Clay Types Study Guide Types of Colloids crystalline











- Slides: 28

Clay Types Study Guide • Types of Colloids – – • • • crystalline silicate clays (covered by this guide) non-crystalline silicate clays (p 314) Fe & Al oxides (p 315, 322 ff) Organic (p 315, 325) Basis for distinguishing silicate clay types Isomorphous substitution Review of clay types Distribution Weathering & generalized distribution in US

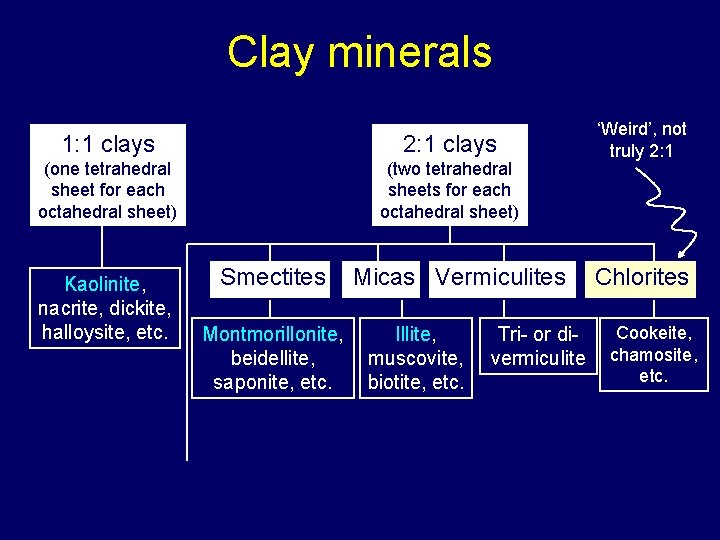
Basis for distinguishing crystalline silicate clays • Based on numbers & combinations of structural units – tetrahedral and octahedral sheets – planes combined sheets combined layers crystals (fig 8. 4) • Two general categories: 1: 1, 2: 1 – 2: 1 types: expanding & nonexpanding – also “ 2: 1: 1” Chlorites • Number of cations in octahedral sheet – tri- vs. di-octahedra (fig 8. 5) • Size and location of layer charge (see also lecture 16 slides) • Type of bonding between layers (see also lecture 16 slides): – Strong: ionic > H-bonding > van der Waals : Weak • Absence or presence of a cation interlayer àfine-grained micas • See lecture 16 slides: review of diff’s in properties of clay types

Clay minerals 1: 1 clays 2: 1 clays (one tetrahedral sheet for each octahedral sheet) (two tetrahedral sheets for each octahedral sheet) Kaolinite, nacrite, dickite, halloysite, etc. Smectites Montmorillonite, beidellite, saponite, etc. Micas Vermiculites Illite, muscovite, biotite, etc. Tri- or divermiculite ‘Weird’, not truly 2: 1 Chlorites Cookeite, chamosite, etc.

