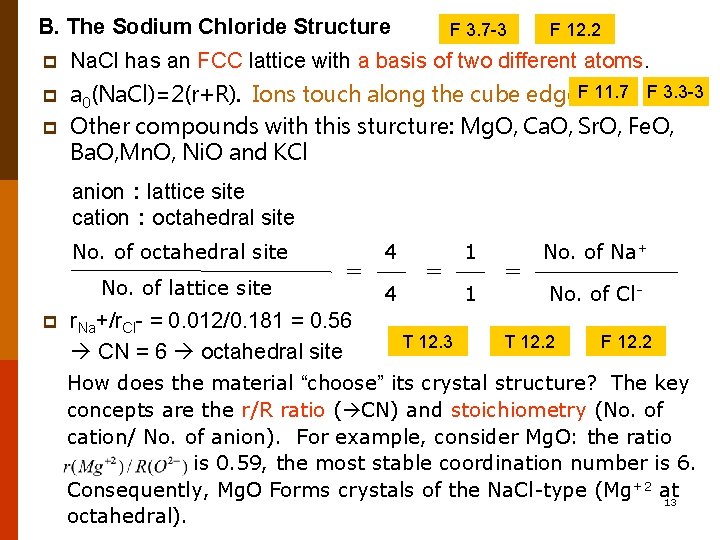
B. The Sodium Chloride Structure F 3. 7 -3 F 12. 2 p Na. Cl has an FCC lattice with a basis of two different atoms. p a 0(Na. Cl)=2(r+R). Ions touch along the cube edge. F 11. 7 F 3. 3 -3 Other compounds with this sturcture: Mg. O, Ca. O, Sr. O, Fe. O, Ba. O, Mn. O, Ni. O and KCl p anion:lattice site cation:octahedral site No. of lattice site p = r. Na+/r. Cl- = 0. 012/0. 181 = 0. 56 CN = 6 octahedral site 4 4 = T 12. 3 1 1 = No. of Na+ No. of Cl- T 12. 2 F 12. 2 How does the material “choose” its crystal structure? The key concepts are the r/R ratio ( CN) and stoichiometry (No. of cation/ No. of anion). For example, consider Mg. O: the ratio is 0. 59, the most stable coordination number is 6. Consequently, Mg. O Forms crystals of the Na. Cl-type (Mg+2 at 13 octahedral).

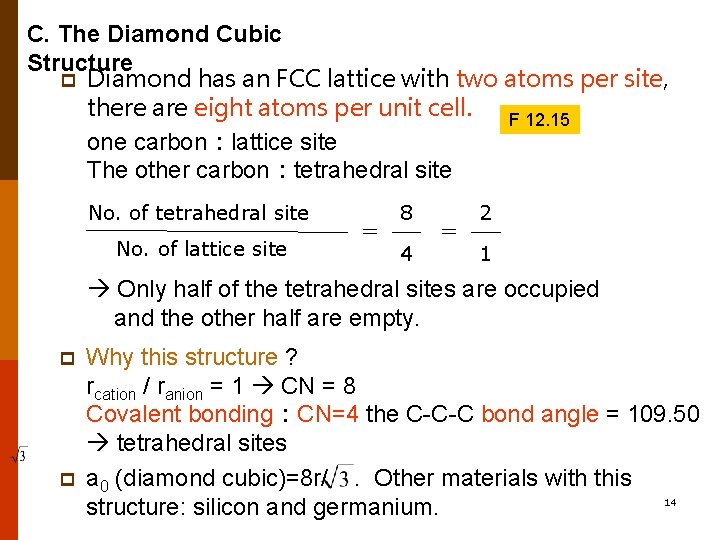
C. The Diamond Cubic Structure p Diamond has an FCC lattice with two atoms per site, there are eight atoms per unit cell. F 12. 15 one carbon:lattice site The other carbon:tetrahedral site No. of lattice site = 8 4 = 2 1 Only half of the tetrahedral sites are occupied and the other half are empty. p p Why this structure ? rcation / ranion = 1 CN = 8 Covalent bonding:CN=4 the C-C-C bond angle = 109. 50 tetrahedral sites a 0 (diamond cubic)=8 r/. Other materials with this 14 structure: silicon and germanium.

