Intermolecular Forces and Liquids and Solids Copyright The



- Slides: 51

Intermolecular Forces and Liquids and Solids Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

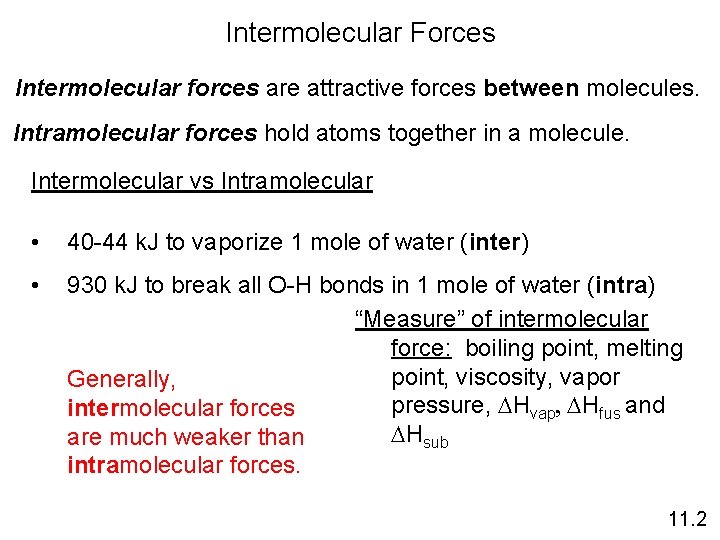
Intermolecular Forces Intermolecular forces are attractive forces between molecules. Intramolecular forces hold atoms together in a molecule. Intermolecular vs Intramolecular • 40 -44 k. J to vaporize 1 mole of water (inter) • 930 k. J to break all O-H bonds in 1 mole of water (intra) “Measure” of intermolecular force: boiling point, melting point, viscosity, vapor Generally, pressure, DHvap, DHfus and intermolecular forces DHsub are much weaker than intramolecular forces. 11. 2

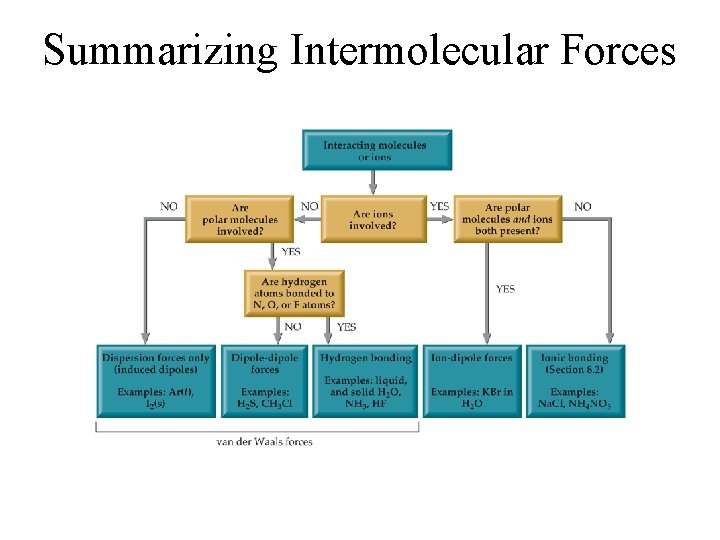
Summarizing Intermolecular Forces

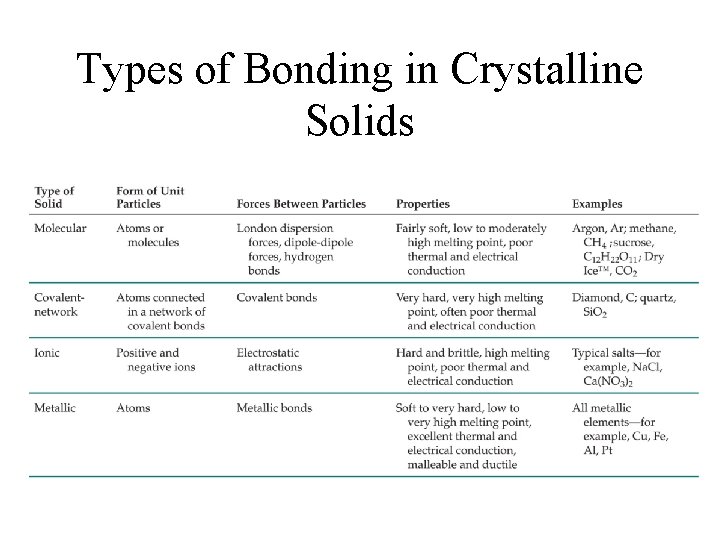
Types of Bonding in Crystalline Solids

Intermolecular Forces Affect Many Physical Properties The strength of the attractions between particles can greatly affect the properties of a substance or solution.

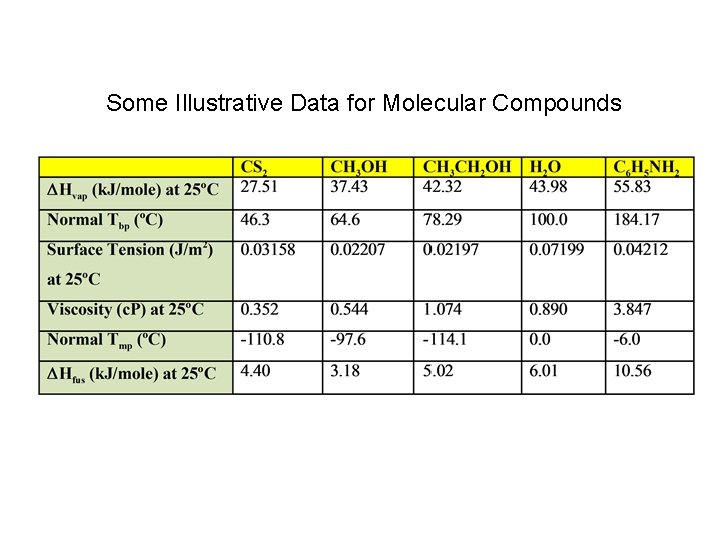
Some Illustrative Data for Molecular Compounds

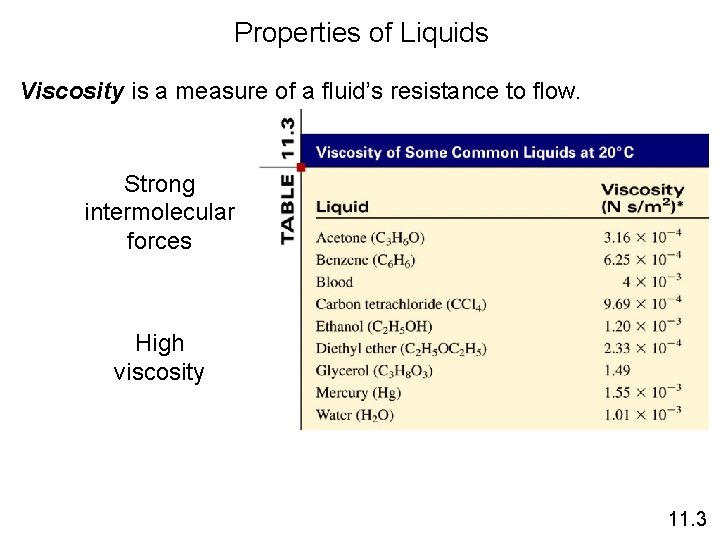
Viscosity • Resistance of a liquid to flow is called viscosity. • It is related to the ease with which molecules can move past each other. • Viscosity increases with stronger intermolecular forces and decreases with higher temperature.

Surface Tension Surface tension results from the net inward force experienced by the molecules on the surface of a liquid.