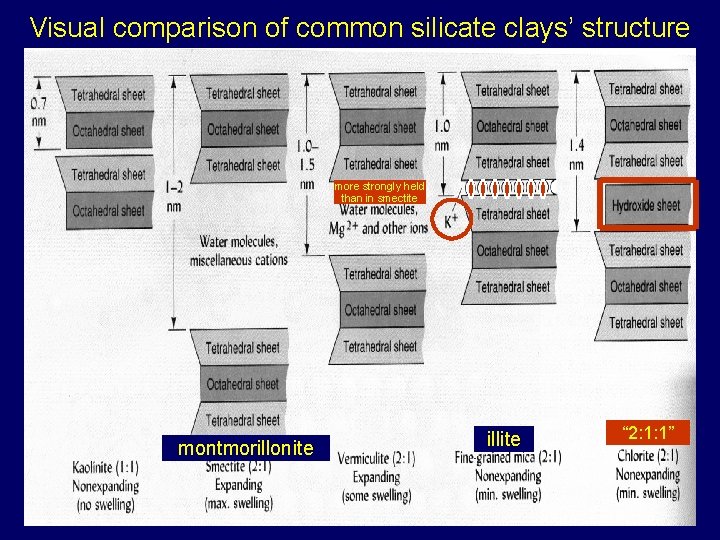
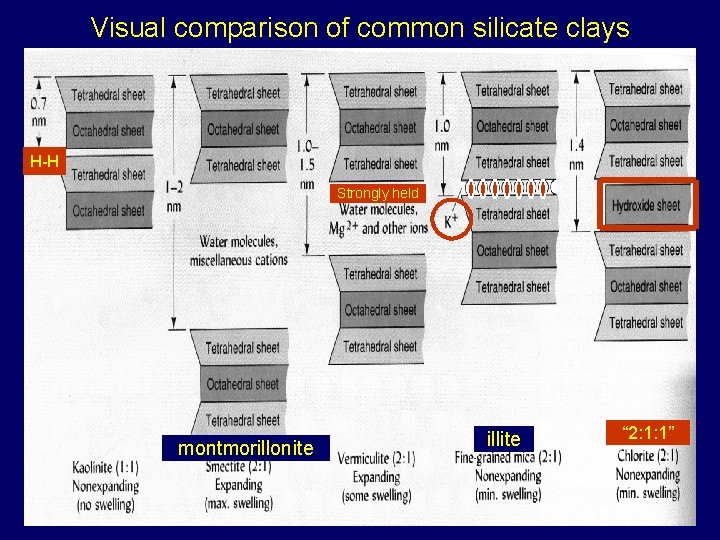
Visual comparison of common silicate clays’ structure more strongly held than in smectite montmorillonite illite “ 2: 1: 1”

Isomorphous substitution equal shape/size • The replacement of one ion for another of similar size within the crystalline structure of the clay • Often results in change in net charge takes eons – doesn’t change rapidly

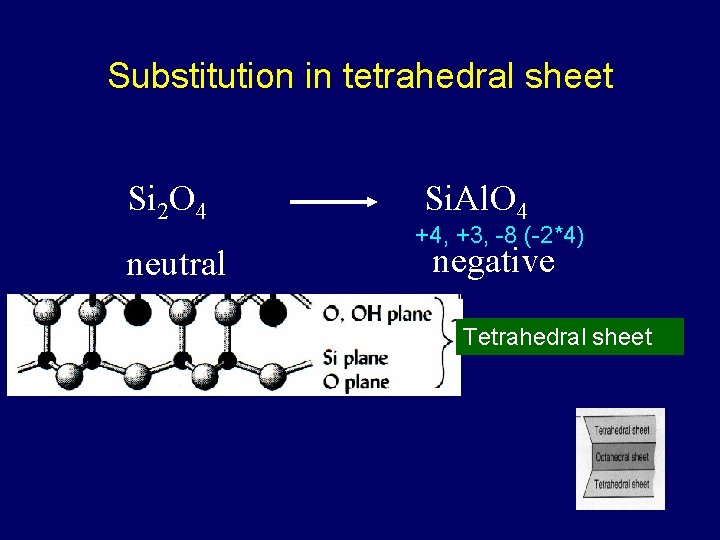
Substitution in tetrahedral sheet Si 2 O 4 neutral Si. Al. O 4 +4, +3, -8 (-2*4) negative Tetrahedral sheet

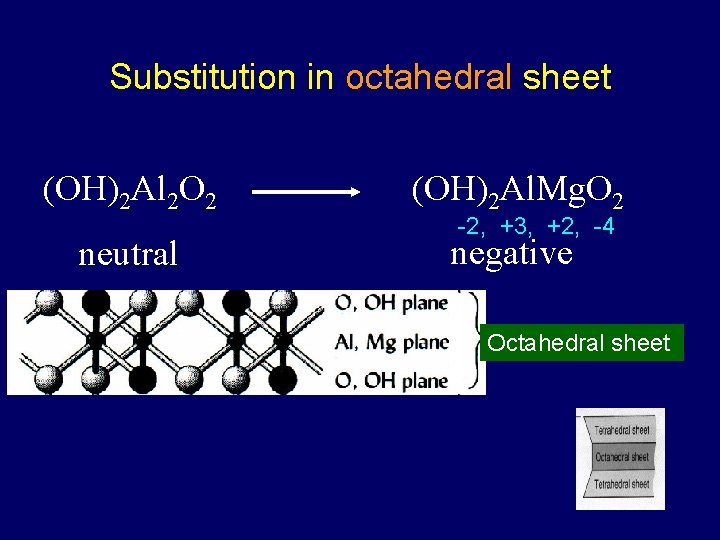
Substitution in octahedral sheet (OH)2 Al 2 O 2 (OH)2 Al. Mg. O 2 neutral negative -2, +3, +2, -4 Octahedral sheet