D. The Zinc-Blende Structure F 3. 7 -4 p The zinc-blende structure is similar to the diamond cubic structure but with two different elements: zinc and sulfur. p Other materials with this structure:Ga. As, Cd. Te. p Why are only half of the tetrahedral sites filled? The answers are the stoichiometry of the compound: there are four FCC sites per cell and eight tetrahedral sites per cell. p Coordination number:four;a 0(zinc-blende)=4(r+R)/ 15

D-2. Crystals with Three Atoms per Lattice Site ◎ Generally, with a basis of three atoms. A. Fluorite Structure p p F 3. 7 -5 MX 2, e. g. , Ca. F 2, UO 2, Th. O 2 and Zr. O 2 , M ions are located in the FCC positions and the X ions fill all the terrahedral sites. CN(M)=8, CN(X)=4. The cations are relatively large compared to ordinary cases. B. Antifluorite Structure p p M 2 X, including Li 2 O, Na 2 O, and K 2 O, simple the inverse of the fluorite structure with the X ions at the FCC positions and the M ions filling all of the F 3. 5 tetrahedral positions. The cations are smaller than the anions as ordinary 16 cases.

VI. Ceramic Crystal Structure based on Chemical Formula (Considering or looking at the packing of one of the ions. ) A. AX-TYPE CRYSTAL STRUCTURES AX compounds, A: cation X: anion (1) Rock Salt Structure F 12. 2 Sodium chloride (Na. Cl), or rock salt type, coordination number: 6, cation-anion radius ratio: 0. 414― 0. 732, unit cell : FCC examples: Na. Cl, Mg. O, Mn. S, Li. F, and Fe. O. F 12. 3 (2) Cesium Chloride Structure Cs. Cl, coordination number: 8, crystal sturcture: SC (not a BCC ) (3) Zinc Blende Structure F 3. 7 -4 F 12. 4 Coordination number: 4; tetrahedrally coordinated. Zinc blende, or sphalerite, structure, e. g. , zinc sulfide (Zn. S): sach Zn atom is bonded to four S atoms, and vice versa. 17 Examples: Zn. S, Zn. Te, and Si. C.

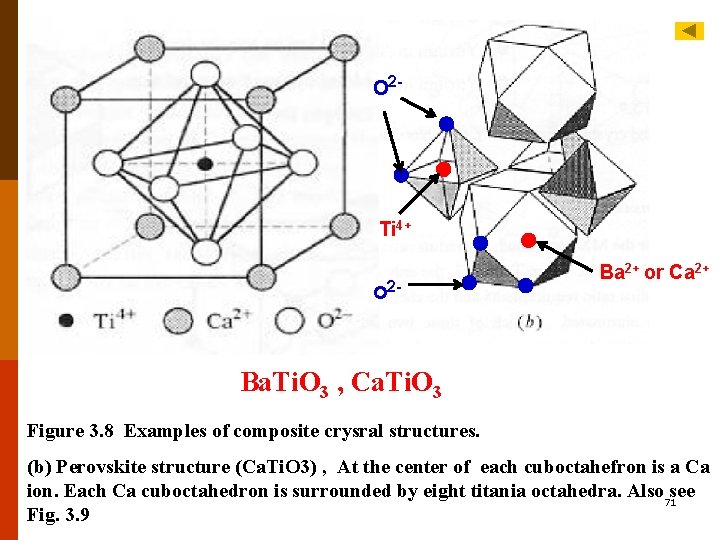
B. Am. Xp— type Crystal Structures The charges on the cations and anions are not the same. Example:fluorite structure (AX 2) and antifluorite structure (A 2 X). fluorite (Ca. F 2) :rc/r. A for Ca. F 2: 0. 8, coordination number: 8. Crystal structure would be similar to Cs. Cl except that only half the center cube positions are occupied by Ca 2+ ions. One unit cell consists of eight cubes. Other compounds: UO 2, PU 2, and Th. O 2 F 3. 7 -5 F 3. 5 F 12. 5 C. Am. Bn. Xp – TYPE CRYSTAL STRUCTURES A typical example:barium titanate (Ba. Ti. O 3), perovskite crystal 2+ ions at all structure. At temperatures above 120℃: cubic, Ba eight corners, single Ti 4+ at the cube center, O 2 - ions at the center of each of the six faces. F 12. 6 T 12 -4 18

