Materials Science Ceramics 1 Definition of ceramics The







![Electrical Properties Ceramics exhibit the largest possible diversity in electrical conductivity [ ( -cm)-1], Electrical Properties Ceramics exhibit the largest possible diversity in electrical conductivity [ ( -cm)-1],](https://slidetodoc.com/presentation_image_h/b765635a05f4f28d4272ceb90eea342b/image-27.jpg)



- Slides: 40

Materials Science Ceramics 1

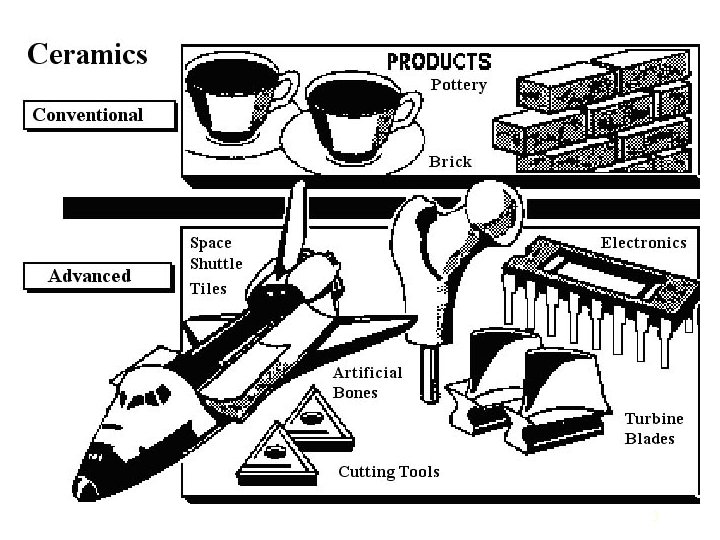
Definition of ceramics The word ceramic can be traced back to the Greek term keramos, meaning "a potter" or "pottery". Keramos in turn is related to an older Sanskrit root meaning "to burn". Thus the early Greeks used the term to mean "burned stuff" or "burned earth" when referring to products obtained through the action of fire upon earthy materials. The art and science of making and using solid articles formed by the action of heat on earthy raw materials. → Conventional ceramics (Traditional ceramics) i. e. clay product, glasses, and cement The art and science of making and using solid articles with have as their essential components, and are composed in large part of inorganic nonmetallic materials. → Conventional ceramics & New ceramics (ceramics which have either unique and outstanding properties, they have been developed in order to fulfill a particular need. Ex. electronic ceramics, bioceramics, 2

3

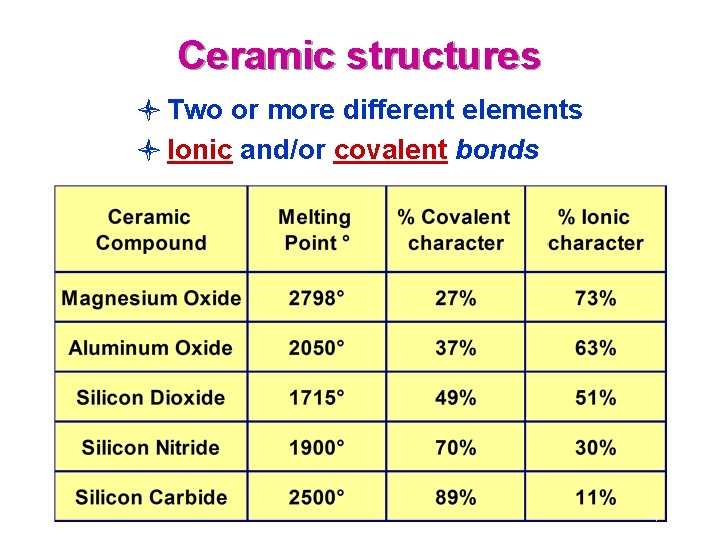
Ceramic structures l Two or more different elements l Ionic and/or covalent bonds 4

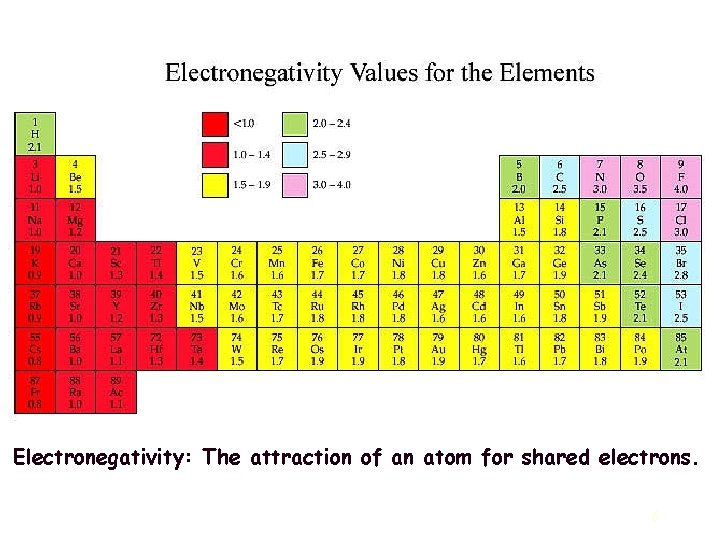
Ionic bonds most often occur between metallic and nonmetallic elements that have large differences in their electronegativities. Ionically-bonded structures tend to have rather high melting points, since the bonds are strong and nondirectional. The other major bonding mechanism in ceramic structures is the covalent bond. Unlike ionic bonds where electrons are transferred, atoms bonded covalently share electrons. Usually the elements involved are nonmetallic and have small electronegativity differences. 5