1: 1 Silicate Clays • Layers composed of one tetrahedral sheet bound to one octahedral sheet • Kaolinite: one of the most widespread clay minerals in soils; most abundant in warm moist climates • Stable at low p. H, the most weathered of the silicate clays • Synthesized under equal concentrations of Al 3+ and Si 4+

Kaolinite • • A 1: 1 clay Little or no isomorphous substitution “nutrient poor” No shrink-swell (stable ‘cuz of Hbonding between adjacent layers) • A product of acid weathering (low p. H, common in soils of the SE USA

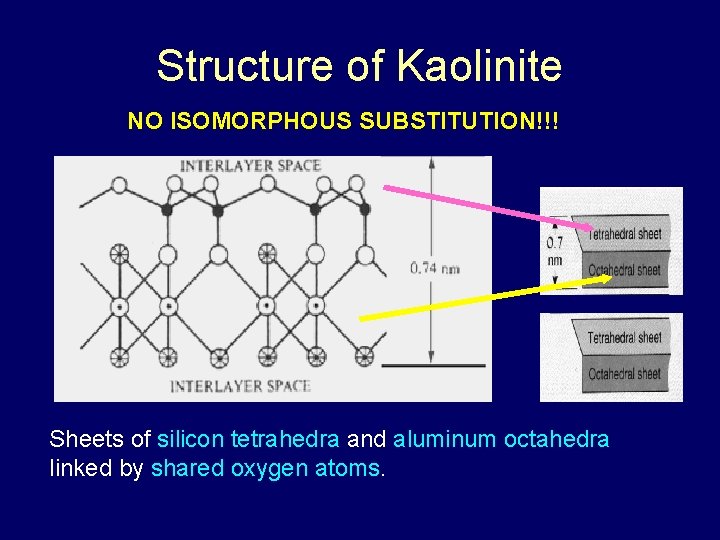
Structure of Kaolinite NO ISOMORPHOUS SUBSTITUTION!!! Sheets of silicon tetrahedra and aluminum octahedra linked by shared oxygen atoms.

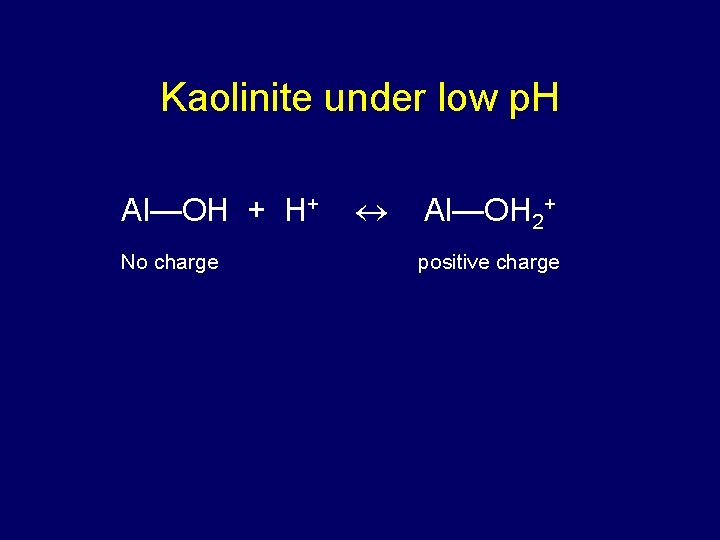
Kaolinite under low p. H Al—OH + H+ No charge Al—OH 2+ positive charge

