Ceramics DMT 125 MATERIALS SCIENCE Def Ceramics are

- Slides: 9

Ceramics DMT 125: MATERIALS SCIENCE

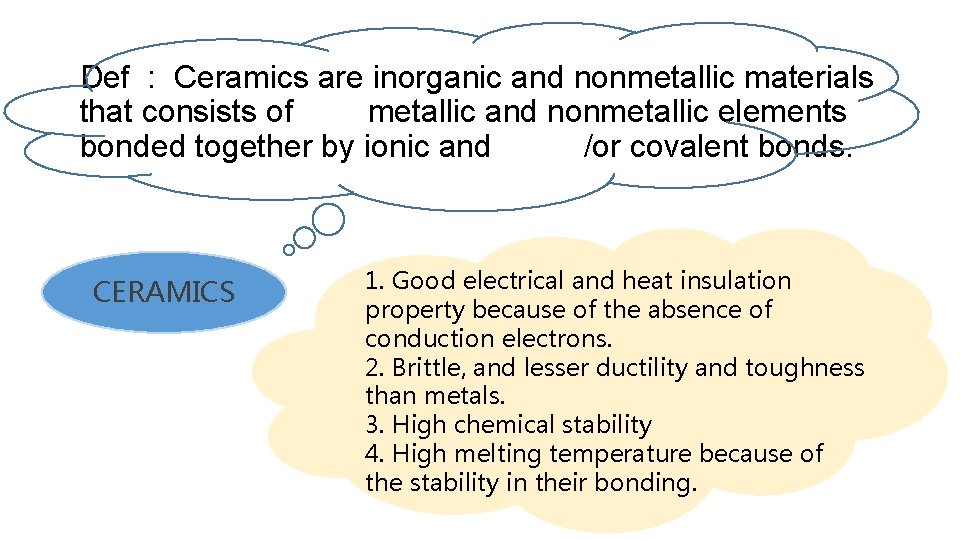
Def : Ceramics are inorganic and nonmetallic materials that consists of metallic and nonmetallic elements bonded together by ionic and /or covalent bonds. CERAMICS 1. Good electrical and heat insulation property because of the absence of conduction electrons. 2. Brittle, and lesser ductility and toughness than metals. 3. High chemical stability 4. High melting temperature because of the stability in their bonding.

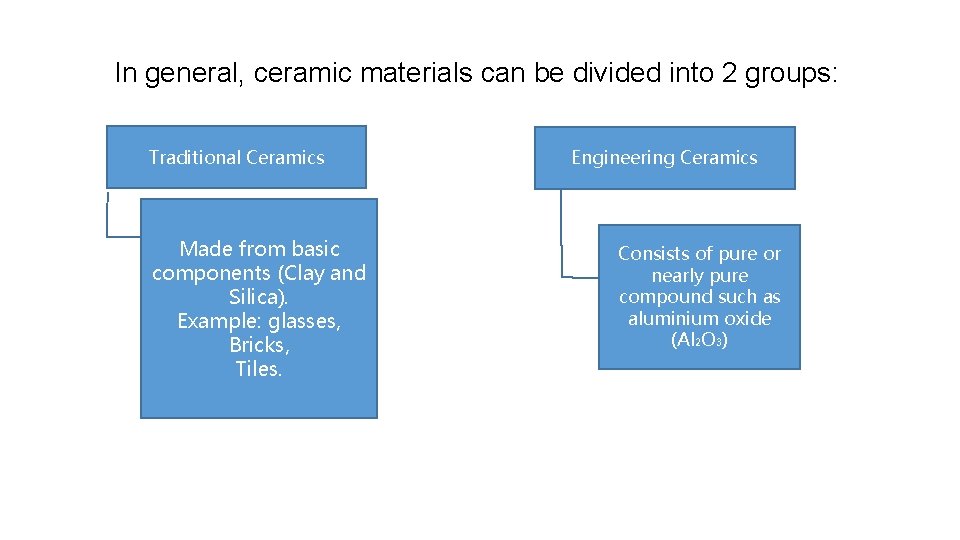
In general, ceramic materials can be divided into 2 groups: Traditional Ceramics Made from basic components (Clay and Silica). Example: glasses, Bricks, Tiles. Engineering Ceramics Consists of pure or nearly pure compound such as aluminium oxide (Al 2 O 3)

