Chapter 13 Ceramics Materials Applications and Processing ISSUES







- Slides: 25

Chapter 13: Ceramics Materials Applications and Processing ISSUES TO ADDRESS. . . • How do we classify ceramics? • What are some applications of ceramics? • How is processing different than for metals? 1

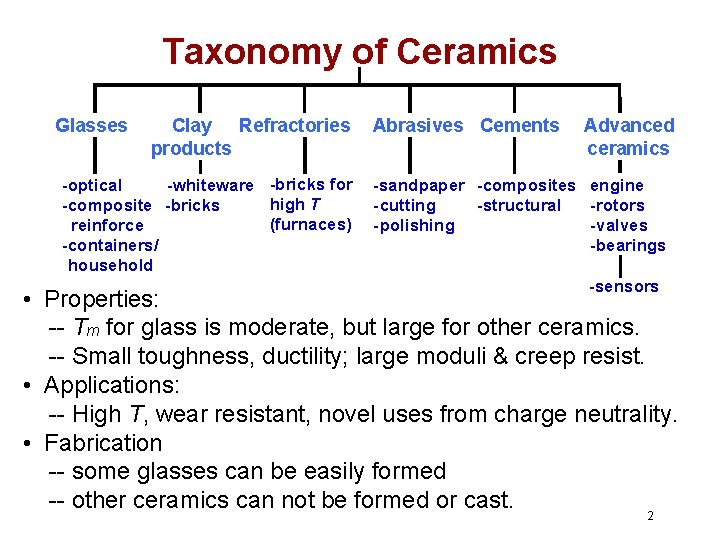
Taxonomy of Ceramics Glasses Clay Refractories products -optical -whiteware -bricks for high T -composite -bricks (furnaces) reinforce -containers/ household Abrasives Cements Advanced ceramics -sandpaper -composites engine -cutting -structural -rotors -polishing -valves -bearings -sensors • Properties: -- Tm for glass is moderate, but large for other ceramics. -- Small toughness, ductility; large moduli & creep resist. • Applications: -- High T, wear resistant, novel uses from charge neutrality. • Fabrication -- some glasses can be easily formed -- other ceramics can not be formed or cast. 2

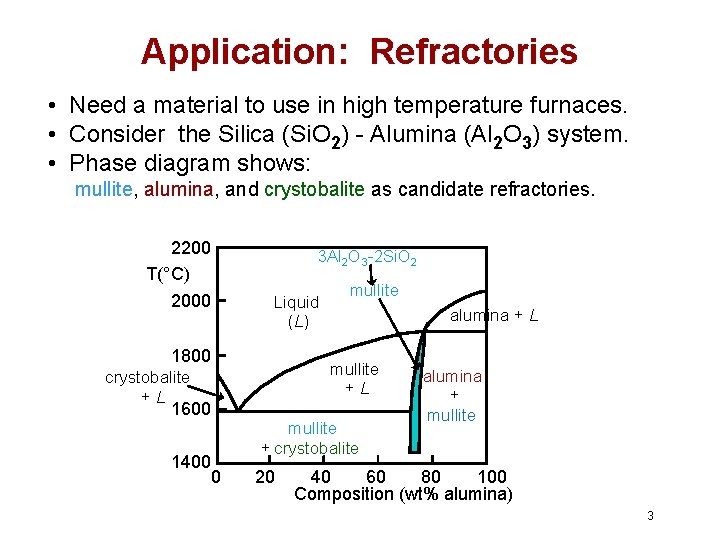
Application: Refractories • Need a material to use in high temperature furnaces. • Consider the Silica (Si. O 2) - Alumina (Al 2 O 3) system. • Phase diagram shows: mullite, alumina, and crystobalite as candidate refractories. 2200 T(°C) 2000 3 Al 2 O 3 -2 Si. O 2 Liquid (L) 1800 mullite alumina + L mullite +L crystobalite +L 1600 1400 0 mullite + crystobalite 20 alumina + mullite 40 60 80 100 Composition (wt% alumina) 3