2: 1 Silicate Clays • Two silica tetrahedral sheets linked to one aluminum octahedral sheet • Three key groups: – Smectites (e. g. , montmorillonite) – Vermiculites – Micas (e. g. , illite) • And one “ 2 -1 -1” (chlorites)

Montmorillonite (2: 1, a Smectite) • Layer charge originates from the substitution of Mg 2+ for Al 3+ in the octahedral sheet • Unstable (weathers to something else) under low p. H and high moisture • Most swelling of all clays • “Nutrient rich”

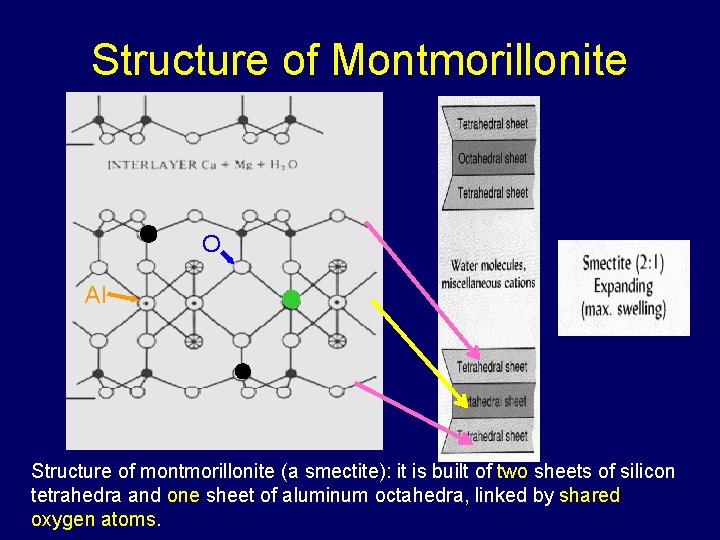
Structure of Montmorillonite O Al Structure of montmorillonite (a smectite): it is built of two sheets of silicon tetrahedra and one sheet of aluminum octahedra, linked by shared oxygen atoms.

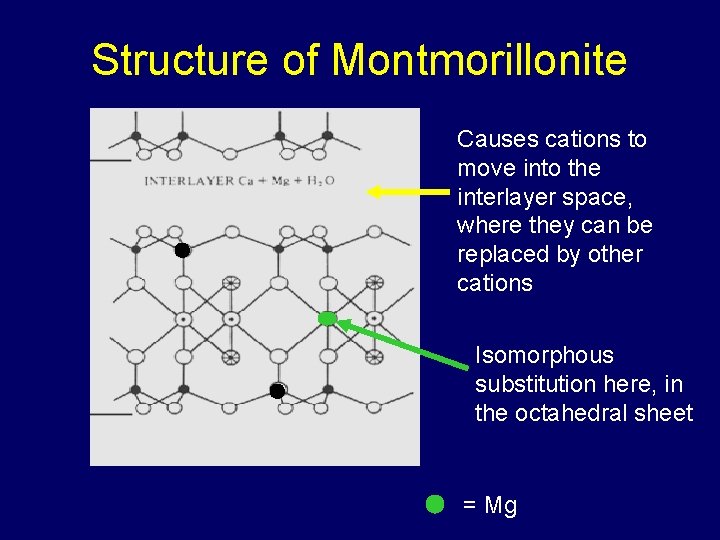
Structure of Montmorillonite Causes cations to move into the interlayer space, where they can be replaced by other cations Isomorphous substitution here, in the octahedral sheet = Mg