VII. Ceramic Crystal Structures based on building blocks imagine the structure to be made of the various building blocks. A. Perovskite Structure Perovskite is a naturally occurring mineral Ca. Ti. O 3, general formula is ABX 3; larger A cations surrounded by 12 oxygens, smaller B(Ti 4+ ) ions by 6 oxygens. Na. WP 3, Ca. Sn. O 3, YAIO 3; AB 3 structures, Re. O 3, WO 3, Nb. F 3, Ta. F 3; Ti. OF. 2, Mo. OF 2 ◎ Calcium titanate, Ca. Ti. O 3 F 3. 7 -8 ◎ Barium titanate, Ba. Ti. O 3: simple tetraggonal, a=b=0. 398 nm, c=0. 403 nm. The central Ti 4+ ion does not lie in the same plane as the four oxygen atoms in the side faces of the tetragonal unit cell. 19 F 12. 6 F 3. 9 F 3. 8

Important electrical properties arising from local electric dipoles: The strength of the dipole can be altered by either an applied force or electric field. Thus, Ba. Ti. O 3 can be used as a transducer to convert electrical voltages into mechanical energy and vice versa. p Applications: telephone receivers, phonograph cartridges, and etc. B. Antifluorite Structure p F 3. 8 C. Spinel Structure F 3. 10 Named after the naturally occurring mineral Mg. Al 2 O 4, general formula is AB 2 O 4, FCC stacking of the oxygen, the cations occupy one-eighth of the tetrahedral sites and one-half of the octahedral. F 3. 6 D. Rutile Structure An idealized version consisting of Ti. O 6 octahedra, each oxygen is shared by three octahedra. Actual structure comprises 20 distorted octahedra rather than the regular ones.

E. STRUCTURE OF COVALENT CERAMICS F 12. 9 F 3. 11 The building block of silicon-based covalent ceramics (silicates, Si. C and Si 3 N 4): Si tetrahedron, e. g. , Si. O 4 in silicates, Si. C 4 in Si. C, Si. N 4 in Si 3 N 4. F. The Crystobalite Structure F 3. 4 -6 F 12. 9 F 12. 10 While Si. O 2 (silica) has three atoms per lattice site, it is much easier to visualize the structure of crystobalite in a different fashion: The basic building block for all Si-O compounds is the negatively charged (Si. O 4)4 - tetrahedron. The crystobalite crystal structure, can be envisioned as the diamond cubic structure with an (Si. O 4)4 - tetrahedron positioned on each lattice site. Thus, crystobalite has an FCC lattice with six atoms, or two tetrahedra, perlattice site. 21

VIII. Ceramic Crystal Structures From The Close Packing of Anions p A number of ceramic crystal structures may be considered in terms of close-packed planes of ions, (the large anions), the cations may reside small interstitial sites. F 3. 6 -1 p Interstitial positions, two different types: tetrahedral position and octahedral position, the coordination numbers for cations: 4 and 6, respectively. p Two factors: (1) the stacking of the close-packed anion layers: FCC or HCP (ABCABC……or ABABAB…… ); (2) the interstitial sites: for example, the rock salt crystal structure. F 3. 5 -3 22

A. Cubic close-Packed p p F 12. 2 F 3. 7 -4 The structure in which the anions are in an FCC arrangement:rock salt, rutile, zinc blende, antifluorite, perovskite and spinel. Rock salt structure:cations on each of the octahedral sites Zinc blende structure:half the tetrahedral sites are filled. B. Hexagonal close-packed p The anion arrangement is HCP: Wurtzite, nickel arsenide, cadmium odide, corundum, illmenite, and olivine. p For example, corundum (Al 2 O 3): the oxygen ions are hexagonally close-packed, Al ions fill two-thirds of octahedral sites. Wurtzite: One-half the tetrahedral sites are filled. 23 F 11. 15

Other, but not all, ceramic crystal structures may be treated in a similar manner, included are the zinc blende and perovskite sturctures. Spinel sturcture (Am. Bn. Xp): magnesium aluminate or spinel (Mg. Al 2 O 4): the O 2 - ions form an FCC lattice, M 2+ ions fill tetrahedral sites and Al 3+ reside in octahedral positions. Magnetic ceramics, or ferrites, have a crystal structure that is a slight variant of this spinel structure, and the magnetic characteristics are affected by the occupancy of tetrahedral and octahedral positions. 24