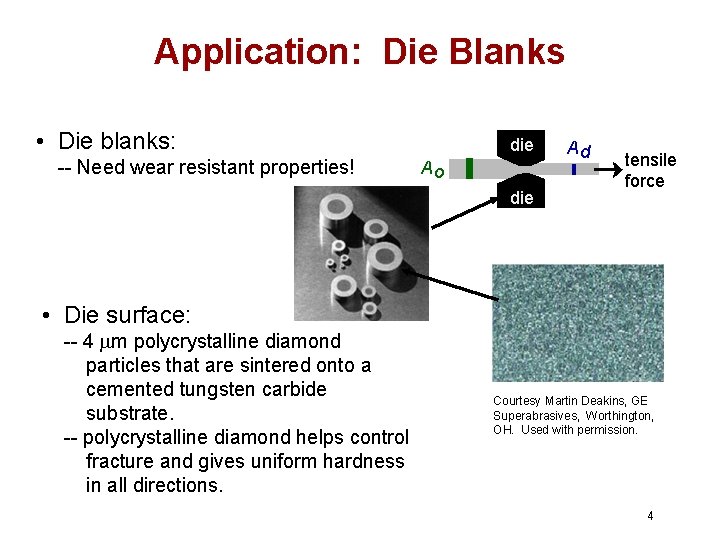
Application: Die Blanks • Die blanks: -- Need wear resistant properties! die Ao die Ad tensile force • Die surface: -- 4 mm polycrystalline diamond particles that are sintered onto a cemented tungsten carbide substrate. -- polycrystalline diamond helps control fracture and gives uniform hardness in all directions. Courtesy Martin Deakins, GE Superabrasives, Worthington, OH. Used with permission. 4

Application: Cutting Tools • Tools: -- for grinding glass, tungsten, carbide, ceramics -- for cutting Si wafers -- for oil drilling • Solutions: -- manufactured single crystal or polycrystalline diamonds in a metal or resin matrix. -- optional coatings (e. g. , Ti to help diamonds bond to a Co matrix via alloying) -- polycrystalline diamonds resharpen by microfracturing along crystalline planes. oil drill bits blades coated single crystal diamonds polycrystalline diamonds in a resin matrix. Photos courtesy Martin Deakins, GE Superabrasives, Worthington, OH. Used with permission. 5

Application: Sensors • Example: Oxygen sensor Zr. O 2 • Principle: Make diffusion of ions Ca 2+ fast for rapid response. • Approach: Add Ca impurity to Zr. O 2: A Ca 2+ impurity removes a Zr 4+ and a O 2 - ion. -- increases O 2 - vacancies -- increases O 2 - diffusion rate • Operation: -- voltage difference produced when O 2 - ions diffuse from the external surface of the sensor to the reference gas. sensor gas with an unknown, higher oxygen content O 2 diffusion + reference gas at fixed oxygen content - voltage difference produced! 6

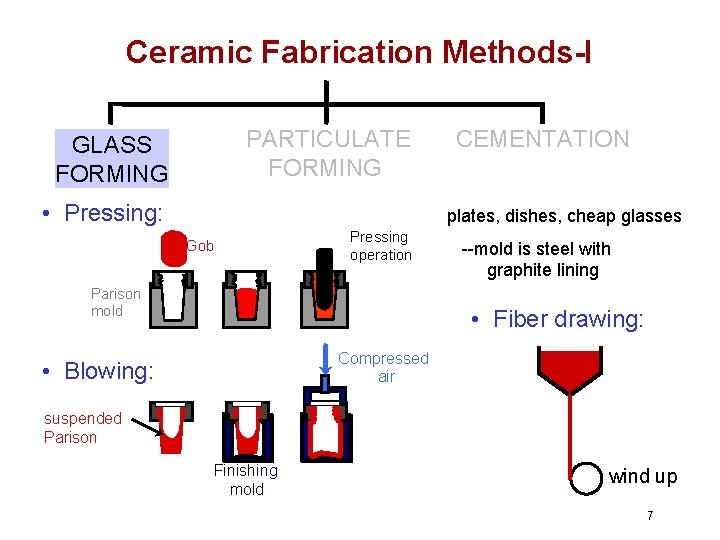
Ceramic Fabrication Methods-I PARTICULATE FORMING GLASS FORMING • Pressing: CEMENTATION plates, dishes, cheap glasses Gob Pressing operation Parison mold --mold is steel with graphite lining • Fiber drawing: Compressed air • Blowing: suspended Parison Finishing mold wind up 7