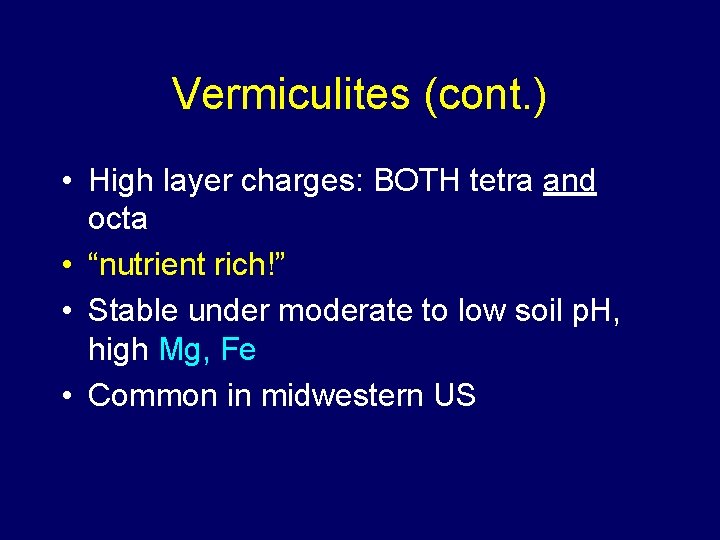
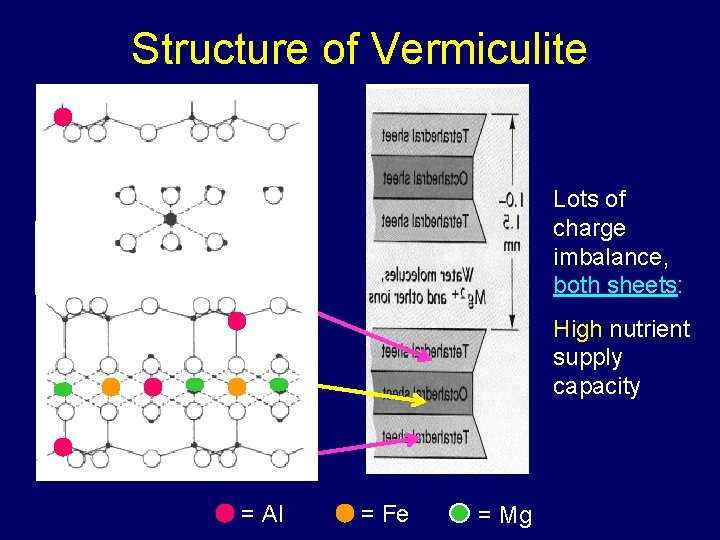
Vermiculites (2: 1) • • Alteration product of micas (rock form) Formed from loss of K+ Interlayer K+ of mica replaced with Mg 2+ Limited shrink-swell …

Vermiculites (cont. ) • High layer charges: BOTH tetra and octa • “nutrient rich!” • Stable under moderate to low soil p. H, high Mg, Fe • Common in midwestern US

Structure of Vermiculite Lots of charge imbalance, both sheets: High nutrient supply capacity = Al = Fe = Mg

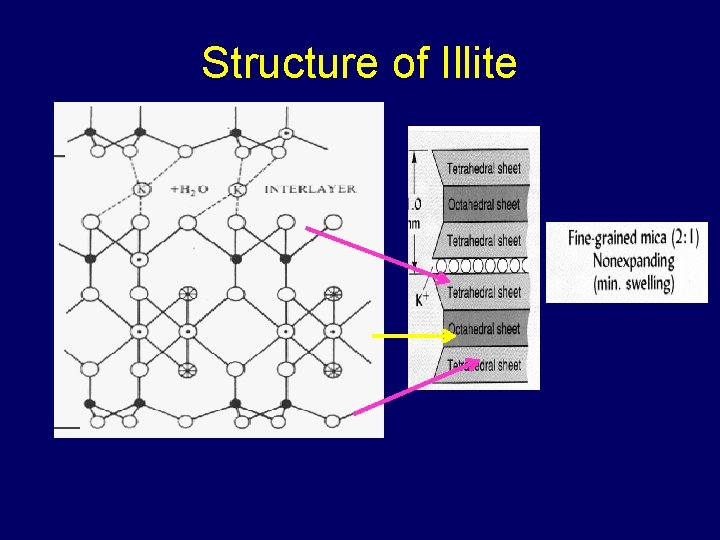
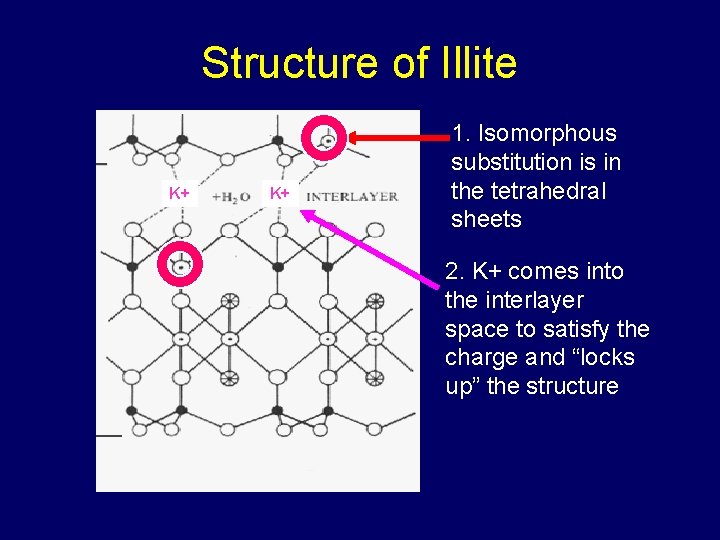
Illite (2: 1, a Mica) • Al 3+ substitution for Si 4+ on the tetrahedral sheet • Strong surface charge • “fairly nutrient poor” • Non-swelling, only moderately plastic • Stable under moderate to low p. H, common in midwestern US

Structure of Illite

Structure of Illite K+ K+ 1. Isomorphous substitution is in the tetrahedral sheets 2. K+ comes into the interlayer space to satisfy the charge and “locks up” the structure

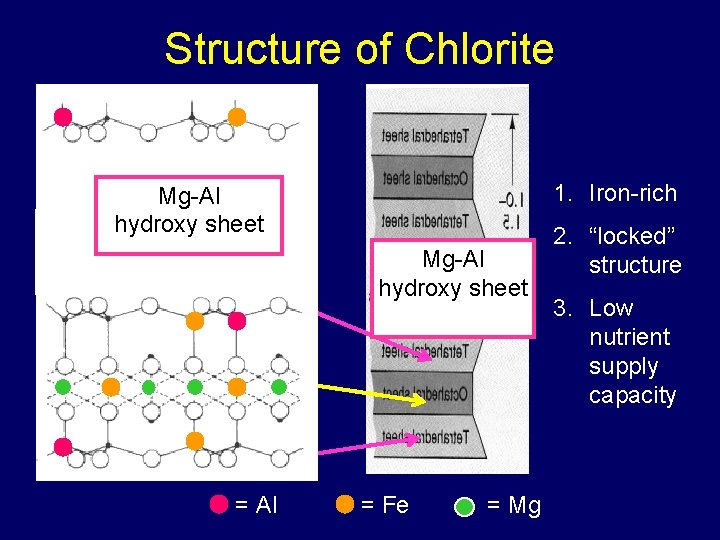
Chlorites (2: 1: 1) • Hydroxy octahedral sheet in the interlayer space • Restricted swelling • “Nutrient poor” • Common in sedimentary rocks and the soils derived from them

Structure of Chlorite 1. Iron-rich Mg-Al hydroxy sheet = Al = Fe = Mg 2. “locked” structure 3. Low nutrient supply capacity

Visual comparison of common silicate clays H-H Strongly held montmorillonite illite “ 2: 1: 1”

Factors affecting mineral stability • Number and type of base cations in the structure (base cations are soluble…) • Number of silica tetrahedra that are linked (more sharing of oxygens = more stable) • Al 3+ proxy for Si 4+ (more proxy = less stable) • Presence of Fe (more Fe = less stable) • Kinds of bonds – Ionic are heat tolerant – Covalent generally stronger ‘cuz shared electrons between atoms, but not heat tolerant