Properties of Liquids Viscosity is a measure of a fluid’s resistance to flow. Strong intermolecular forces High viscosity 11. 3

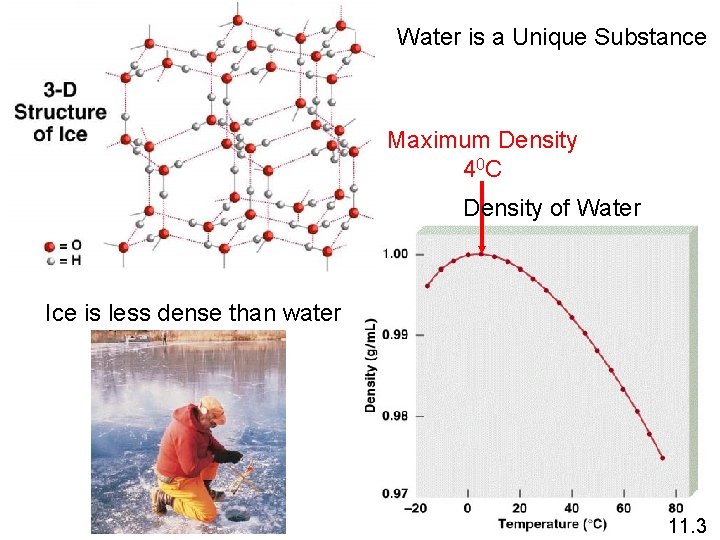
Water is a Unique Substance Maximum Density 4 0 C Density of Water Ice is less dense than water 11. 3

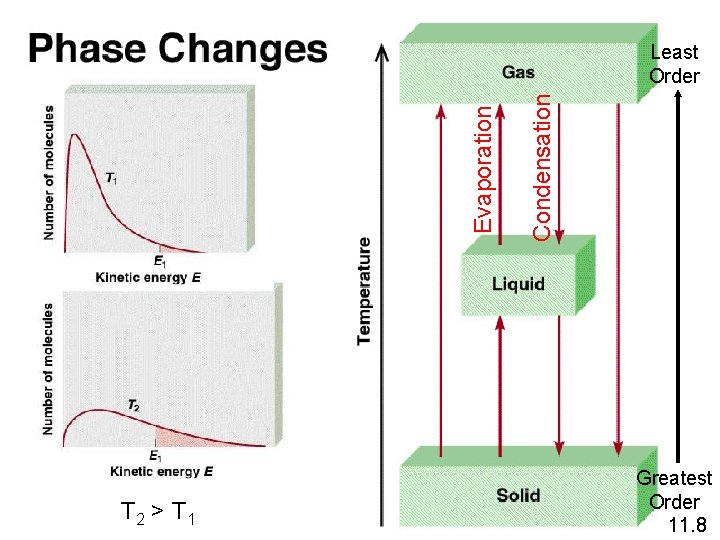
T 2 > T 1 Condensation Evaporation Least Order Greatest Order 11. 8

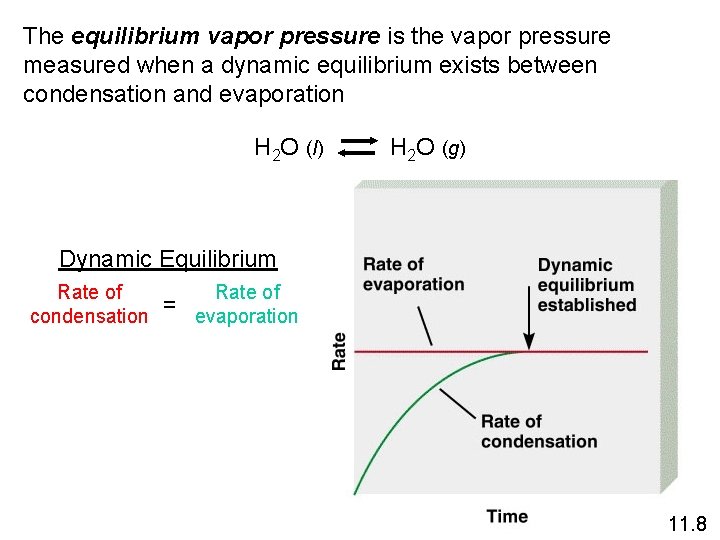
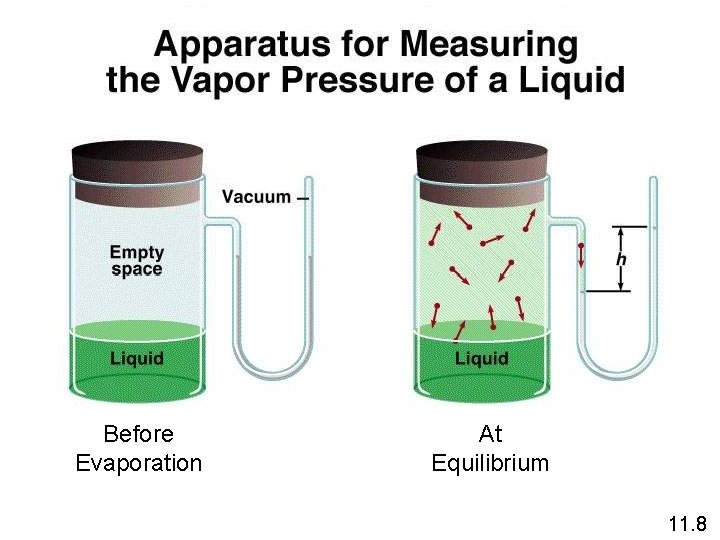
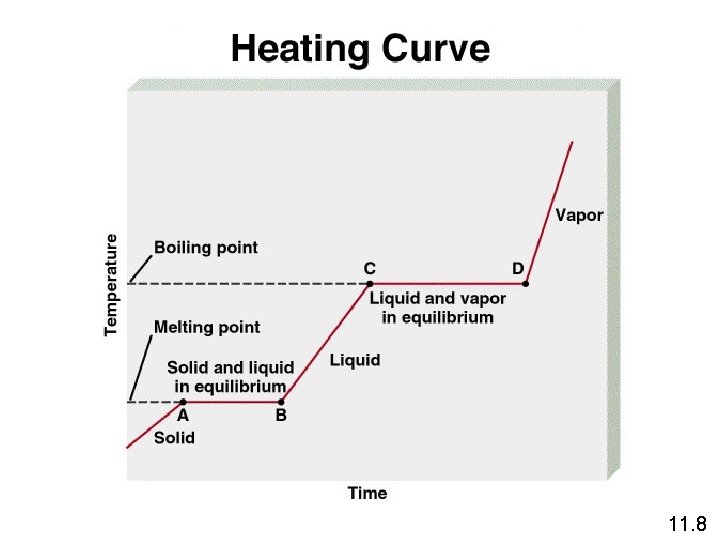
The equilibrium vapor pressure is the vapor pressure measured when a dynamic equilibrium exists between condensation and evaporation H 2 O (l) H 2 O (g) Dynamic Equilibrium Rate of condensation = Rate of evaporation 11. 8

Before Evaporation At Equilibrium 11. 8

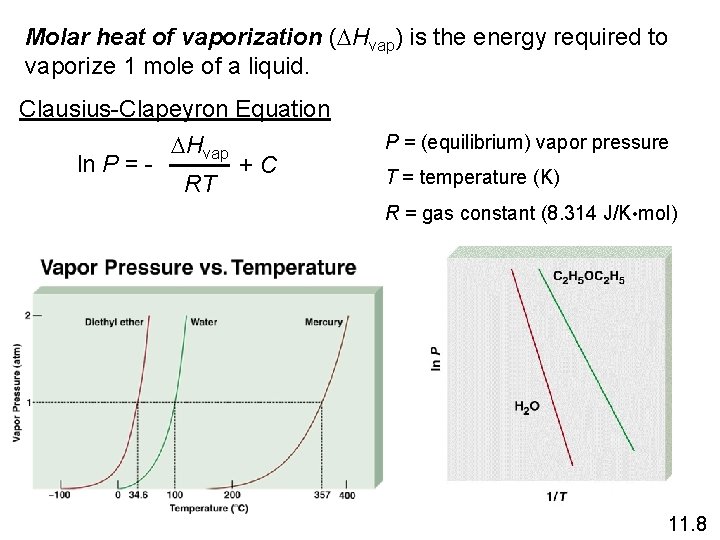
Molar heat of vaporization (DHvap) is the energy required to vaporize 1 mole of a liquid. Clausius-Clapeyron Equation DHvap ln P = + C RT P = (equilibrium) vapor pressure T = temperature (K) R = gas constant (8. 314 J/K • mol) 11. 8

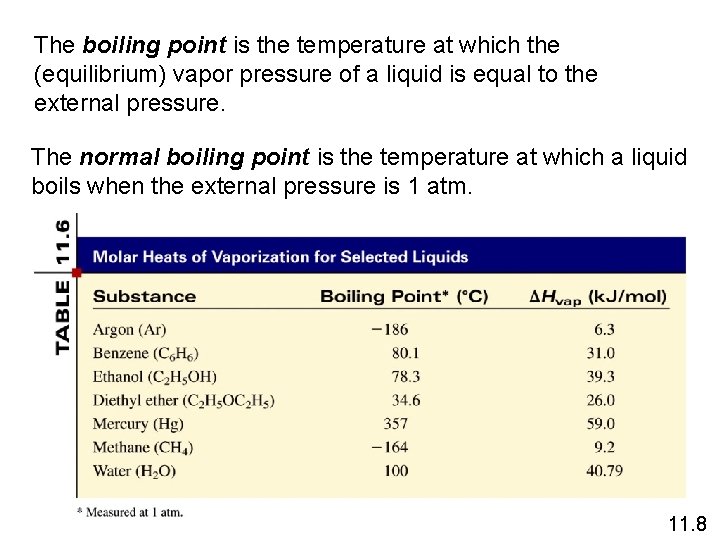
The boiling point is the temperature at which the (equilibrium) vapor pressure of a liquid is equal to the external pressure. The normal boiling point is the temperature at which a liquid boils when the external pressure is 1 atm. 11. 8