Electronegativity: The attraction of an atom for shared electrons. 6

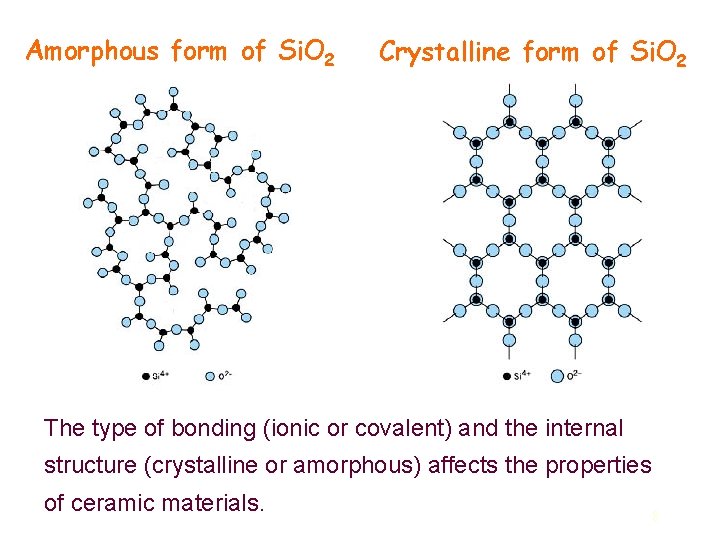
Ceramic structures l Ceramic materials can be divided into two classes: crystalline and amorphous (noncrystalline). l In crystalline materials, atoms (or ions) are arranged in a regularly repeating pattern in three dimensions (i. e. , they have long-range order). l In amorphous materials, the atoms exhibit only short-range order. l Some ceramic materials, like silicon dioxide (Si. O 2), can exist in either form. A crystalline form of Si. O 2 results when this material is slowly cooled from a high temperature (Tm>1723˙C). Rapid cooling favors noncrystalline (amorphous) formation since time is not allowed for ordered arrangements to form. 7

Amorphous form of Si. O 2 Crystalline form of Si. O 2 The type of bonding (ionic or covalent) and the internal structure (crystalline or amorphous) affects the properties of ceramic materials. 8

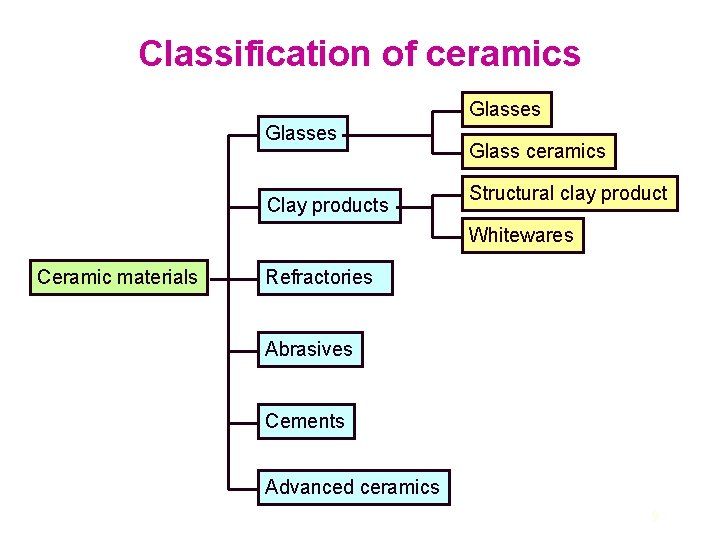
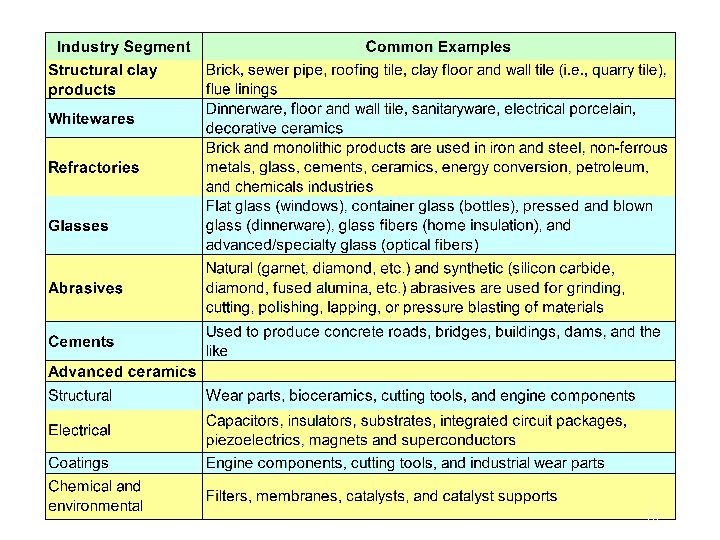
Classification of ceramics Glasses Clay products Glass ceramics Structural clay product Whitewares Ceramic materials Refractories Abrasives Cements Advanced ceramics 9

10

Typical properties of ceramics F Light weight F Corrosion resistance F Very brittle F Low and variable tensile strengths F High compressive strengths - generally much higher than tensile strength F Very high hardness, high wear and abrasion resistance F High heat capacity and low heat conductance F Electrically insulating, semiconducting, or superconducting F Nonmagnetic and magnetic 11

Chemical Properties Chemical properties describe the chemical stability of materials. The high chemical durability of the great majority of ceramic products makes them resistant to almost all acids, alkalis, and organic solvents. Ceramics are more resistant to corrosion than plastics and metals are. Of further importance is the fact that ceramic materials are not affected by oxygen. Most ceramics have very high melting points, and certain ceramics can be used up to temperatures approaching their melting points. Ceramics also remain stable over long time periods. 12