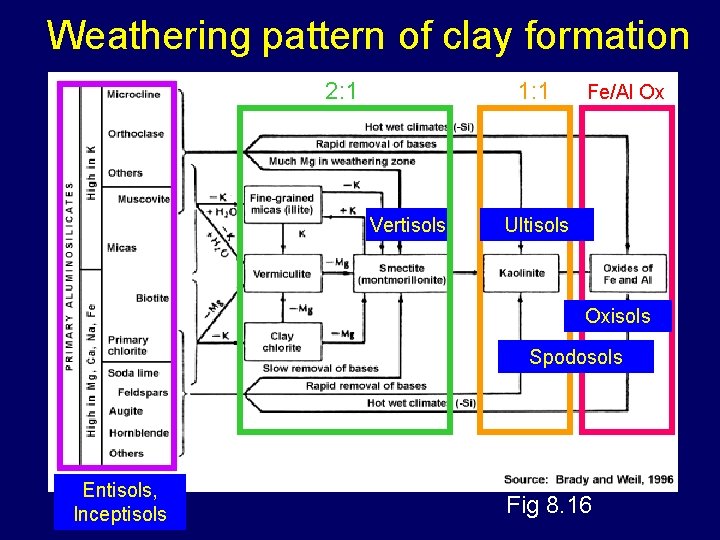
Weathering pattern of clay formation 2: 1 1: 1 Vertisols Fe/Al Ox Ultisols Oxisols Spodosols Entisols, Inceptisols Fig 8. 16

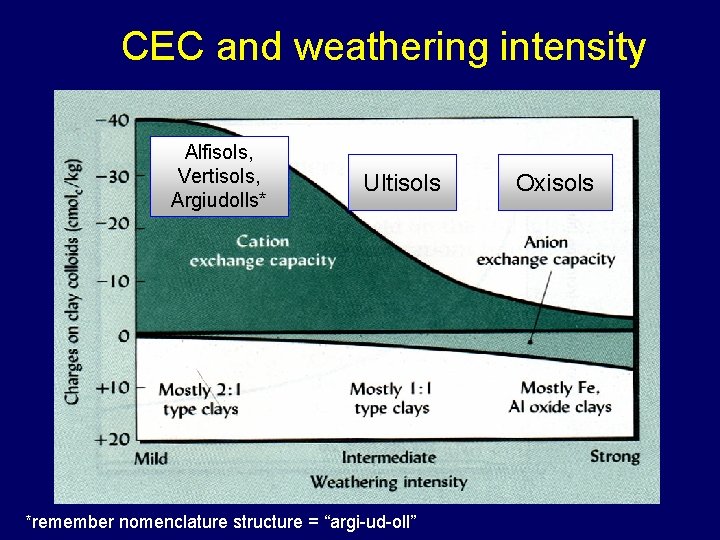
CEC and weathering intensity Alfisols, Vertisols, Argiudolls* Ultisols *remember nomenclature structure = “argi-ud-oll” Oxisols

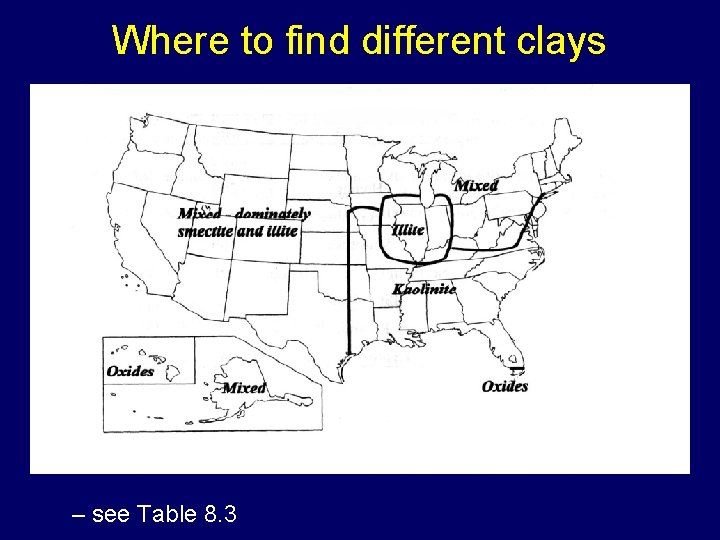
Where to find different clays – see Table 8. 3