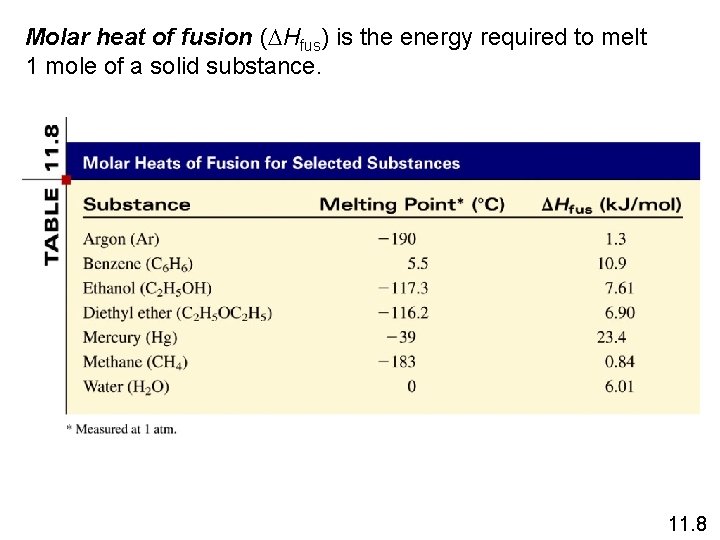
Molar heat of fusion (DHfus) is the energy required to melt 1 mole of a solid substance. 11. 8

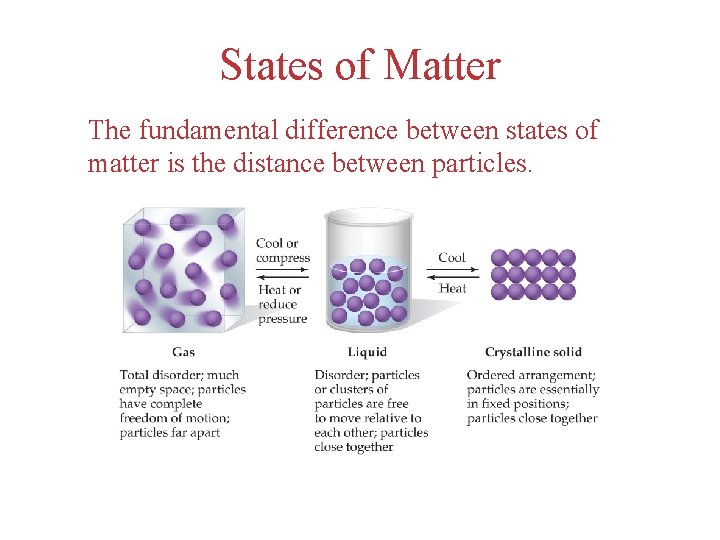
States of Matter The fundamental difference between states of matter is the distance between particles.

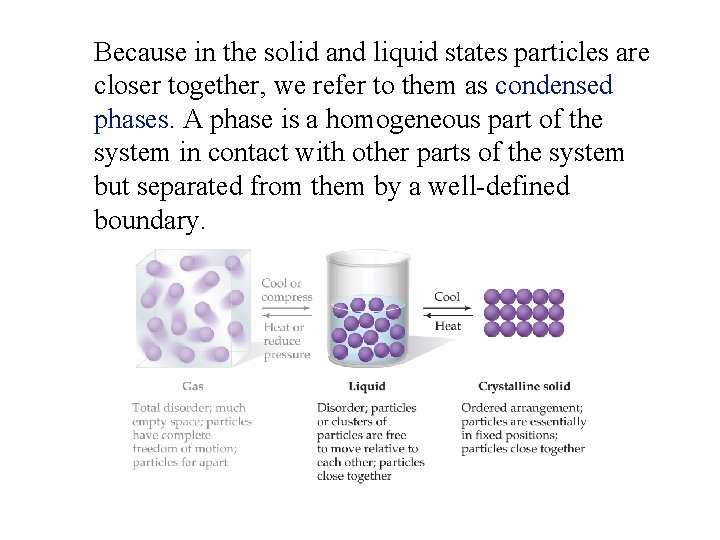
Because in the solid and liquid states particles are closer together, we refer to them as condensed phases. A phase is a homogeneous part of the system in contact with other parts of the system but separated from them by a well-defined boundary.

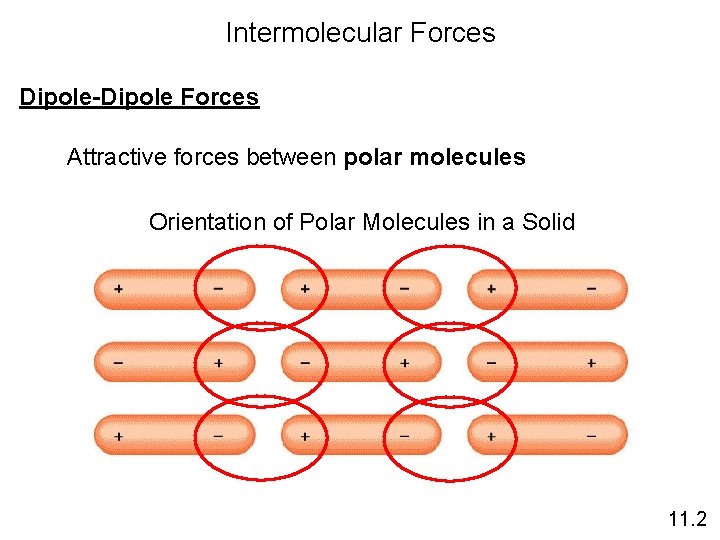
Intermolecular Forces Dipole-Dipole Forces Attractive forces between polar molecules Orientation of Polar Molecules in a Solid 11. 2

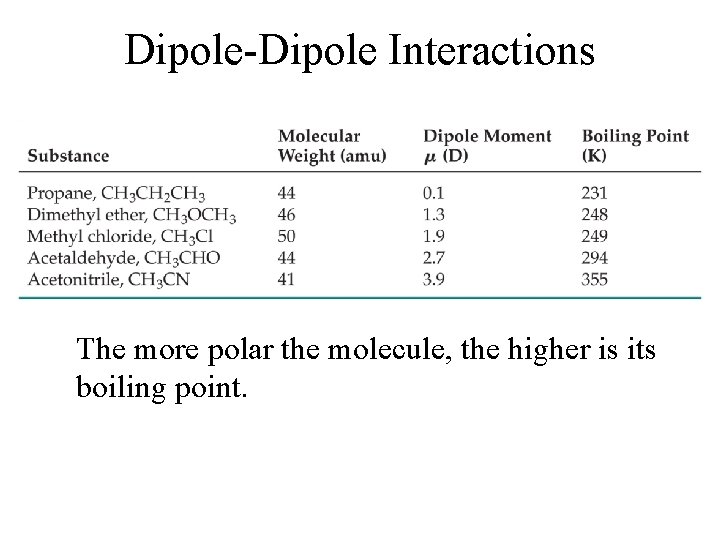
Dipole-Dipole Interactions The more polar the molecule, the higher is its boiling point.