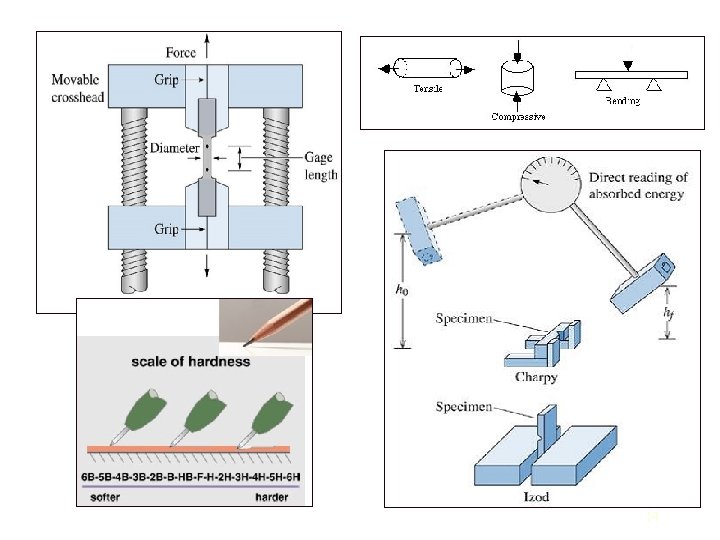
Mechanical Properties : Overview Mechanical properties describe the way that a material responds to forces, loads, and impacts. The following characteristics are commonly tested: • Tensile strength - failure under tension • Compression strength – failure under compression • Stiffness – resistance to bending (elastic deformation) • Hardness – resistance to surface penetration or scratching • Impact (Toughness) – resistance to abrupt forces • Fatigue failure – resistance to continued usage (cyclic deformation) 13

14

Deformation When materials are put into use, they undergo changes in dimensions in response to the forces they are exposed to. This is called deformation. Elastic deformation: the object reverts to its original size and shape when the load is removed. Plastic deformation: when load is removed, object has permanent change in shape Fracture occurs when the load causes the object to break into two or more pieces. 15

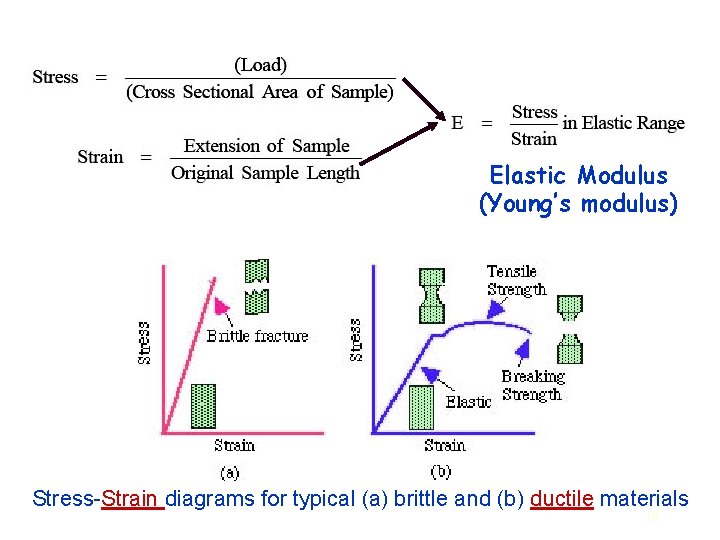
Elastic Modulus (Young’s modulus) Stress-Strain diagrams for typical (a) brittle and (b) ductile materials 16

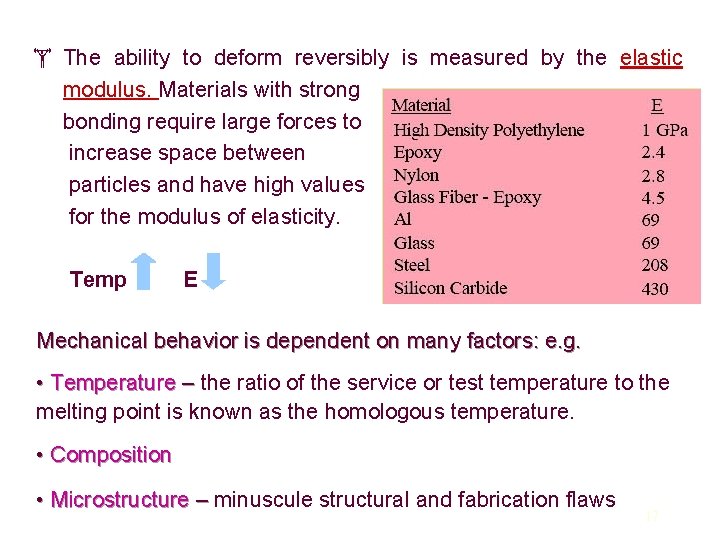
The ability to deform reversibly is measured by the elastic modulus. Materials with strong bonding require large forces to increase space between particles and have high values for the modulus of elasticity. Temp E Mechanical behavior is dependent on many factors: e. g. • Temperature – the ratio of the service or test temperature to the melting point is known as the homologous temperature. • Composition • Microstructure – minuscule structural and fabrication flaws 17

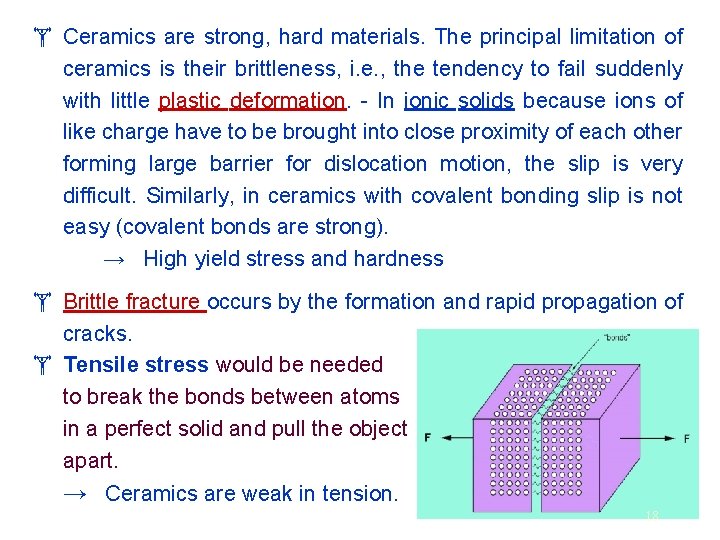
Ceramics are strong, hard materials. The principal limitation of ceramics is their brittleness, i. e. , the tendency to fail suddenly with little plastic deformation. - In ionic solids because ions of like charge have to be brought into close proximity of each other forming large barrier for dislocation motion, the slip is very difficult. Similarly, in ceramics with covalent bonding slip is not easy (covalent bonds are strong). → High yield stress and hardness Brittle fracture occurs by the formation and rapid propagation of cracks. Tensile stress would be needed to break the bonds between atoms in a perfect solid and pull the object apart. → Ceramics are weak in tension. 18