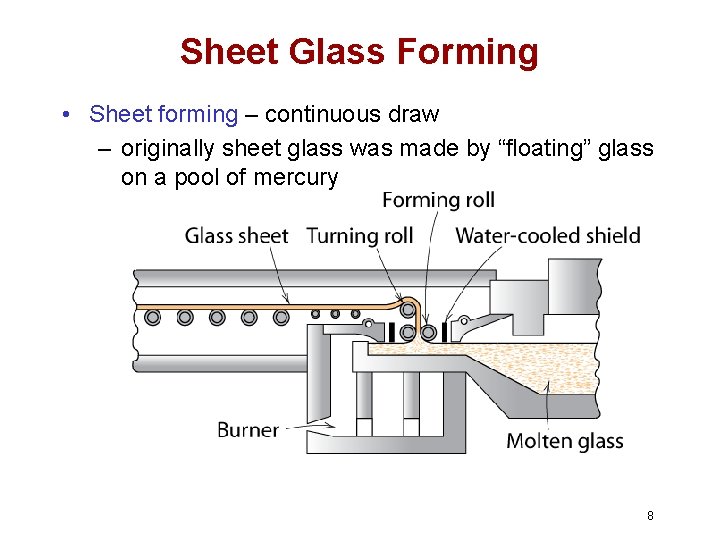
Sheet Glass Forming • Sheet forming – continuous draw – originally sheet glass was made by “floating” glass on a pool of mercury 8

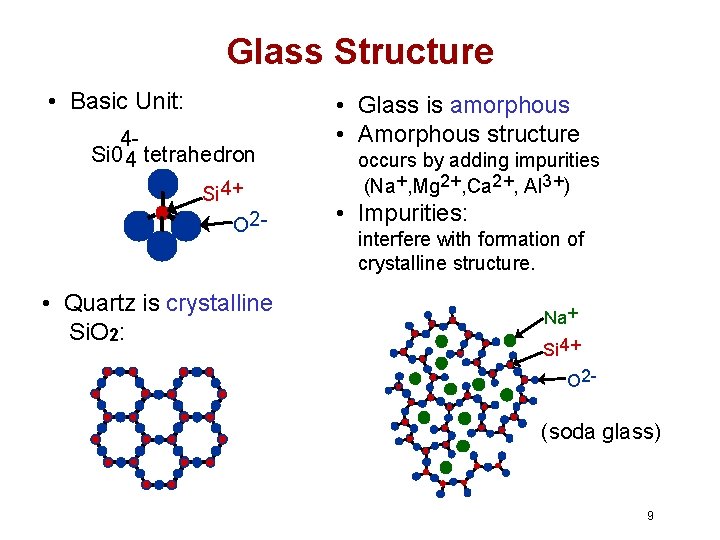
Glass Structure • Basic Unit: 4 Si 0 4 tetrahedron Si 4+ O 2 - • Quartz is crystalline Si. O 2: • Glass is amorphous • Amorphous structure occurs by adding impurities (Na+, Mg 2+, Ca 2+, Al 3+) • Impurities: interfere with formation of crystalline structure. Na + Si 4+ O 2 - (soda glass) 9

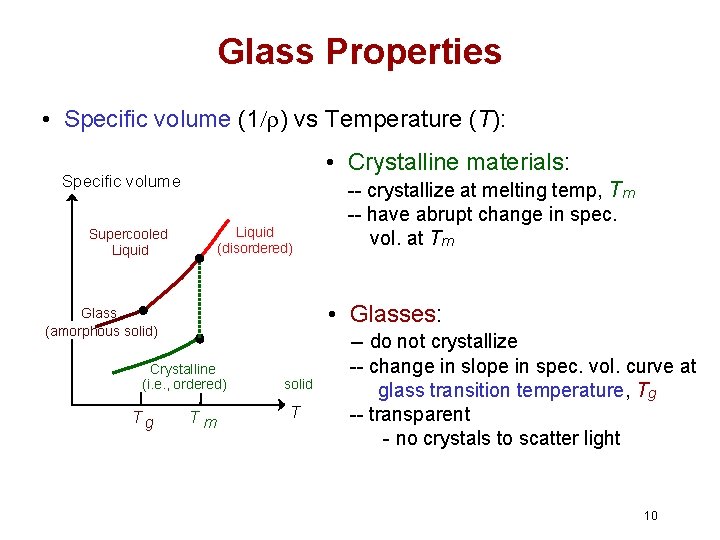
Glass Properties • Specific volume (1/r) vs Temperature (T): • Crystalline materials: Specific volume Liquid (disordered) Supercooled Liquid • Glasses: Glass (amorphous solid) Crystalline (i. e. , ordered) Tg -- crystallize at melting temp, Tm -- have abrupt change in spec. vol. at Tm Tm solid T -- do not crystallize -- change in slope in spec. vol. curve at glass transition temperature, Tg -- transparent - no crystals to scatter light 10