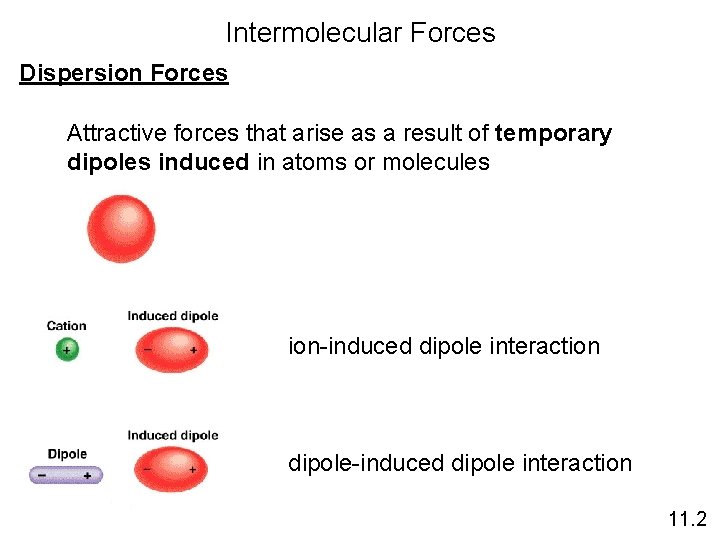
Intermolecular Forces Dispersion Forces Attractive forces that arise as a result of temporary dipoles induced in atoms or molecules ion-induced dipole interaction dipole-induced dipole interaction 11. 2

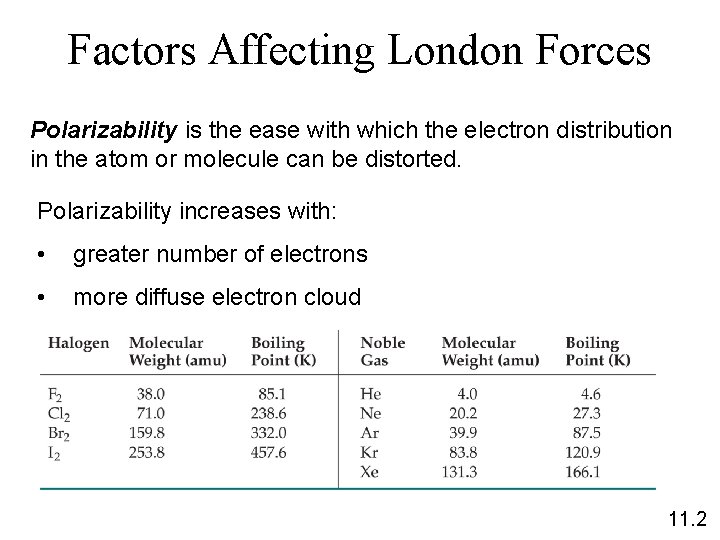
Factors Affecting London Forces Polarizability is the ease with which the electron distribution in the atom or molecule can be distorted. Polarizability increases with: • greater number of electrons • more diffuse electron cloud 11. 2

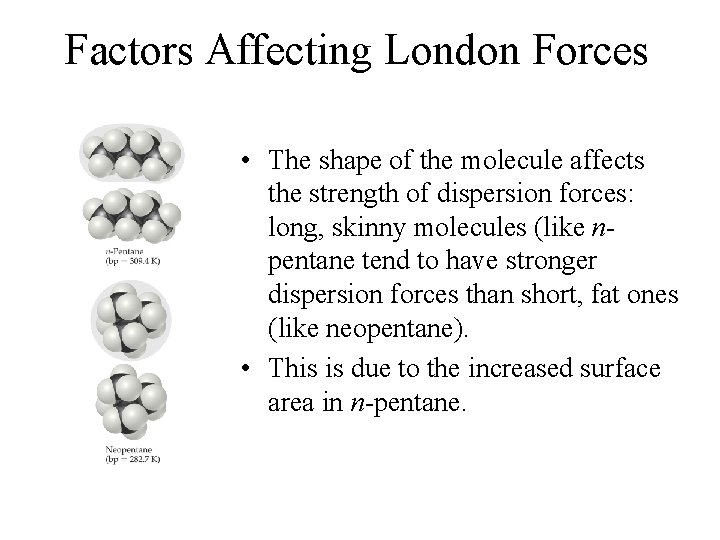
Factors Affecting London Forces • The shape of the molecule affects the strength of dispersion forces: long, skinny molecules (like npentane tend to have stronger dispersion forces than short, fat ones (like neopentane). • This is due to the increased surface area in n-pentane.

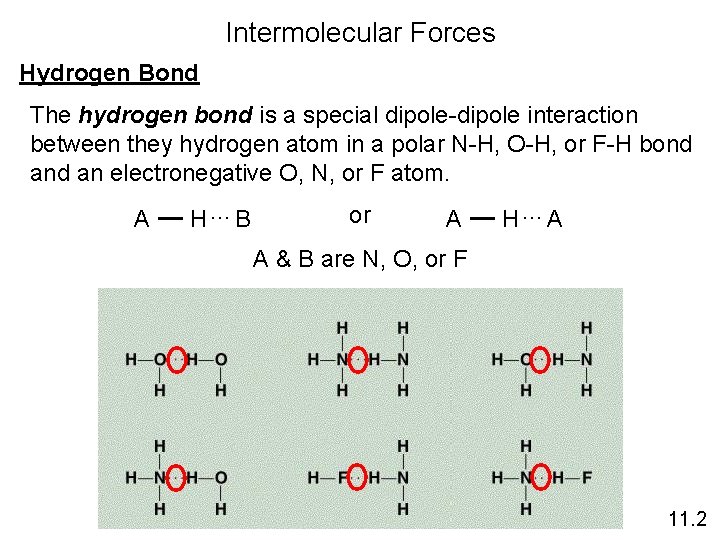
Intermolecular Forces Hydrogen Bond The hydrogen bond is a special dipole-dipole interaction between they hydrogen atom in a polar N-H, O-H, or F-H bond an electronegative O, N, or F atom. A H…B or A H…A A & B are N, O, or F 11. 2

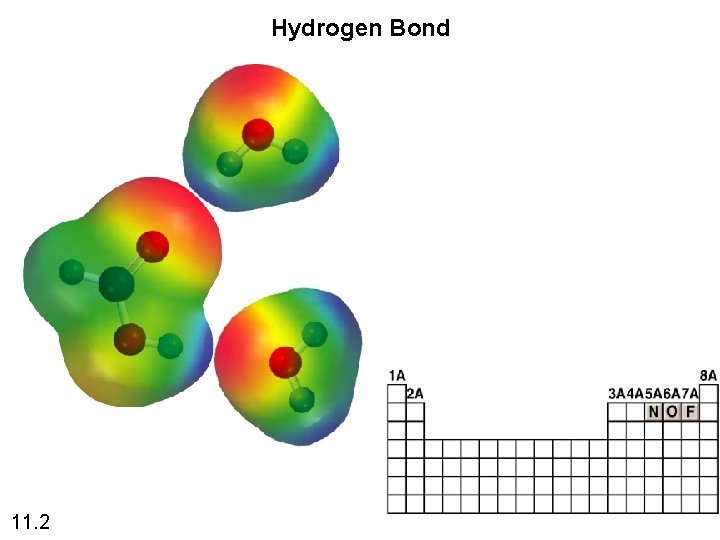
Hydrogen Bond 11. 2

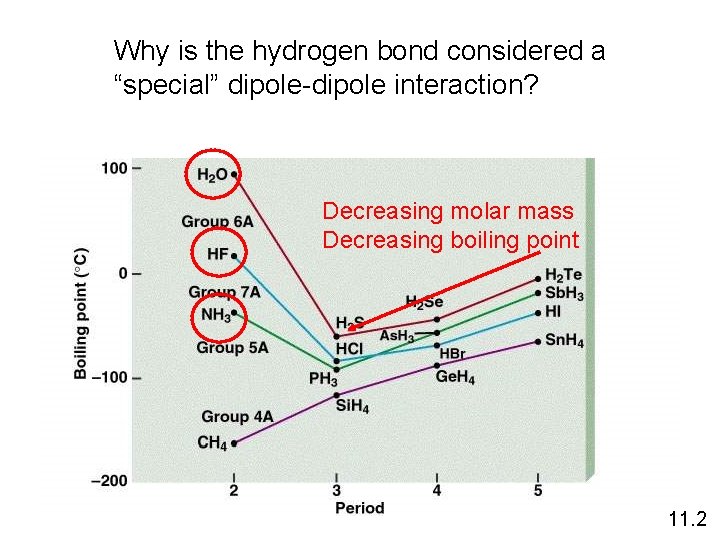
Why is the hydrogen bond considered a “special” dipole-dipole interaction? Decreasing molar mass Decreasing boiling point 11. 2

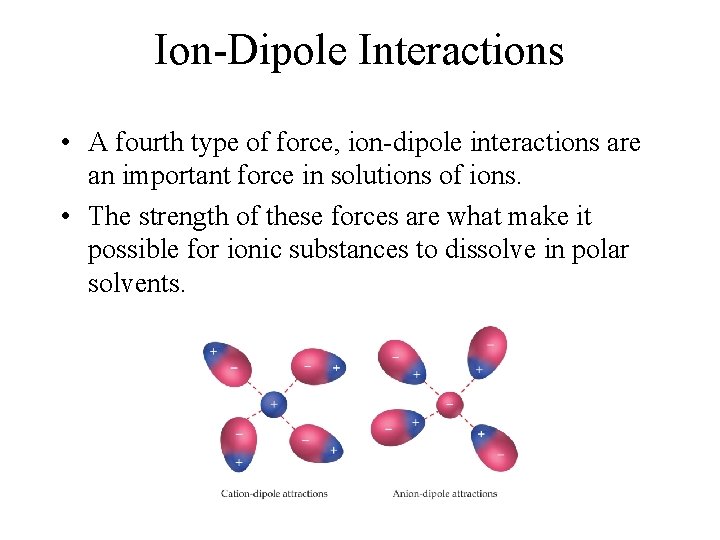
Ion-Dipole Interactions • A fourth type of force, ion-dipole interactions are an important force in solutions of ions. • The strength of these forces are what make it possible for ionic substances to dissolve in polar solvents.

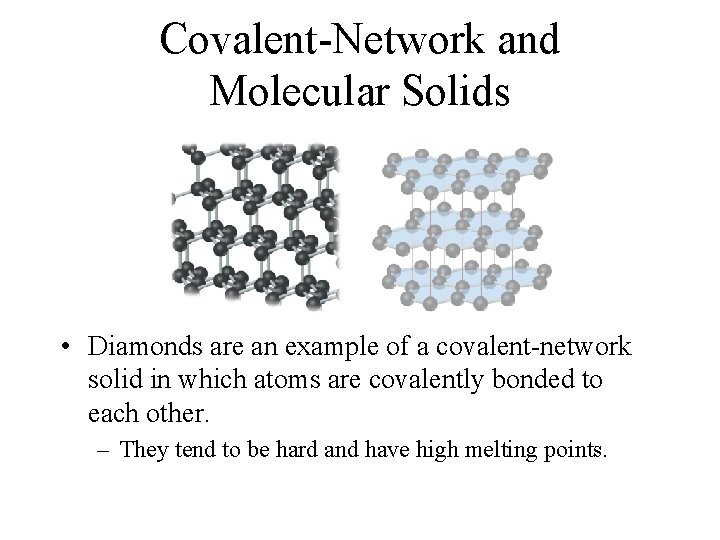
Covalent-Network and Molecular Solids • Diamonds are an example of a covalent-network solid in which atoms are covalently bonded to each other. – They tend to be hard and have high melting points.

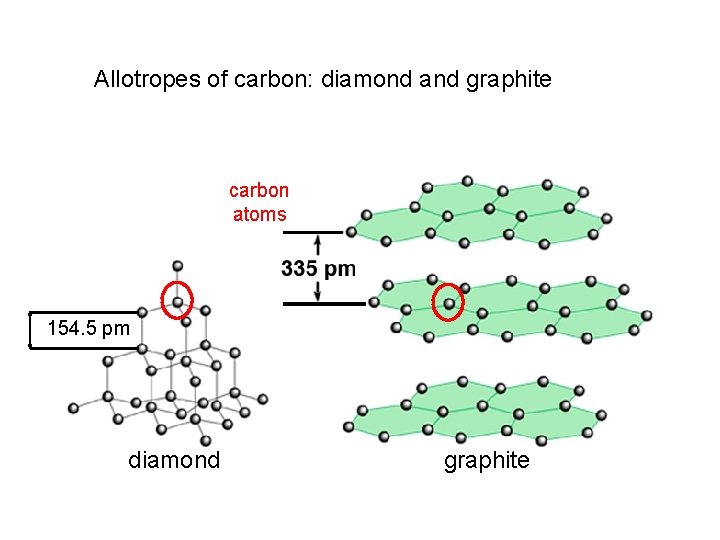
Allotropes of carbon: diamond and graphite carbon atoms 154. 5 pm diamond graphite

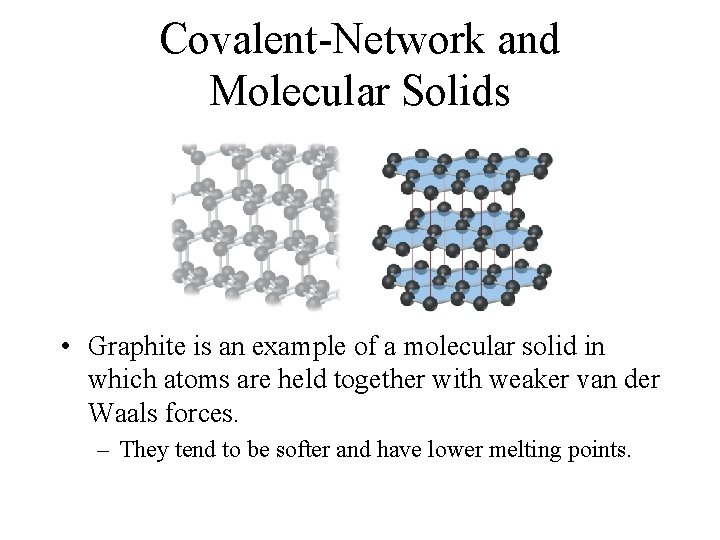
Covalent-Network and Molecular Solids • Graphite is an example of a molecular solid in which atoms are held together with weaker van der Waals forces. – They tend to be softer and have lower melting points.

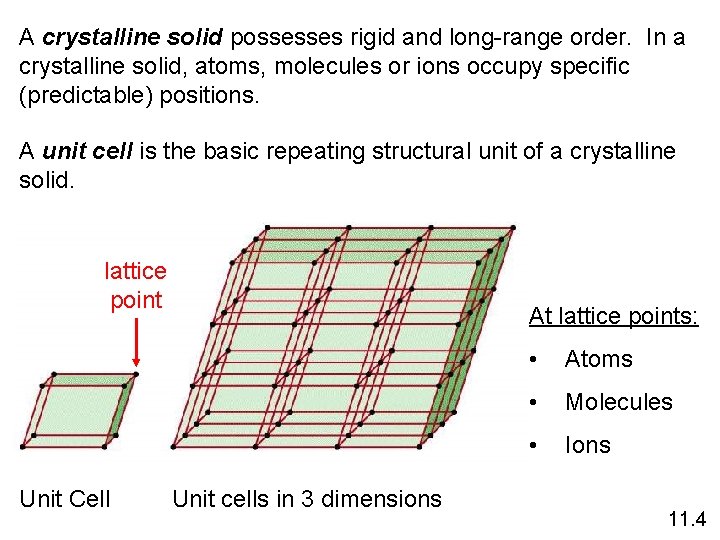
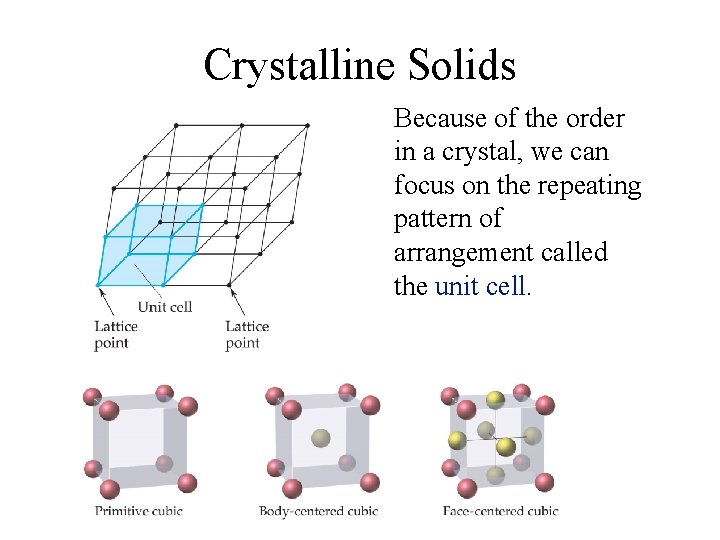
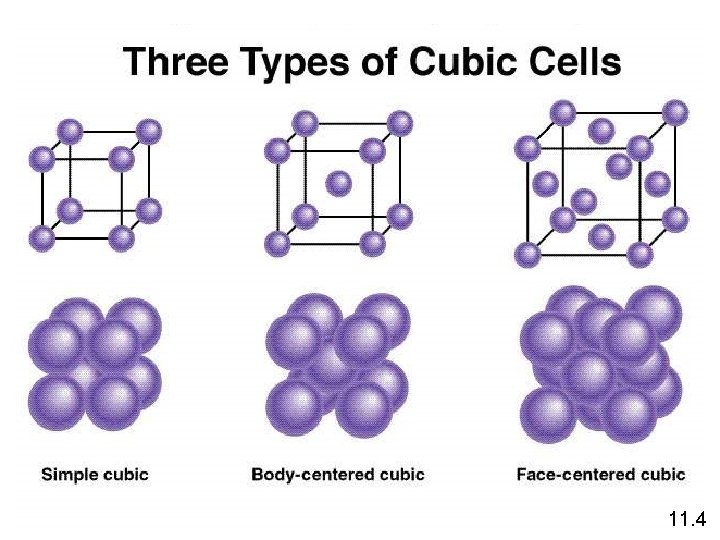
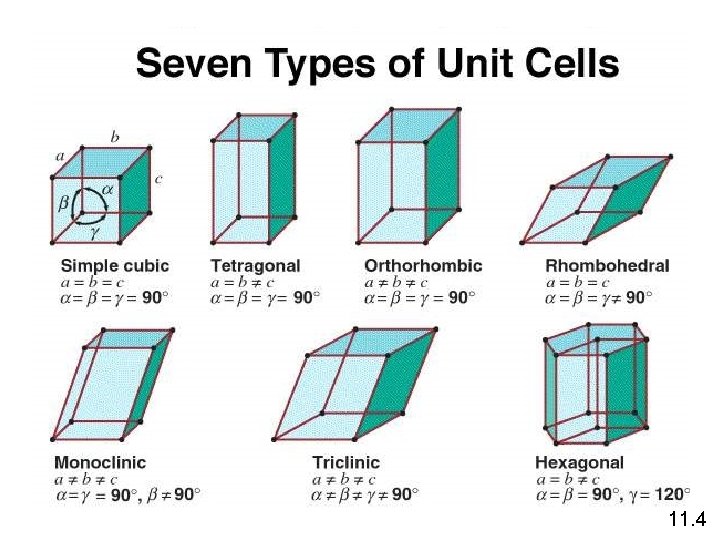
A crystalline solid possesses rigid and long-range order. In a crystalline solid, atoms, molecules or ions occupy specific (predictable) positions. A unit cell is the basic repeating structural unit of a crystalline solid. lattice point Unit Cell At lattice points: Unit cells in 3 dimensions • Atoms • Molecules • Ions 11. 4