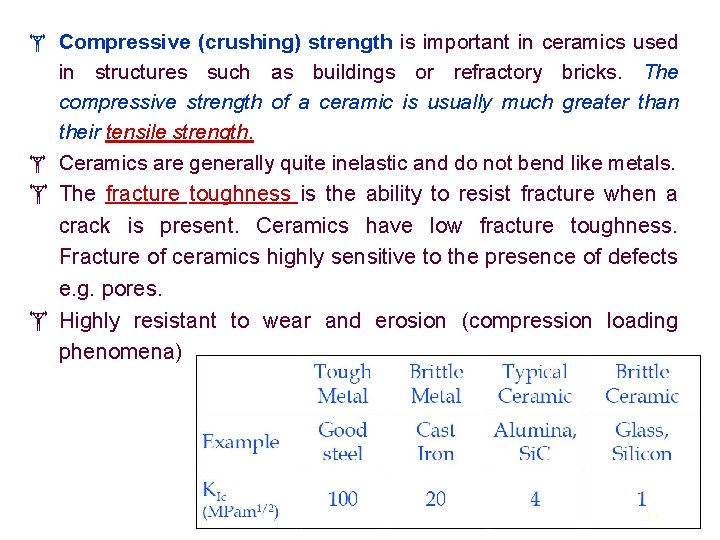
Compressive (crushing) strength is important in ceramics used in structures such as buildings or refractory bricks. The compressive strength of a ceramic is usually much greater than their tensile strength. Ceramics are generally quite inelastic and do not bend like metals. The fracture toughness is the ability to resist fracture when a crack is present. Ceramics have low fracture toughness. Fracture of ceramics highly sensitive to the presence of defects e. g. pores. Highly resistant to wear and erosion (compression loading phenomena) 19

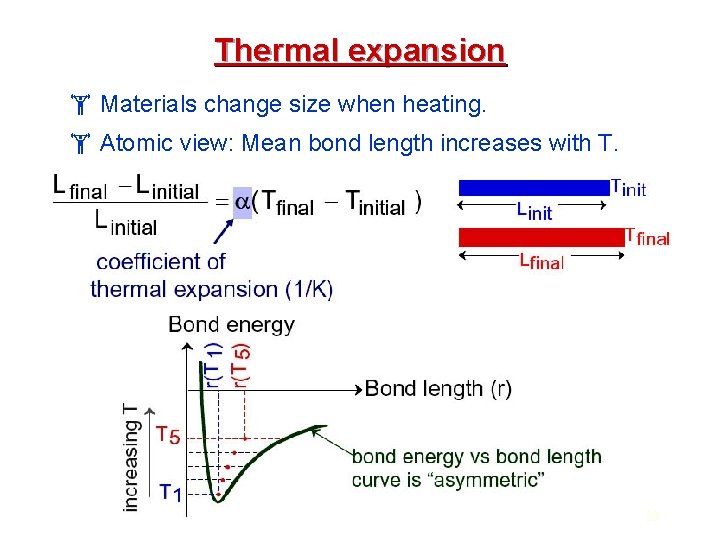
Thermal Properties The most important thermal properties of ceramic materials are heat capacity, thermal expansion coefficient, and thermal conductivity. In solid materials at T > 0 K, atoms are constantly vibrating. Thermal conductivity : The ability to carry thermal energy (heat). Thermal energy can be either stored or transmitted by a solid. The ability of a material to absorb heat from its surrounding is its heat capacity (The ability of a material to absorb heat). Thermal expansion coefficient Fractional change in length divided by change in temperature, a measure of a materials tendency to expand when heated. 20

21

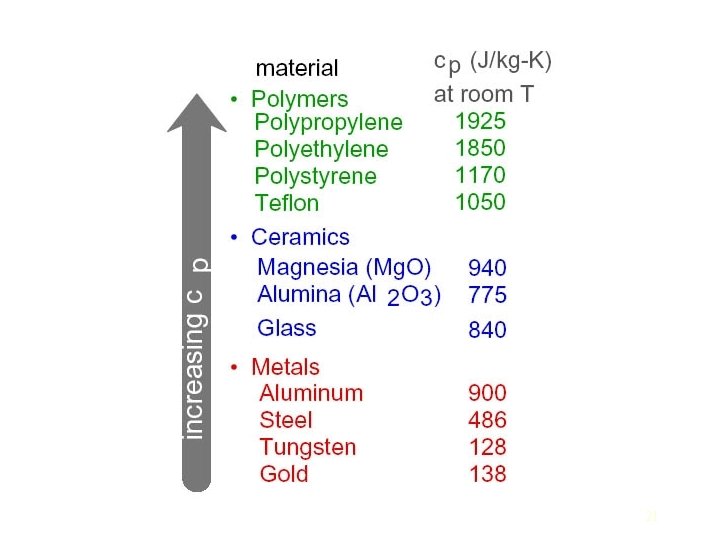
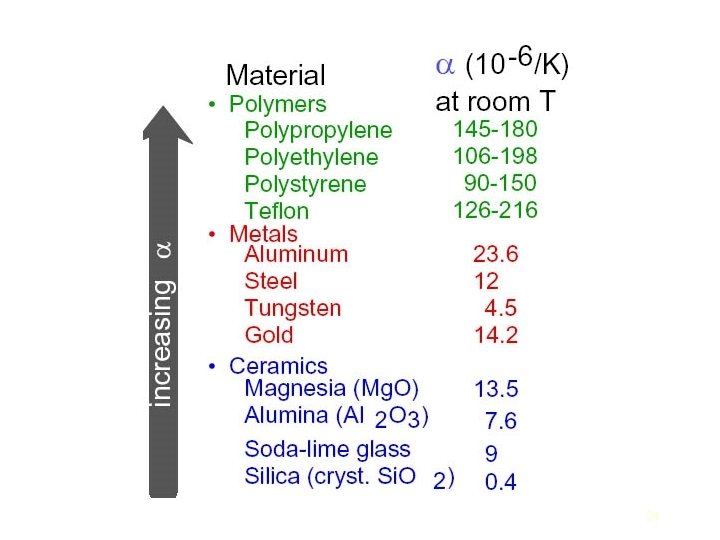
The potential energy between two bonded atoms is related to their bond length. Ceramics generally have strong bonds and light atoms. The result is that they typically have both high heat capacities and high melting temperatures. The conduction of heat through a solid involves the transfer of energy between vibrating atoms. The vibration of each atom affects the motion of neighboring atoms, and the result is elastic waves that propagate through the solid. Amorphous ceramics which lack the ordered lattice undergo even greater scattering, and therefore are poor conductors. Those ceramic materials that are composed of particles of similar size and mass with simple structures (such as diamond or Be. O) undergo the smallest amount of scattering and therefore have the greatest conductivity. 22