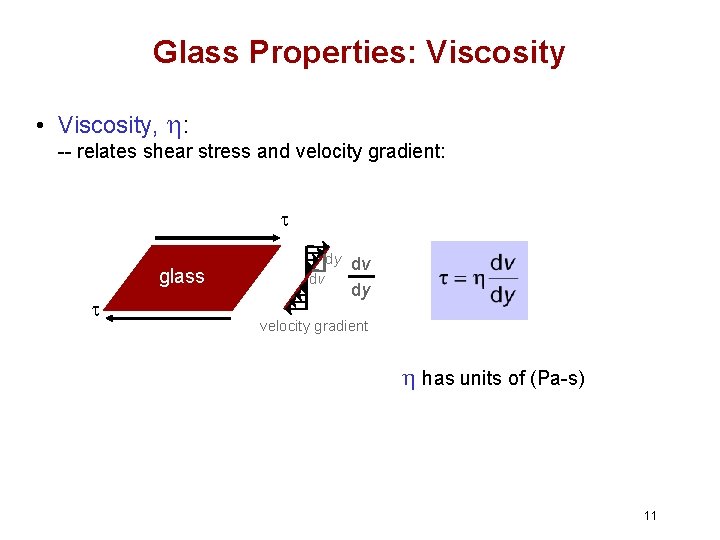
Glass Properties: Viscosity • Viscosity, h: -- relates shear stress and velocity gradient: t glass t dy dv dv dy velocity gradient h has units of (Pa-s) 11

Glass Viscosity vs. T and Impurities • Viscosity decreases with T • Impurities lower Tdeform Viscosity [Pa × s] a ilic ds a se ilic s fu x % 96 yre e P -lim da so ss gla 10 14 10 10 10 6 10 2 1 200 • soda-lime glass: 70% Si. O 2 balance Na 2 O (soda) & Ca. O (lime) • borosilicate (Pyrex): 13% B 2 O 3, 3. 5% Na 2 O, 2. 5% Al 2 O 3 • Vycor: 96% Si. O 2, 4% B 2 O 3 • fused silica: > 99. 5 wt% Si. O 2 strain point annealing range Tg Softening Point Tdeform : soft enough to deform or “work” Tmelt 600 1000 1400 1800 T(°C) Working Point 12

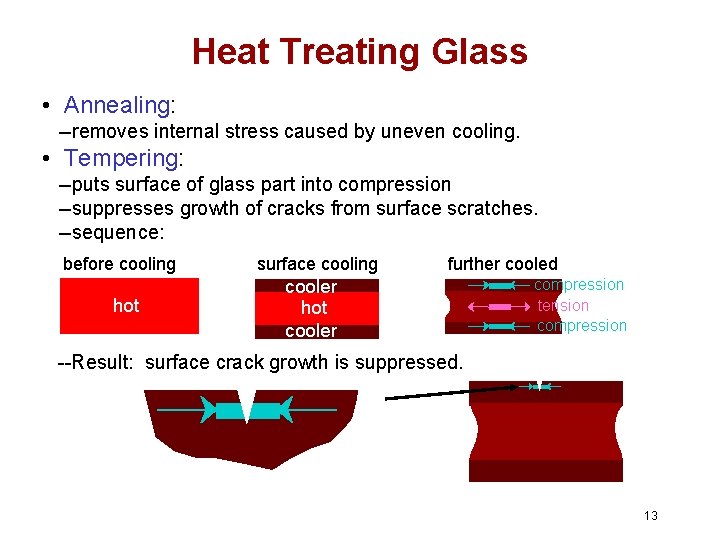
Heat Treating Glass • Annealing: --removes internal stress caused by uneven cooling. • Tempering: --puts surface of glass part into compression --suppresses growth of cracks from surface scratches. --sequence: before cooling hot surface cooling further cooled cooler hot cooler compression tension compression --Result: surface crack growth is suppressed. 13

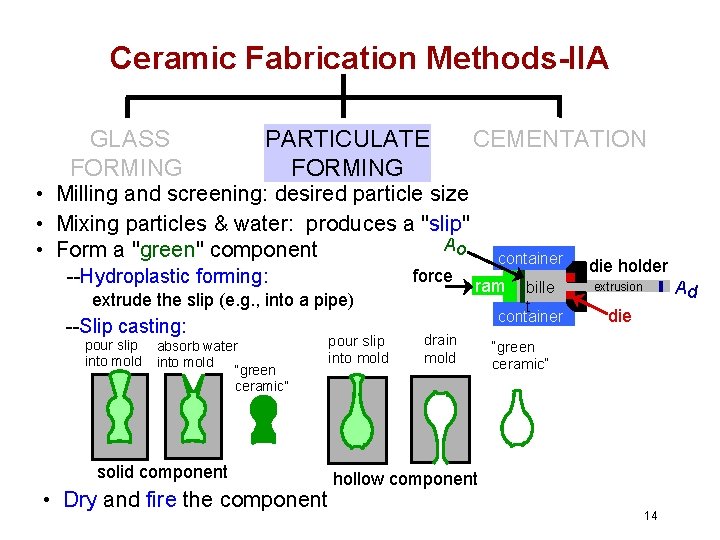
Ceramic Fabrication Methods-IIA GLASS FORMING PARTICULATE FORMING CEMENTATION • Milling and screening: desired particle size • Mixing particles & water: produces a "slip" Ao • Form a "green" component --Hydroplastic forming: force extrude the slip (e. g. , into a pipe) --Slip casting: pour slip into mold absorb water into mold “green ceramic” pour slip into mold solid component • Dry and fire the component container ram drain mold bille t container die holder Ad extrusion die “green ceramic” hollow component 14

Clay Composition A mixture of components used (50%) 1. Clay (25%) 2. Filler – e. g. quartz (finely ground) (25%) 3. Fluxing agent (Feldspar) binds it together aluminosilicates + K+, Na+, Ca+ 15

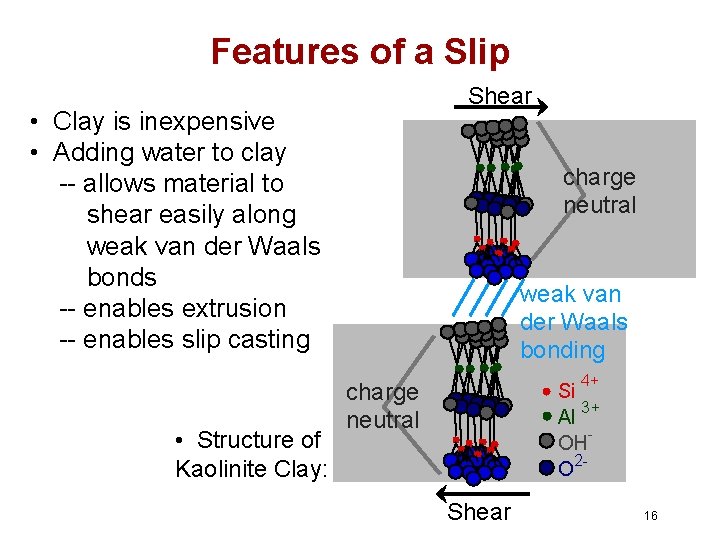
Features of a Slip Shear • Clay is inexpensive • Adding water to clay -- allows material to shear easily along weak van der Waals bonds -- enables extrusion -- enables slip casting • Structure of Kaolinite Clay: charge neutral weak van der Waals bonding 4+ charge neutral Si 3+ Al OH 2 O Shear 16

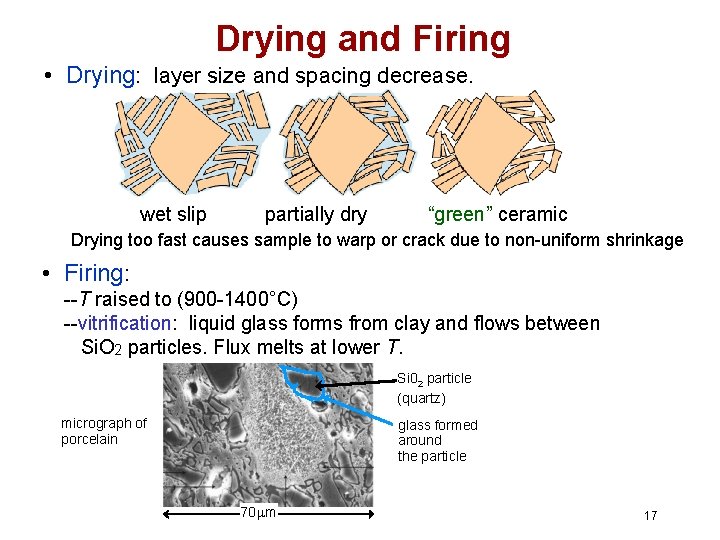
Drying and Firing • Drying: layer size and spacing decrease. wet slip partially dry “green” ceramic Drying too fast causes sample to warp or crack due to non-uniform shrinkage • Firing: --T raised to (900 -1400°C) --vitrification: liquid glass forms from clay and flows between Si. O 2 particles. Flux melts at lower T. Si 02 particle (quartz) micrograph of porcelain glass formed around the particle 70 mm 17

Ceramic Fabrication Methods-IIB GLASS FORMING PARTICULATE FORMING Sintering: useful for both clay and non-clay compositions. • Procedure: -- produce ceramic and/or glass particles by grinding -- place particles in mold -- press at elevated T to reduce pore size. • Aluminum oxide powder: -- sintered at 1700°C for 6 minutes. CEMENTATION 15 m 18

Powder Pressing Sintering • powder touches & forms neck & gradually neck thickens • add processing aids to help form neck • little or no plastic deformation Uniaxial compression - compacted in single direction Isostatic (hydrostatic) compression - pressure applied by fluid - powder in rubber envelope Hot pressing - pressure + heat 19

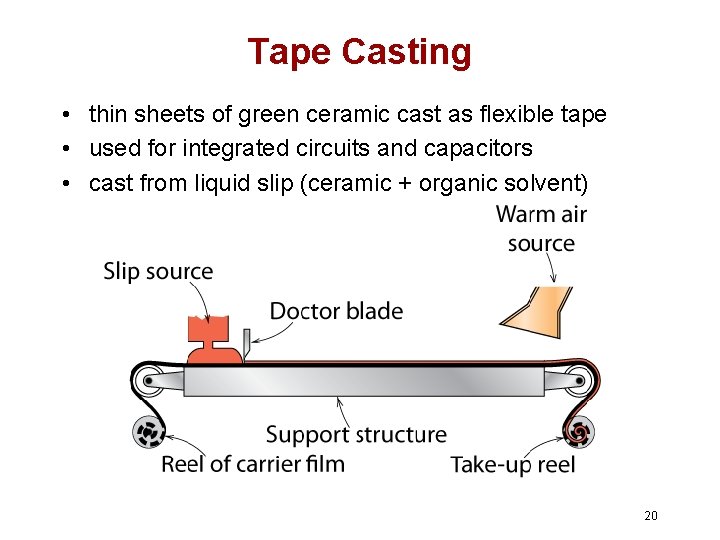
Tape Casting • thin sheets of green ceramic cast as flexible tape • used for integrated circuits and capacitors • cast from liquid slip (ceramic + organic solvent) 20

Ceramic Fabrication Methods-III • • GLASS PARTICULATE CEMENTATION FORMING Produced in extremely large quantities. Portland cement: -- mix clay and lime bearing materials -- calcinate (heat to 1400°C) -- primary constituents: tri-calcium silicate di-calcium silicate Adding water -- produces a paste which hardens -- hardening occurs due to hydration (chemical reactions with the water). Forming: done usually minutes after hydration begins. 21

Applications: Advanced Ceramics Heat Engines • Advantages: – Run at higher temperature – Excellent wear & corrosion resistance – Low frictional losses – Ability to operate without a cooling system – Low density • Disadvantages: – Brittle – Too easy to have voidsweaken the engine – Difficult to machine • Possible parts – engine block, piston coatings, jet engines Ex: Si 3 N 4, Si. C, & Zr. O 2 22

Applications: Advanced Ceramics Electronic Packaging • Chosen to securely hold microelectronics & provide heat transfer • Must match thermal expansion coefficient of the microelectronic chip & the electronic packaging material. Additional requirements include: – good heat transfer coefficient – poor electrical conductivity • Materials currently used include: • Boron nitride (BN) • Silicon Carbide (Si. C) • Aluminum nitride (Al. N) – thermal conductivity 10 x that for Alumina – good expansion match with Si 23

Applications: Advanced Ceramics • • • Ceramic Armor MEMs (Micro. Electronic. Mechanical Systems) Optical Fiber Ceramics Ball Bearing Piezoelectric Ceramics 24

Summary • Basic categories of ceramics: -- glasses -- clay products -- refractories -- cements -- advanced ceramics • Fabrication Techniques: -- glass forming (impurities affect forming temp). -- particulate forming (needed if ductility is limited) -- cementation (large volume, room T process) • Heat treating: Used to -- alleviate residual stress from cooling, -- produce fracture resistant components by putting surface into compression. 25