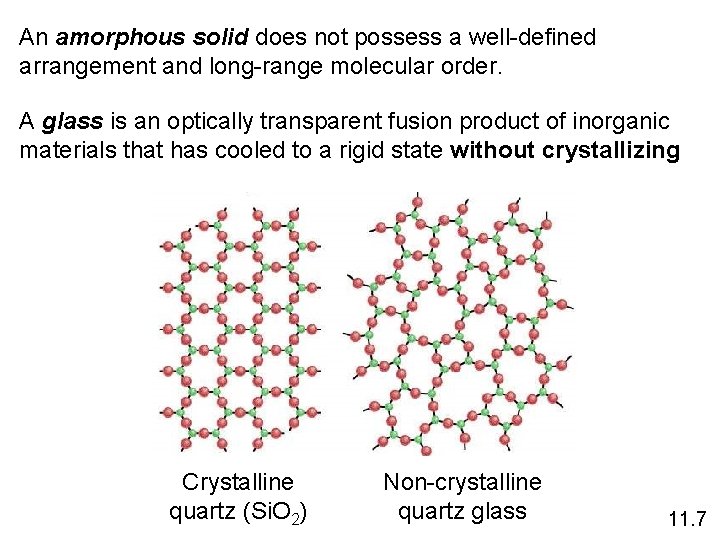
An amorphous solid does not possess a well-defined arrangement and long-range molecular order. A glass is an optically transparent fusion product of inorganic materials that has cooled to a rigid state without crystallizing Crystalline quartz (Si. O 2) Non-crystalline quartz glass 11. 7

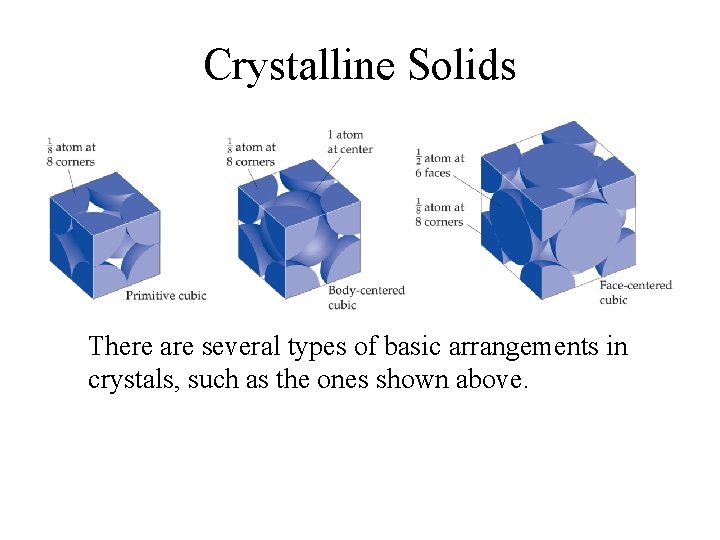
Crystalline Solids Because of the order in a crystal, we can focus on the repeating pattern of arrangement called the unit cell.

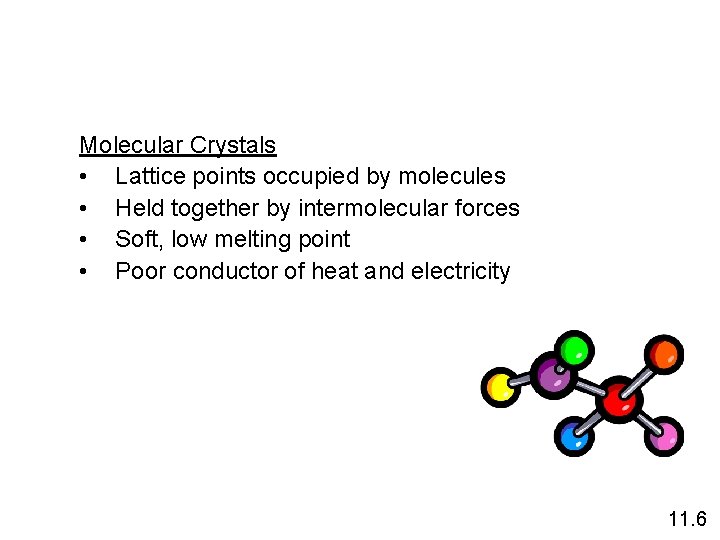
Molecular Crystals • Lattice points occupied by molecules • Held together by intermolecular forces • Soft, low melting point • Poor conductor of heat and electricity 11. 6

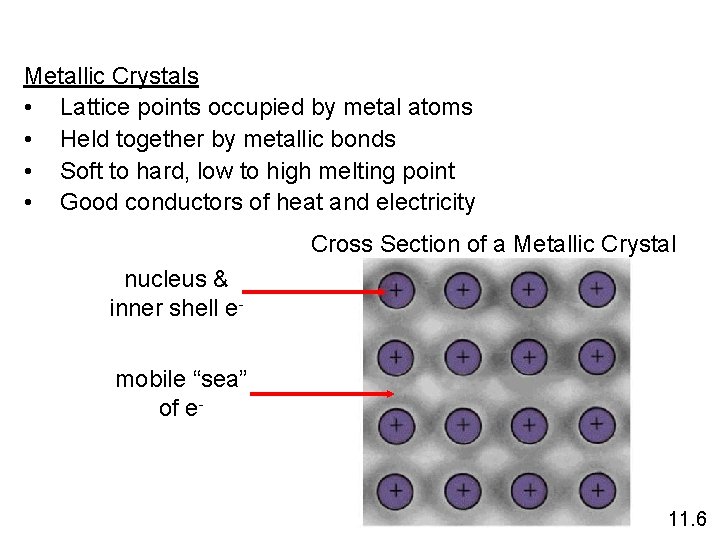
Metallic Crystals • Lattice points occupied by metal atoms • Held together by metallic bonds • Soft to hard, low to high melting point • Good conductors of heat and electricity Cross Section of a Metallic Crystal nucleus & inner shell emobile “sea” of e- 11. 6

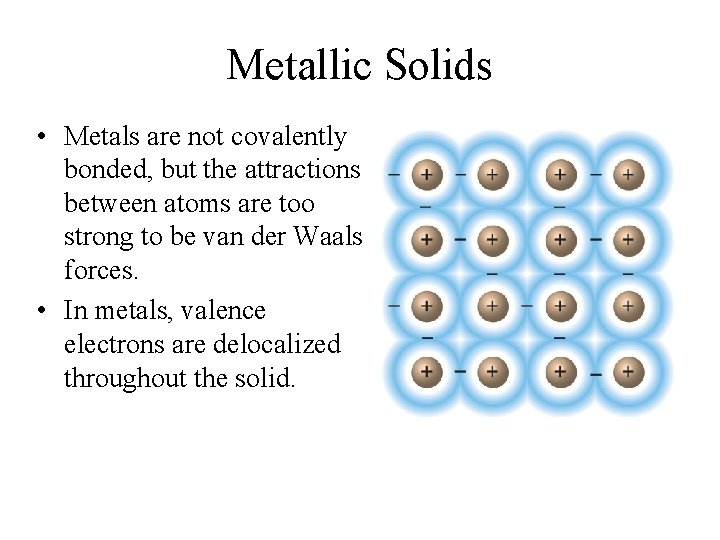
Metallic Solids • Metals are not covalently bonded, but the attractions between atoms are too strong to be van der Waals forces. • In metals, valence electrons are delocalized throughout the solid.

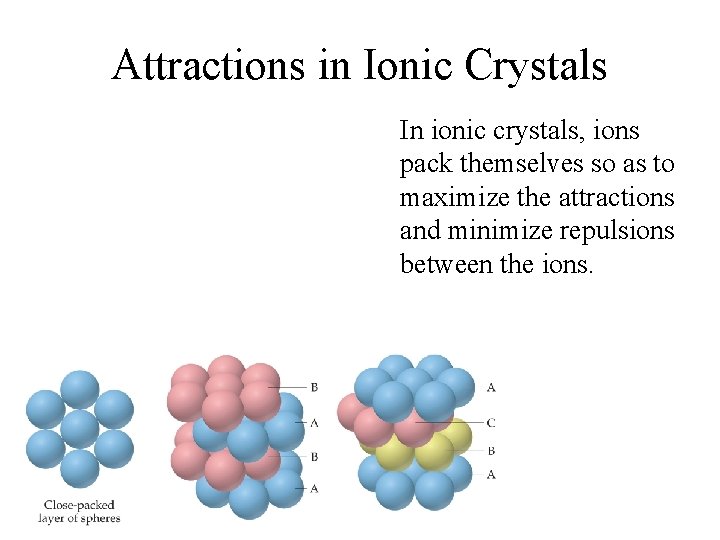
Attractions in Ionic Crystals In ionic crystals, ions pack themselves so as to maximize the attractions and minimize repulsions between the ions.

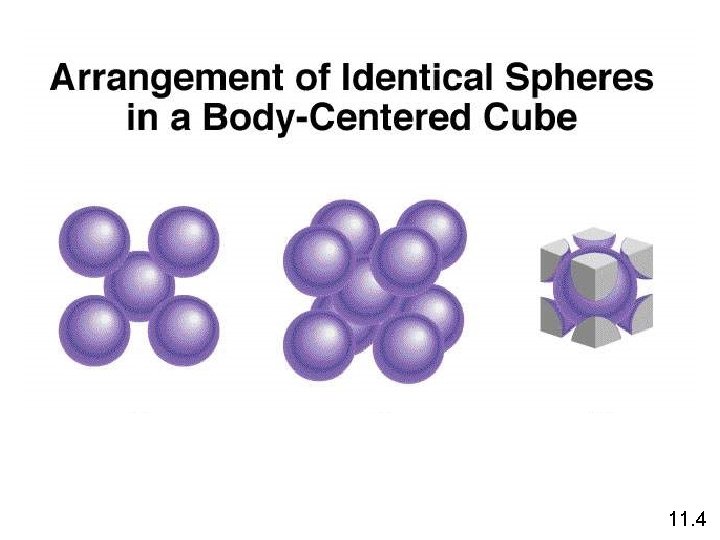
11. 4

11. 4

11. 4

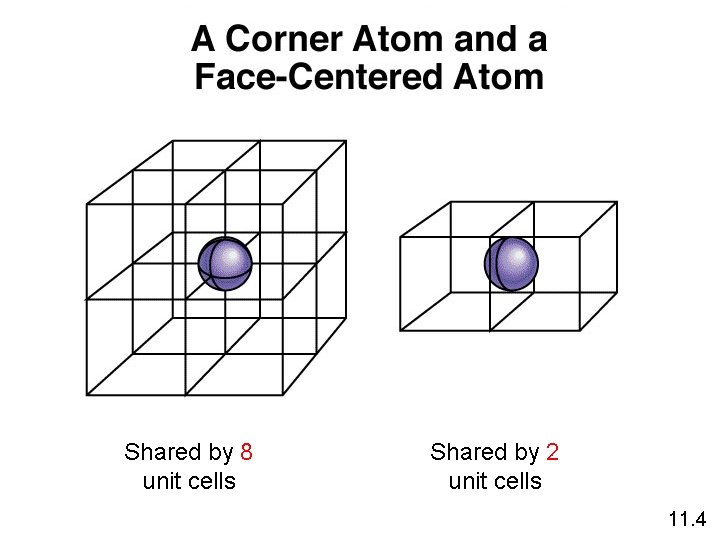
Shared by 8 unit cells Shared by 2 unit cells 11. 4

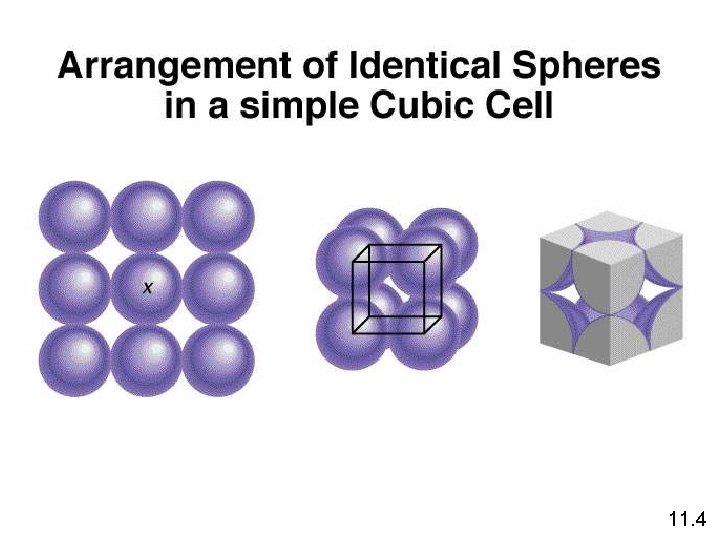
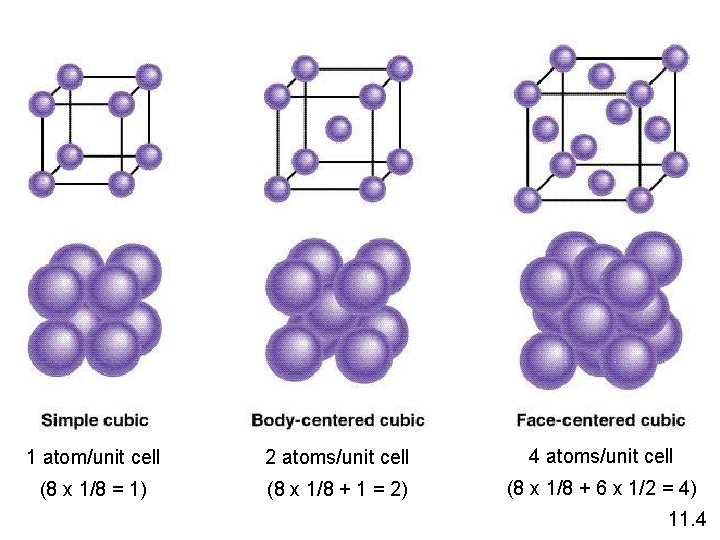
1 atom/unit cell 2 atoms/unit cell 4 atoms/unit cell (8 x 1/8 = 1) (8 x 1/8 + 1 = 2) (8 x 1/8 + 6 x 1/2 = 4) 11. 4

Crystalline Solids There are several types of basic arrangements in crystals, such as the ones shown above.

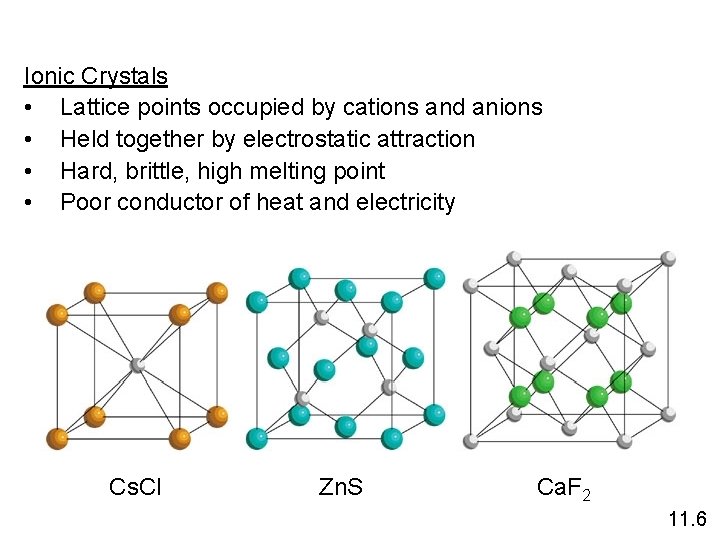
Ionic Crystals • Lattice points occupied by cations and anions • Held together by electrostatic attraction • Hard, brittle, high melting point • Poor conductor of heat and electricity Cs. Cl Zn. S Ca. F 2 11. 6

11. 4

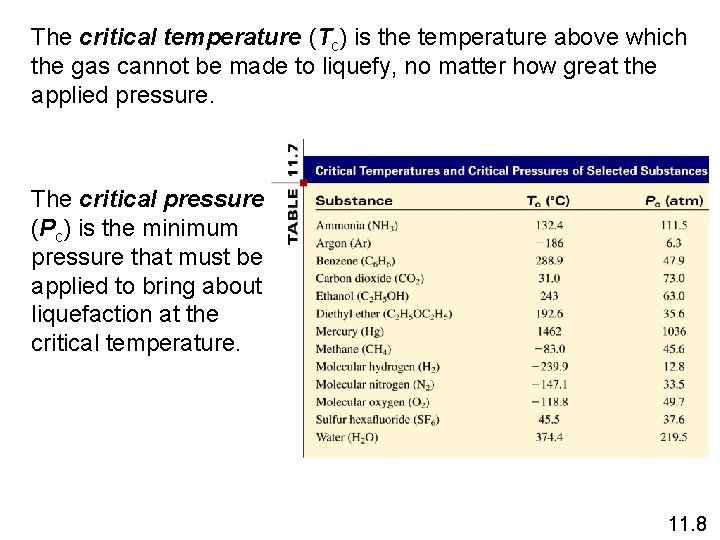
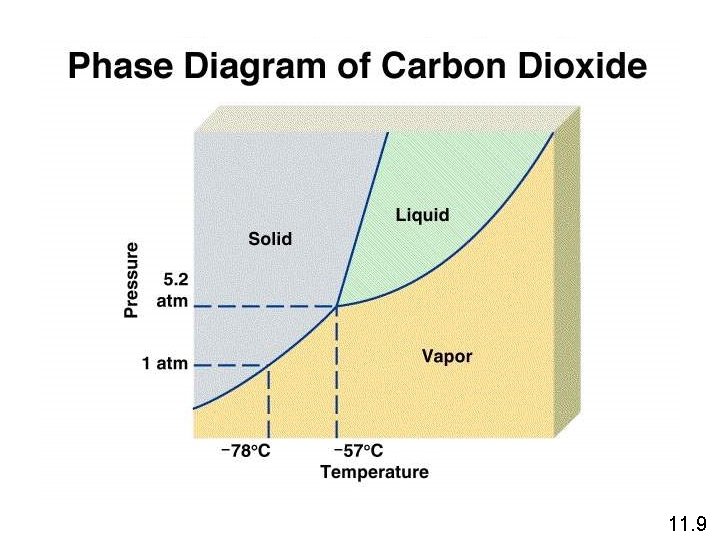
The critical temperature (Tc) is the temperature above which the gas cannot be made to liquefy, no matter how great the applied pressure. The critical pressure (Pc) is the minimum pressure that must be applied to bring about liquefaction at the critical temperature. 11. 8

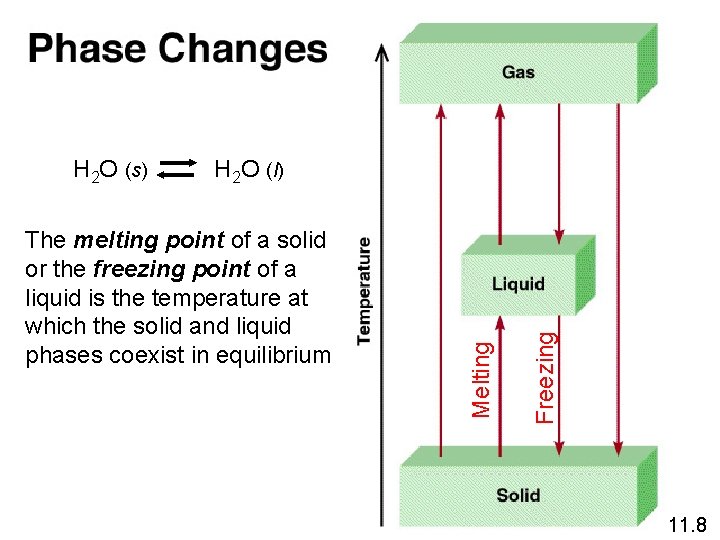
Freezing The melting point of a solid or the freezing point of a liquid is the temperature at which the solid and liquid phases coexist in equilibrium Melting H 2 O (s) H 2 O (l) 11. 8

11. 8

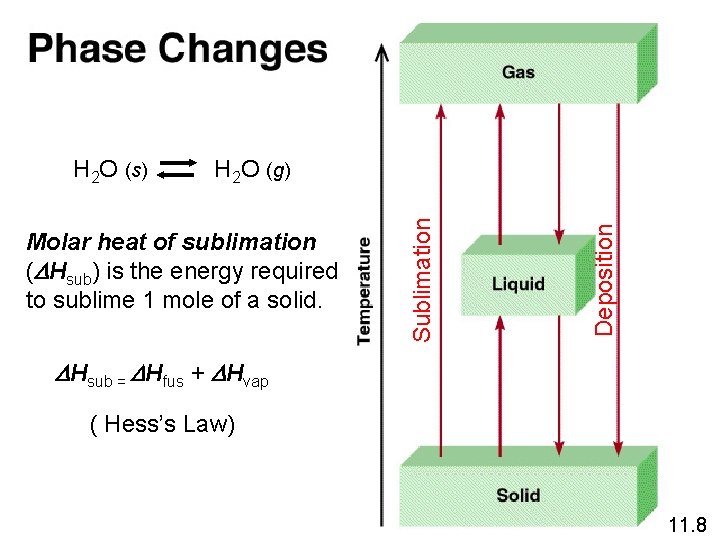
Deposition Molar heat of sublimation (DHsub) is the energy required to sublime 1 mole of a solid. Sublimation H 2 O (s) H 2 O (g) DHsub = DHfus + DHvap ( Hess’s Law) 11. 8

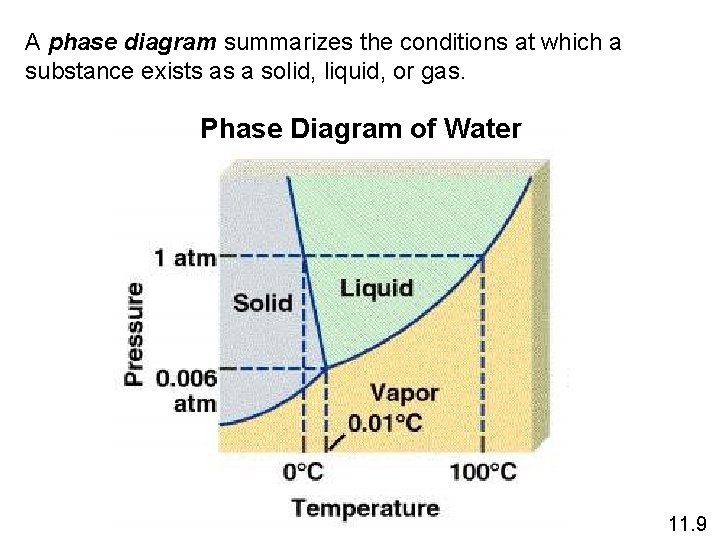
A phase diagram summarizes the conditions at which a substance exists as a solid, liquid, or gas. Phase Diagram of Water 11. 9

11. 9