Thermal expansion Materials change size when heating. Atomic view: Mean bond length increases with T. 23

24

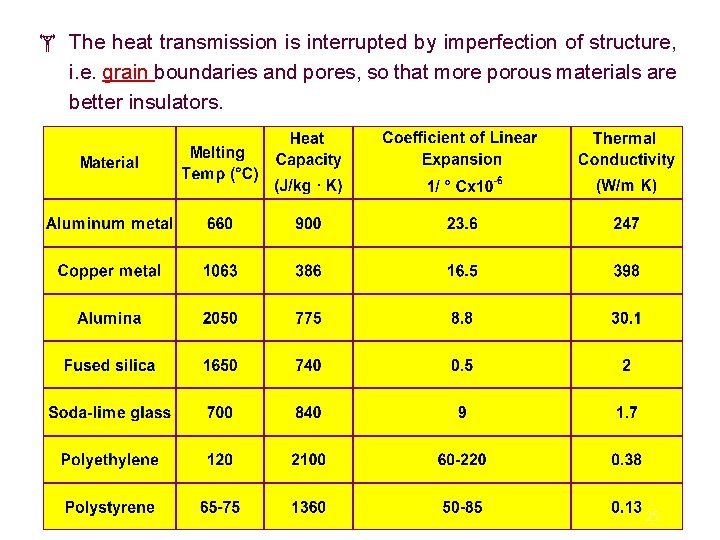
The heat transmission is interrupted by imperfection of structure, i. e. grain boundaries and pores, so that more porous materials are better insulators. 25

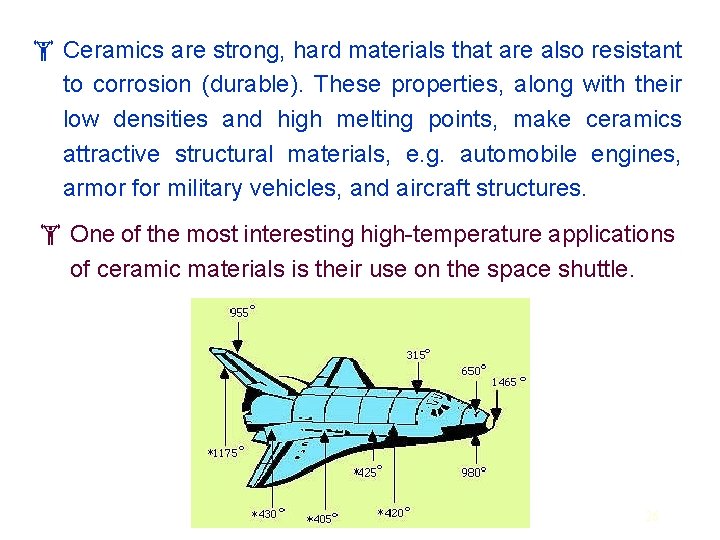
Ceramics are strong, hard materials that are also resistant to corrosion (durable). These properties, along with their low densities and high melting points, make ceramics attractive structural materials, e. g. automobile engines, armor for military vehicles, and aircraft structures. One of the most interesting high-temperature applications of ceramic materials is their use on the space shuttle. 26
![Electrical Properties Ceramics exhibit the largest possible diversity in electrical conductivity cm1 Electrical Properties Ceramics exhibit the largest possible diversity in electrical conductivity [ ( -cm)-1],](https://slidetodoc.com/presentation_image_h/b765635a05f4f28d4272ceb90eea342b/image-27.jpg)
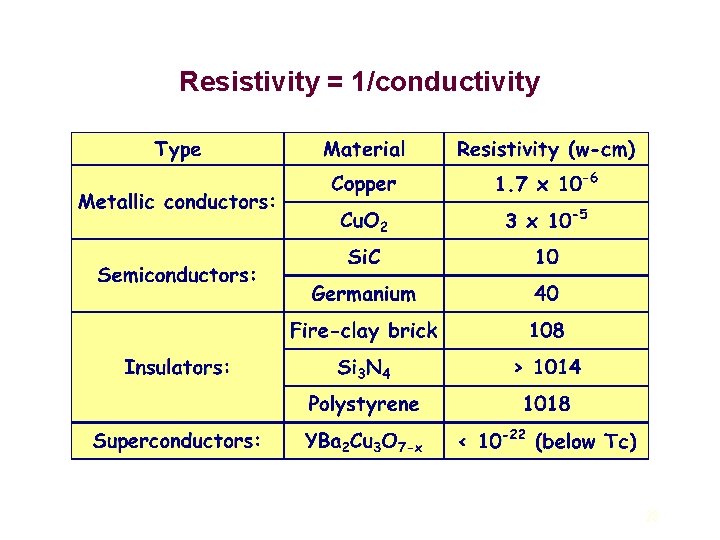
Electrical Properties Ceramics exhibit the largest possible diversity in electrical conductivity [ ( -cm)-1], in terms of the type and magnitude of the conductivity: insulators, < 10 -22 ( -cm)-1 (such as alumina) ionic conductors, ~ 10 -2 ( -cm)-1 (such as Ag. I) electronic semi-conductors, ~ 100 ( -cm)-1 (such as Si. C) electronic conductors, >103 ( -cm)-1 (such as Ti. N) electronic superconductors, (such as YBa 2 Cu 3 O 7 -x) Electrical current in solids is most often the result of the flow of electrons (electronic conduction). 27