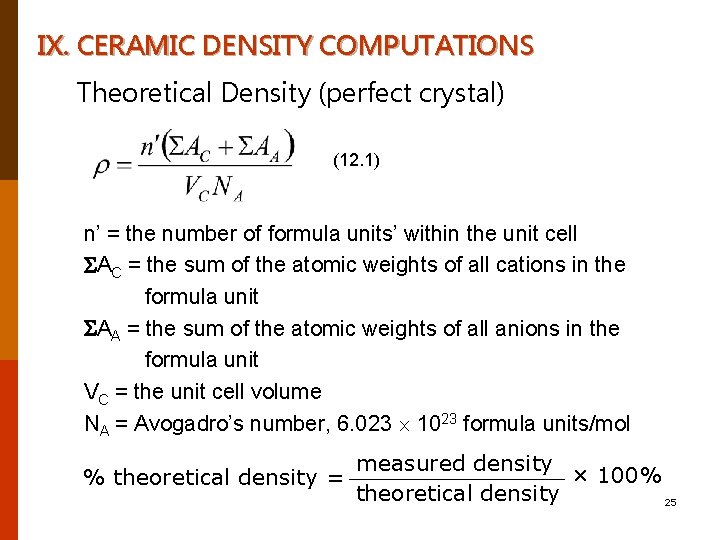
IX. CERAMIC DENSITY COMPUTATIONS Theoretical Density (perfect crystal) (12. 1) n’ = the number of formula units’ within the unit cell AC = the sum of the atomic weights of all cations in the formula unit AA = the sum of the atomic weights of all anions in the formula unit VC = the unit cell volume NA = Avogadro’s number, 6. 023 1023 formula units/mol measured density × 100% % theoretical density = theoretical density 25

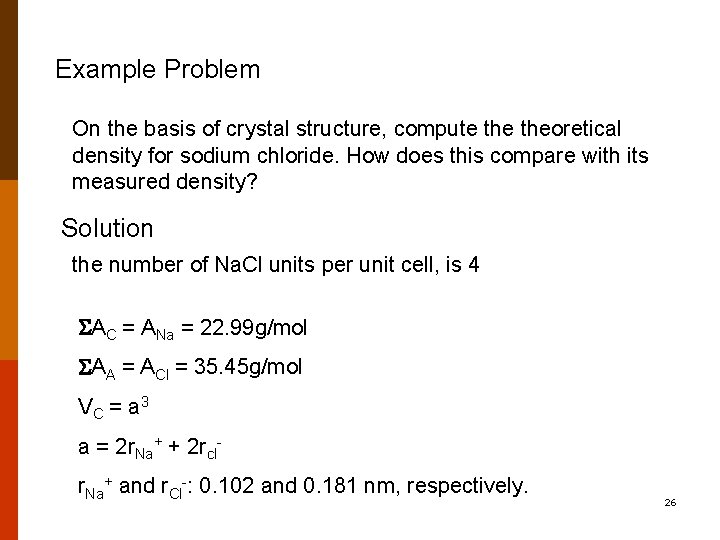
Example Problem On the basis of crystal structure, compute theoretical density for sodium chloride. How does this compare with its measured density? Solution the number of Na. Cl units per unit cell, is 4 AC = ANa = 22. 99 g/mol AA = ACl = 35. 45 g/mol VC = a 3 a = 2 r. Na+ + 2 rclr. Na+ and r. Cl-: 0. 102 and 0. 181 nm, respectively. 26
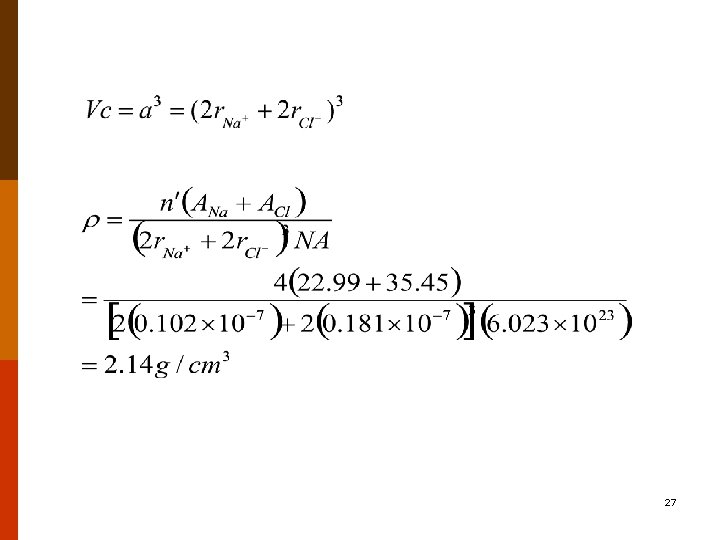
27