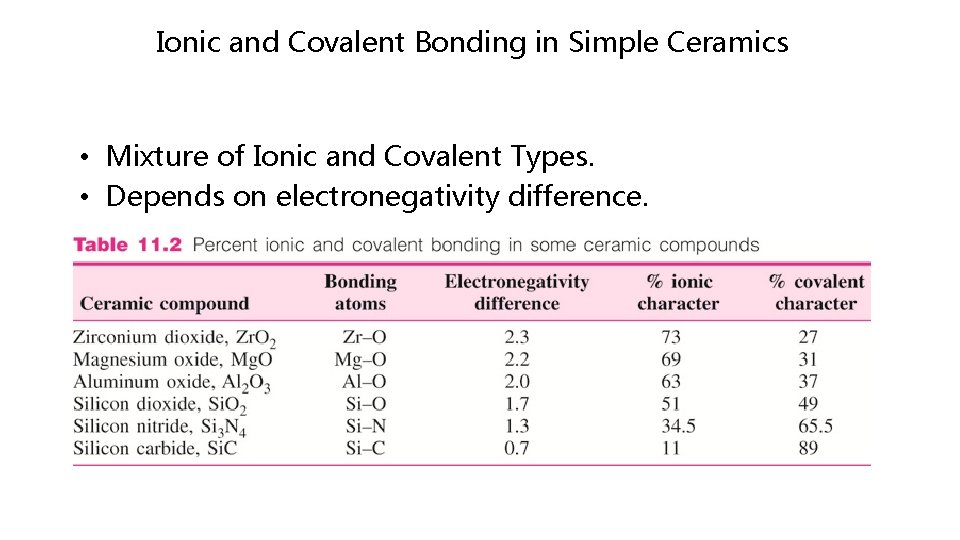
Ionic and Covalent Bonding in Simple Ceramics • Mixture of Ionic and Covalent Types. • Depends on electronegativity difference.

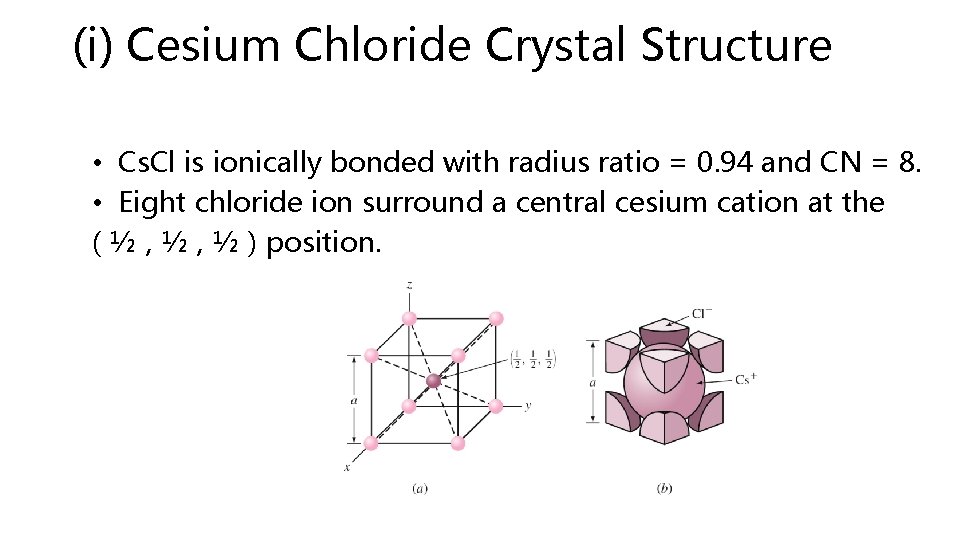
(i) Cesium Chloride Crystal Structure • Cs. Cl is ionically bonded with radius ratio = 0. 94 and CN = 8. • Eight chloride ion surround a central cesium cation at the ( ½ , ½ ) position.

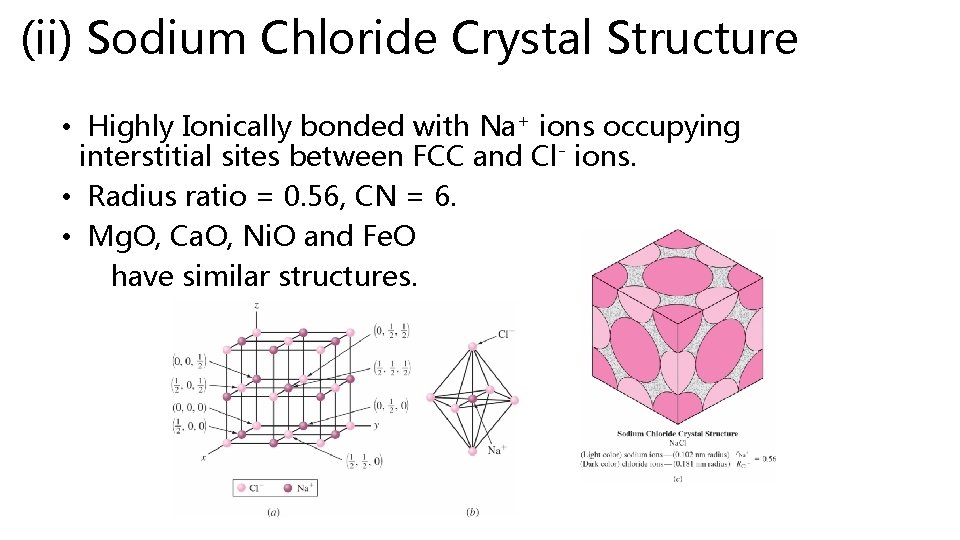
(ii) Sodium Chloride Crystal Structure • Highly Ionically bonded with Na+ ions occupying interstitial sites between FCC and Cl- ions. • Radius ratio = 0. 56, CN = 6. • Mg. O, Ca. O, Ni. O and Fe. O have similar structures.

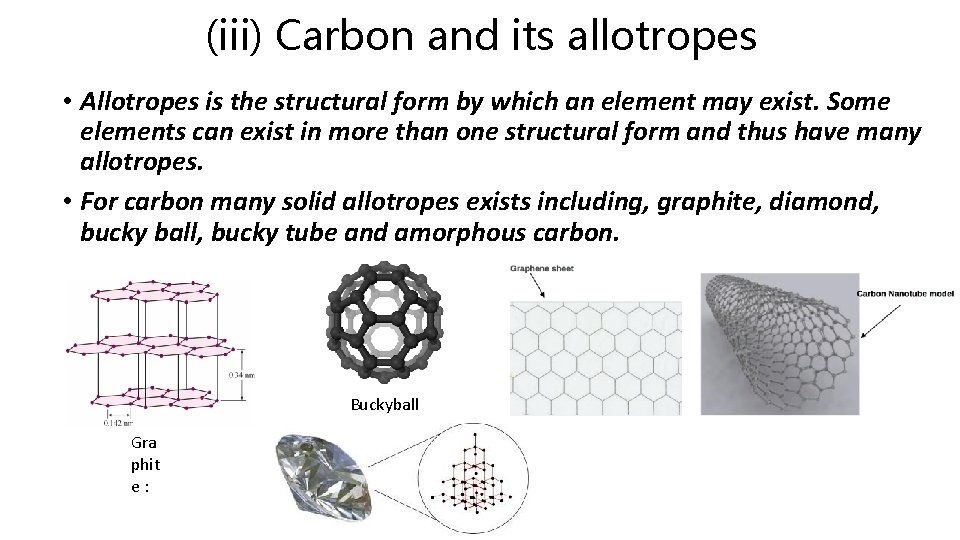
(iii) Carbon and its allotropes • Allotropes is the structural form by which an element may exist. Some elements can exist in more than one structural form and thus have many allotropes. • For carbon many solid allotropes exists including, graphite, diamond, bucky ball, bucky tube and amorphous carbon. Buckyball Gra phit e:

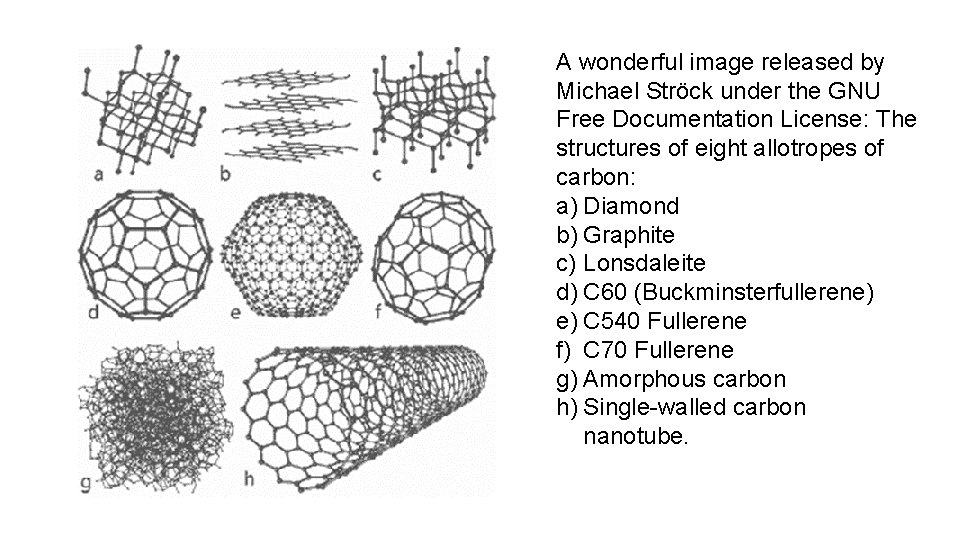
A wonderful image released by Michael Ströck under the GNU Free Documentation License: The structures of eight allotropes of carbon: a) Diamond b) Graphite c) Lonsdaleite d) C 60 (Buckminsterfullerene) e) C 540 Fullerene f) C 70 Fullerene g) Amorphous carbon h) Single-walled carbon nanotube.

Processing of Ceramics • Produced by compacting powder or particles into shapes and heated to bond particles together. 3 basic steps in the processing of ceramics by the agglomeration of particles are: 1. Material preparation: Particles and binders and lubricants are (sometimes ground) and blend wet or dry. 2. Forming: Formed in dry, plastic or liquid conditions. 3. Thermal treatment by drying and firing to a high enough temperature for the particles to bond