Resistivity = 1/conductivity 28

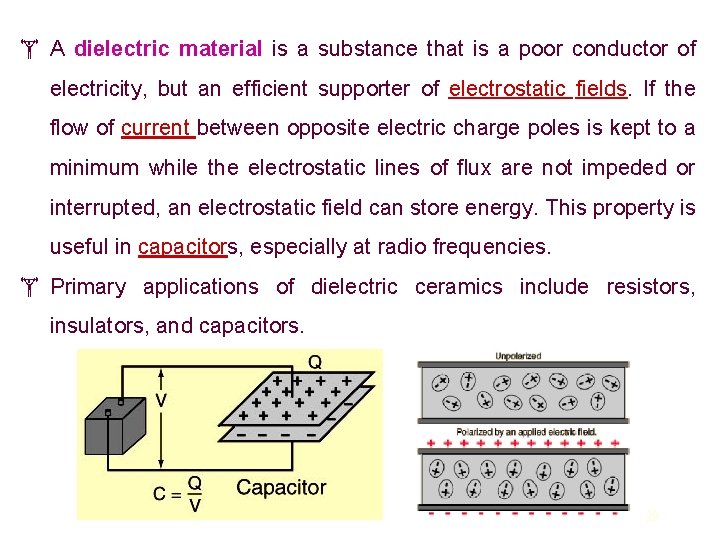
A dielectric material is a substance that is a poor conductor of electricity, but an efficient supporter of electrostatic fields. If the flow of current between opposite electric charge poles is kept to a minimum while the electrostatic lines of flux are not impeded or interrupted, an electrostatic field can store energy. This property is useful in capacitors, especially at radio frequencies. Primary applications of dielectric ceramics include resistors, insulators, and capacitors. 29

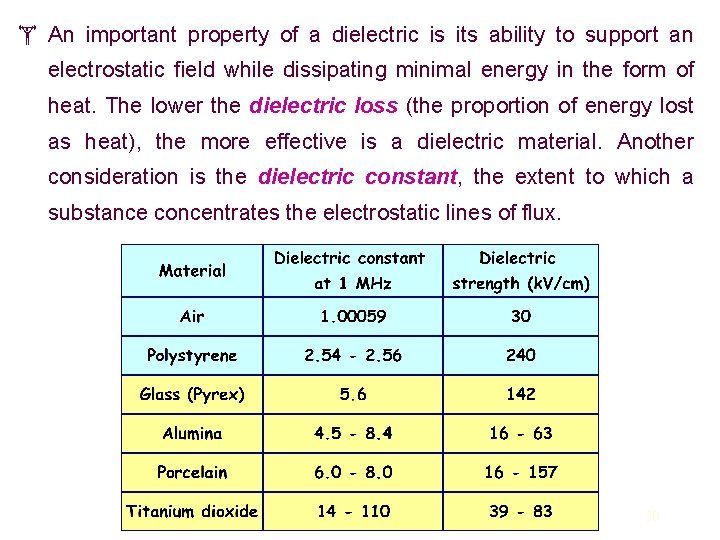
An important property of a dielectric is its ability to support an electrostatic field while dissipating minimal energy in the form of heat. The lower the dielectric loss (the proportion of energy lost as heat), the more effective is a dielectric material. Another consideration is the dielectric constant, the extent to which a substance concentrates the electrostatic lines of flux. 30

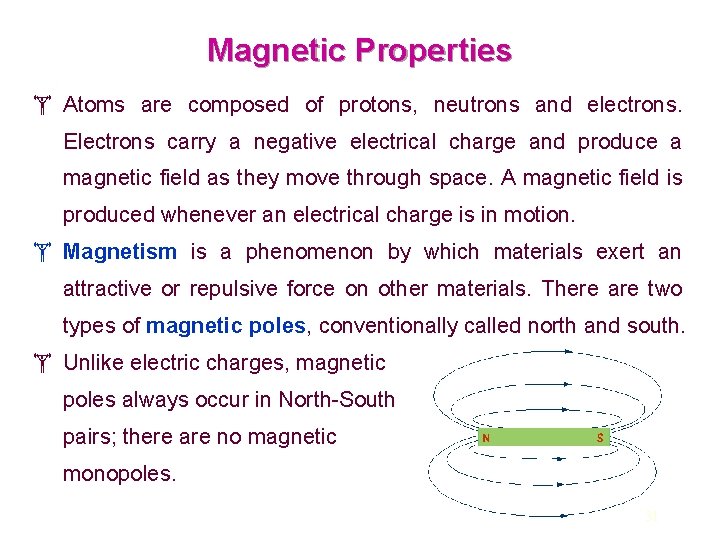
Magnetic Properties Atoms are composed of protons, neutrons and electrons. Electrons carry a negative electrical charge and produce a magnetic field as they move through space. A magnetic field is produced whenever an electrical charge is in motion. Magnetism is a phenomenon by which materials exert an attractive or repulsive force on other materials. There are two types of magnetic poles, conventionally called north and south. Unlike electric charges, magnetic poles always occur in North-South pairs; there are no magnetic monopoles. 31