12. 3 Silicate Ceramics Silicates are materials composed primarily of silicon and oxygen: soils, rocks, clays, and sand. Rather than unit cells, it is more convenient to use various arrangements of an Si. O 44 - tetrahedron (Figure 12. 9) SILICA F 12 -9 Every corner oxygen atom in each tetrahedron is shared by adjacent tetrahedra. Three primary polymorphic crystalline forms: quarttz, cristobalite, and tridymite. The atoms are not closely packed to gether, silicas have relatively low densities. F 12 -10 28

Silica Glasses Noncrystalline solid or glass, called fused silica, or vitreous silica. Other oxides (e. g. , B 2 O 3 and Ge. O 2) may also form glassy structures these materials, as well as Si. O 2, are termed network formers. Common inorganic glasses: silica glasses with added other oxides such as Ca. O and Na 2 O. These oxides do not form polyhedral networks, rather modify the Si. O 44 - network: network modifiers Other oxides, such as Ti. O 2 and Al 2 O 3, while not network formers, substitute for silicon and become part of and stabilize the network; these are called intermediates. These modifiers and intermediates lowers the melting point and viscosity of a glass, and makes it easier to form at lower 29 temperatures. F 12 -11

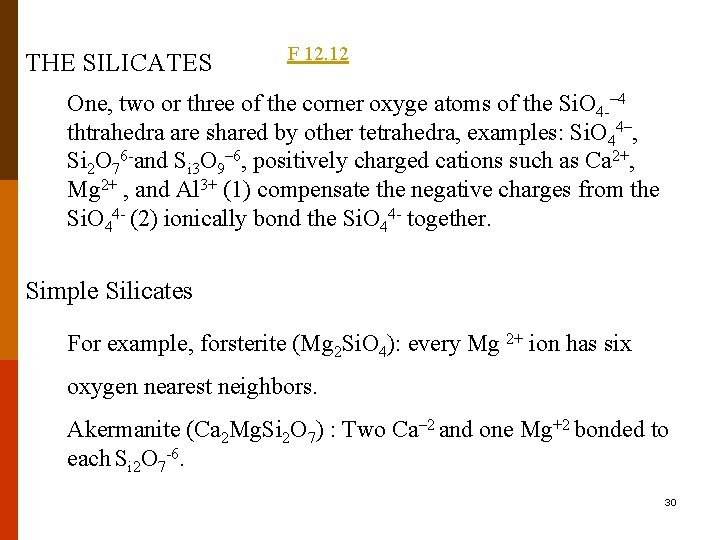
THE SILICATES F 12. 12 One, two or three of the corner oxyge atoms of the Si. O 4 -– 4 thtrahedra are shared by other tetrahedra, examples: Si. O 44–, Si 2 O 76 -and Si 3 O 9– 6, positively charged cations such as Ca 2+, Mg 2+ , and Al 3+ (1) compensate the negative charges from the Si. O 44 - (2) ionically bond the Si. O 44 - together. Simple Silicates For example, forsterite (Mg 2 Si. O 4): every Mg 2+ ion has six oxygen nearest neighbors. Akermanite (Ca 2 Mg. Si 2 O 7) : Two Ca– 2 and one Mg+2 bonded to each Si 2 O 7 -6. 30