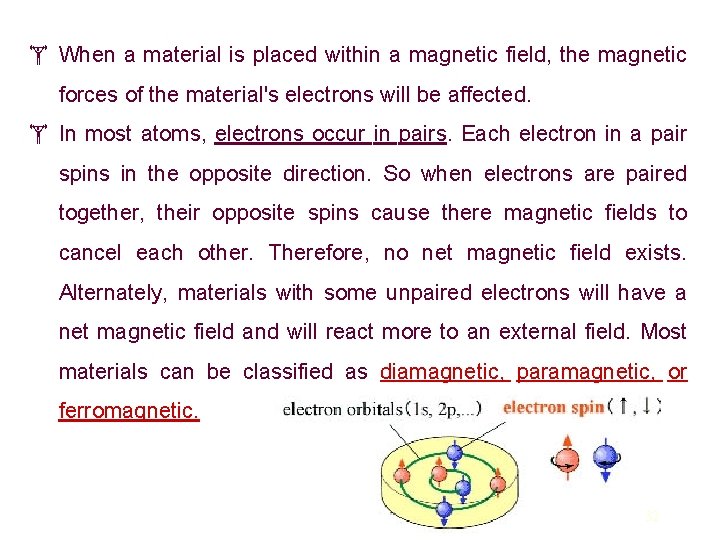
When a material is placed within a magnetic field, the magnetic forces of the material's electrons will be affected. In most atoms, electrons occur in pairs. Each electron in a pair spins in the opposite direction. So when electrons are paired together, their opposite spins cause there magnetic fields to cancel each other. Therefore, no net magnetic field exists. Alternately, materials with some unpaired electrons will have a net magnetic field and will react more to an external field. Most materials can be classified as diamagnetic, paramagnetic, or ferromagnetic. 32

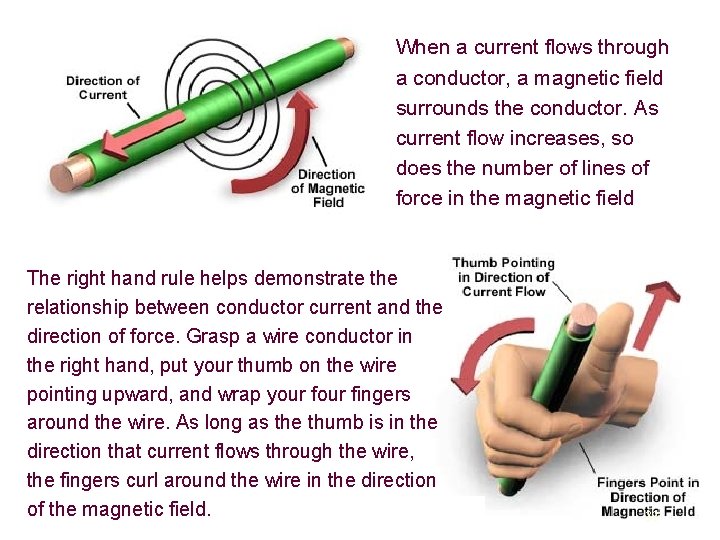
When a current flows through a conductor, a magnetic field surrounds the conductor. As current flow increases, so does the number of lines of force in the magnetic field The right hand rule helps demonstrate the relationship between conductor current and the direction of force. Grasp a wire conductor in the right hand, put your thumb on the wire pointing upward, and wrap your fingers around the wire. As long as the thumb is in the direction that current flows through the wire, the fingers curl around the wire in the direction of the magnetic field. 33

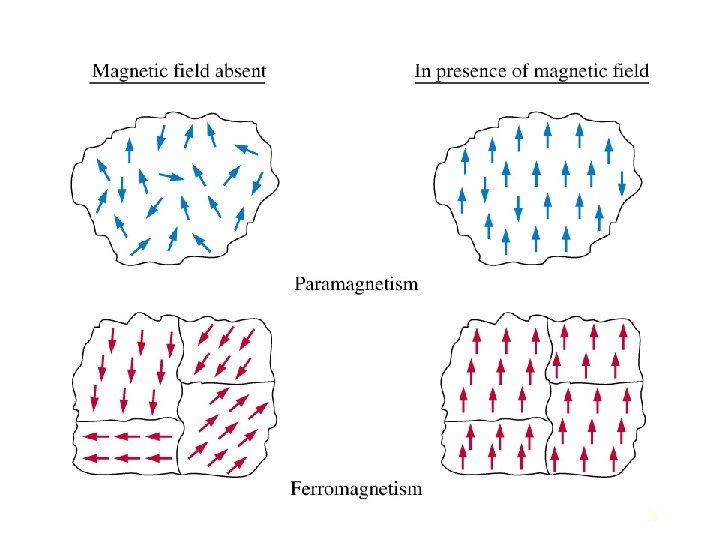
Diamagnetic materials have a very weak and negative susceptibility to magnetic fields. Diamagnetic materials are slightly repelled by a magnetic field and the material does not retain the magnetic properties when the external field is removed. Diamagnetic materials are solids with all paired electron and, therefore, no permanent net magnetic moment per atom. Diamagnetic properties arise from the realignment of the electron orbits under the influence of an external magnetic field. Most elements in the periodic table, including copper, silver, and gold, are diamagnetic. Paramagnetic materials have a small and positive susceptibility to magnetic fields. These materials are slightly attracted by a magnetic field and the material does not retain the magnetic properties when the external field is removed. Paramagnetic properties are due to the presence of some unpaired electrons and from the realignment of the electron orbits caused by the external magnetic field. Paramagnetic materials include magnesium, molybdenum, lithium, and tantalum. 34

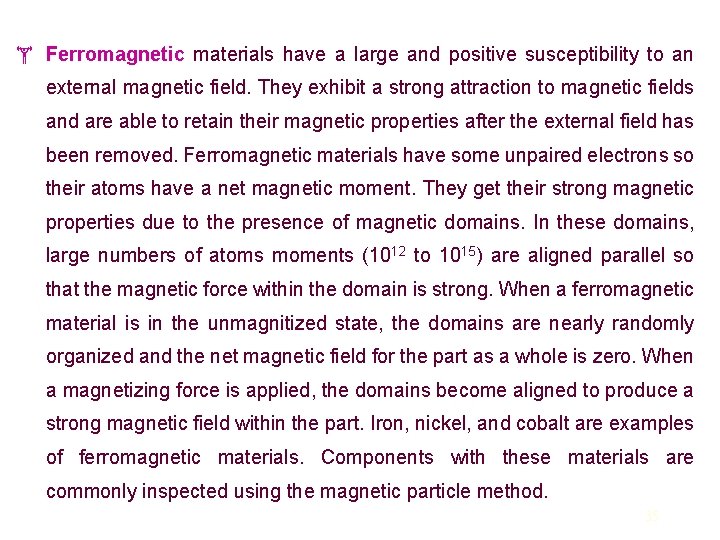
Ferromagnetic materials have a large and positive susceptibility to an external magnetic field. They exhibit a strong attraction to magnetic fields and are able to retain their magnetic properties after the external field has been removed. Ferromagnetic materials have some unpaired electrons so their atoms have a net magnetic moment. They get their strong magnetic properties due to the presence of magnetic domains. In these domains, large numbers of atoms moments (1012 to 1015) are aligned parallel so that the magnetic force within the domain is strong. When a ferromagnetic material is in the unmagnitized state, the domains are nearly randomly organized and the net magnetic field for the part as a whole is zero. When a magnetizing force is applied, the domains become aligned to produce a strong magnetic field within the part. Iron, nickel, and cobalt are examples of ferromagnetic materials. Components with these materials are commonly inspected using the magnetic particle method. 35