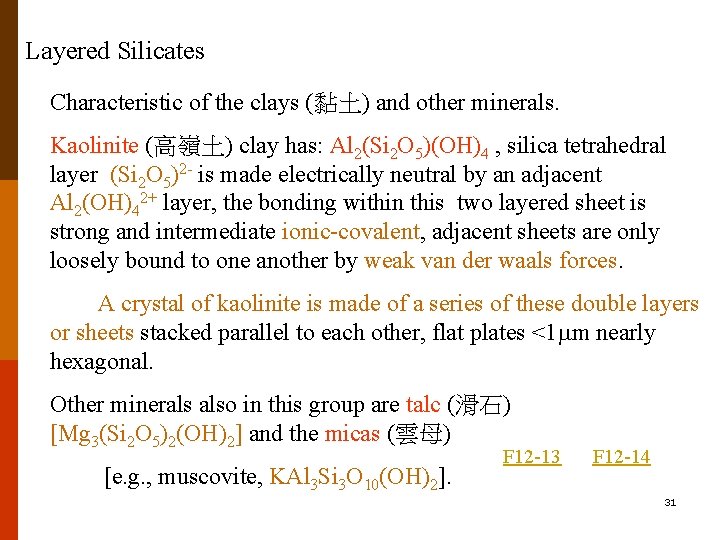
Layered Silicates Characteristic of the clays (黏土) and other minerals. Kaolinite (高嶺土) clay has: Al 2(Si 2 O 5)(OH)4 , silica tetrahedral layer (Si 2 O 5)2 - is made electrically neutral by an adjacent Al 2(OH)42+ layer, the bonding within this two layered sheet is strong and intermediate ionic-covalent, adjacent sheets are only loosely bound to one another by weak van der waals forces. A crystal of kaolinite is made of a series of these double layers or sheets stacked parallel to each other, flat plates <1 m nearly hexagonal. Other minerals also in this group are talc (滑石) [Mg 3(Si 2 O 5)2(OH)2] and the micas (雲母) [e. g. , muscovite, KAl 3 Si 3 O 10(OH)2]. F 12 -13 F 12 -14 31

12. 4 CARBON Various polymorphic forms: graphite, diamond, fullerenes, carbon nanotubes, as well as in the amorphous state. DIAMOND F 12 -15 A metastable carbon polymorph at room temperature and atmospheric pressure. Crystal structure: a variant of the zinc blende, carbon atoms occupy all positions (both Zn and S). Each carbon bonds to four other carbons and totally covalent: diamond cubic crystal structure [also: germanium, silicon, and gray tin, below 13℃ (55℉)]. 32

Physical properties: extremely hard (the hardest known material ), a very low electrical conductivity, an unusually high thermal conductivity, optically transparent in the visible and infrared regions, high index of refraction. Industrial applications: to grind or cut other softer materials. Synthetic diamonds beginning in the mid-1950 s, today a large proportion of the industrial-quality materials are man-made. Diamond thin films, for example, the surfaces of drills, dies, bearings, knives, and other tools have been coated with diamond films to increase surface hardness; some lenses and radomes. Potential applications: gears, optical recording heads and disks, and as substrates for semiconductor devices. F 12 -16 33

GRAPHTTE F 12 -17 Crystal structure: more stable than diamond at ambient temperature and pressure. Layers of hexagonally arranged carbon atoms; within the layers: strong covalent bonds; between the layers: van der waals type of bond. Weak interplanar bonds: excellent lubricative properties of graphite. Electrical conductivity is relatively high in crystallographic directions parallel to the hexagonal sheets. Other desirable properties: high strength, and good chemical stability at elevated temperatures and in nonoxidizing atmospheres, high thermal conductivity, low coefficient of thermal expansion, high resistance to thermal shock, high adsorption of gases, good machinability. Applications: heating elements, electrodes for arc welding, metallurgical crucibles, insulations in rocket nozzles, chemical reactor vessels, electrical contacts, brushes and resistors, electrodes in batteries in air purification 34 devices.

Today a large proportion of the industrial-quality materials are man-made, diammond thin films. For example, the surfaces of drills, dies, bearings, knives, and other tools have been coated with diamond films to increase surface hardness; some lenses and radomes. Potential applications: gears, to optical recording heads and disks, and as substrates for F 12 -16 semiconductor devices. GRAPHITE Crystal structure more stable than diamond at ambient temperature and pressure. layers of hexagonally arranged carbon atoms; within the layers: strong covalent bonds. Van der Waals type of bond between the layers. Weak interplanar bonds, excellent lubricative properties of graphite. Electrical conductivity is reatively high in crystallographic directions 35 parallel to the hexagonal sheets.

Other desirable properties high strength and good chemical stability at elevated temperatures and in nonoxidizing atmospheres, high thermal conductivity, low coefficient of thermal expansion high resistance to thermal shock, high adsorption of gases, good machinability. Applications: heating elements electrodes for arc welding, metallurgical crucibles, Casting molds high-temperature refractories insulations, in rocket nozzles, chemical reactor vessels, electrical contacts, brushes and resistors, electrodes in Batteries in air purification devices. 36

FULLERENES AND CARBON NANOTUBES Fullerenes Another polymorphic form of carbon discovered in 1985. Discrete molecular form consisting of a hollow spherical cluster of sixty carbon atoms: a single molecule denonted by C 60. Each molecule is composed of both hexagon (six-carbon atom) and pentagon (five-carbon atom) One such molecule: 20 hexagons and 12 pentagons. F 12 -18 37

C 60 (soccer ball. ) : buckminsterfullerene, (in honor of R. Buck -minster Fuller, ) Often referred to as “buckyball” or fullerene. Diamond and graphite: network solids; buckminsterfullerene : molecular solids In the solid state, the C 60 units form a crystalline structrue and pack together in a face-centered cubic array. As a pure crystalline solid: electrically insulating. However, with proper impuity additions: highly conductive and semiconductive. 38

Carbon Nanotubes F 12 -19 Another molecular form of carbon. Its structure consists of a single sheet of graphite, rolled into a tube, both ends of which are capped with C 60 fullerene hemispheres. Tube diameters are of a nanometer(i. e. , 100 nm or less). Each nanotube is a single molecule composed of millions of atoms; Multiple-walled carbon nanotubes also exist. These nanotubes are extremely strong and stiff, relatively ductile, and have low densities. For single-walled nanotubes, tensile strengths range between 50 and 200 Gpa (approximately an order of magnitude greater than for carbon fibers); this is the strongest known material. 39

The carbon nanotube has been termed the “ultimate fiber” and is extremely promising as a reinforcement in composite materials. Carbon nanotubes have unique and structrue-sensitive electrical charac-teristics: may behave electrically as either a metal or a semiconductor. Reported applications: flat-panel and full-color displays(i. e. , TV and computer monitors) Future electronic applications: diodes and transistors. 40

O 2 - Ti 4+ o 2 - Ba 2+ or Ca 2+ Ba. Ti. O 3 , Ca. Ti. O 3 Figure 3. 8 Examples of composite crysral structures. (b) Perovskite structure (Ca. Ti. O 3) , At the center of each cuboctahefron is a Ca ion. Each Ca cuboctahedron is surrounded by eight titania octahedra. Also 71 see Fig. 3. 9

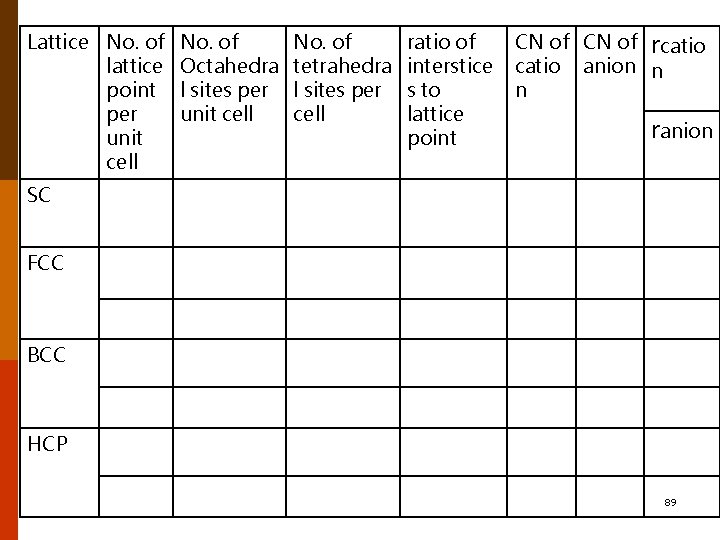
Lattice No. of lattice point per unit cell No. of Octahedra l sites per unit cell No. of tetrahedra l sites per cell ratio of interstice s to lattice point CN of rcatio anion n n ranion SC FCC BCC HCP 89