36

Ceramics containing iron oxide (Fe 2 O 3) can have magnetic properties similar to those of iron, nickel, and cobalt magnets. These iron oxide-based ceramics are called ferrites. Other magnetic ceramics include oxides of nickel, manganese, and barium. Ceramic magnets, used in electric motors and electronic circuits, can be manufactured with high resistance to demagnetization. When electrons become highly aligned, as they do in ceramic magnets, they create a powerful magnetic field which is more difficult to disrupt (demagnetize) by breaking the alignment of the electrons. 37

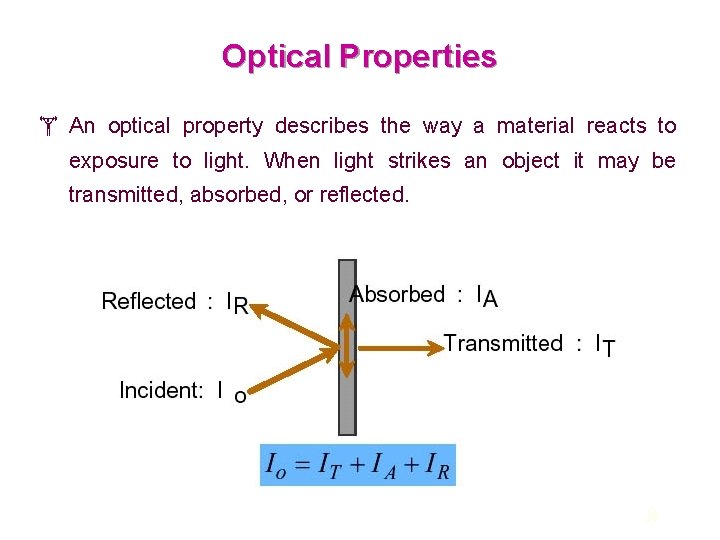
Optical Properties An optical property describes the way a material reacts to exposure to light. When light strikes an object it may be transmitted, absorbed, or reflected. 38

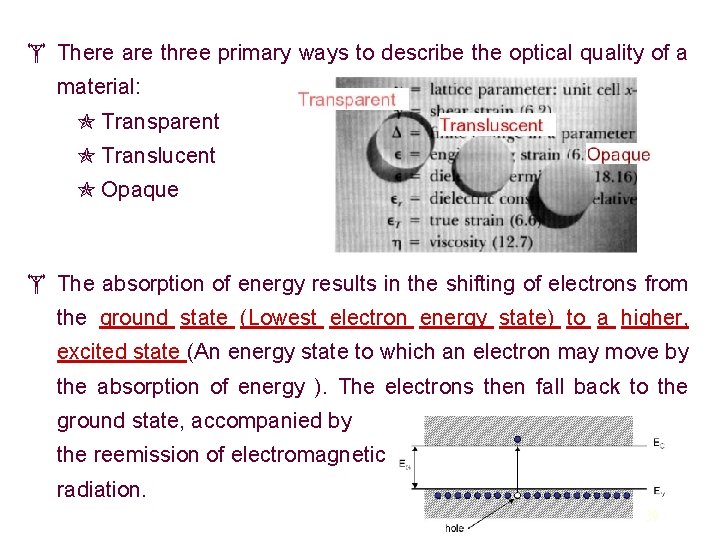
There are three primary ways to describe the optical quality of a material: Transparent Translucent Opaque The absorption of energy results in the shifting of electrons from the ground state (Lowest electron energy state) to a higher, excited state (An energy state to which an electron may move by the absorption of energy ). The electrons then fall back to the ground state, accompanied by the reemission of electromagnetic radiation. 39

The energy range for visible light is from 1. 8 to 3. 1 e. V. Materials with band gap energies (Eg) in this range will absorb those corresponding colors (energies) and transmit the others. They will appear transparent and colored. Materials with band gap energies less than 1. 8 e. V will be opaque because all visible light will be absorbed by electron Light that is emitted from electron transitions in solids is called luminescence. If it occurs for a short time it is fluorescence, and if it lasts for a longer time it is phosphorescence. Conversions between light and electricity are the basis for the use of semiconducting materials such as gallium arsenide in lasers and the widespread use of LED's (light-emitting diodes) in electronic devices. Fluorescent and phosphorescent ceramics are used in electric lamps and television screens. 40
 Ceramics science definition
Ceramics science definition Eric’s favourite .......... is science.
Eric’s favourite .......... is science. Slip and score ceramics definition
Slip and score ceramics definition Clay vocabulary
Clay vocabulary Hand building ceramics definition
Hand building ceramics definition Opaque ceramics definition
Opaque ceramics definition Ietf mud
Ietf mud Sprigging ceramics definition
Sprigging ceramics definition Score definition ceramics
Score definition ceramics Coiling definition art
Coiling definition art Raku-nn
Raku-nn Greenware def
Greenware def Pinch pot ceramics definition
Pinch pot ceramics definition Define bisqueware
Define bisqueware Hand building ceramics definition
Hand building ceramics definition Dicor
Dicor Pinching ceramics definition
Pinching ceramics definition Drape mold ceramics definition
Drape mold ceramics definition Natural materials and man made materials
Natural materials and man made materials Useful and harmful
Useful and harmful Natural man made
Natural man made Adopting materials
Adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra