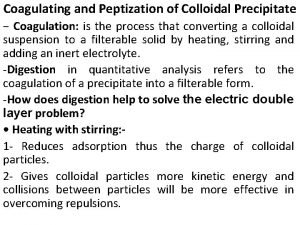
CHAPTER 3 STRUCTURE OF CRYSTALLINE SOLIDS Crystalline repetitive

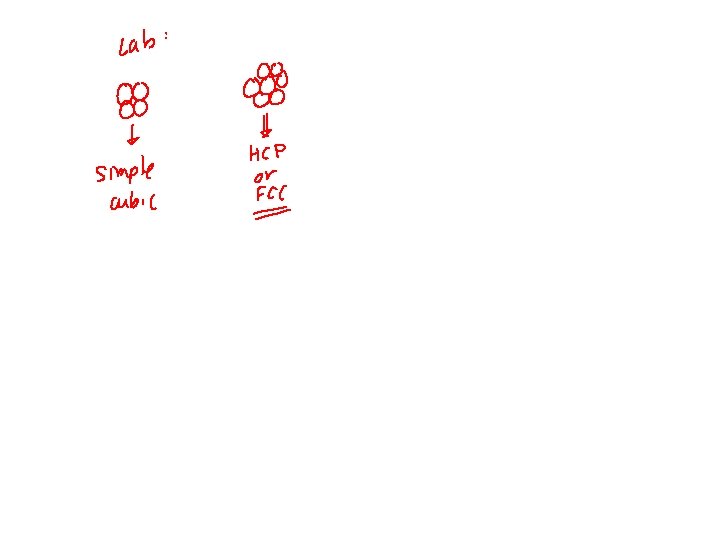





![8. Identify the crystallographic direction of the vector: A. [-1 1 1] B. [1 8. Identify the crystallographic direction of the vector: A. [-1 1 1] B. [1](https://slidetodoc.com/presentation_image/229b31d6f2e8b9d3c62addb8d13f7dc8/image-35.jpg)
![9. Identify the crystallographic direction of the vector: A. [-2 1 2] B. [-2 9. Identify the crystallographic direction of the vector: A. [-2 1 2] B. [-2](https://slidetodoc.com/presentation_image/229b31d6f2e8b9d3c62addb8d13f7dc8/image-36.jpg)
- Slides: 39

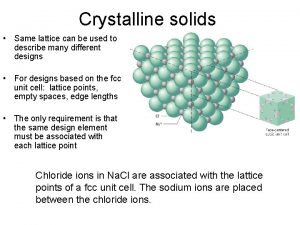
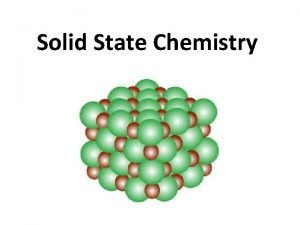
CHAPTER 3: STRUCTURE OF CRYSTALLINE SOLIDS • Crystalline – repetitive 3 -D structure - all metals, many ceramics and some polymers • Atomic Hard Sphere Model represent atoms as solid spheres touching each other. • Lattice 3 -D array of points coinciding with atom positions

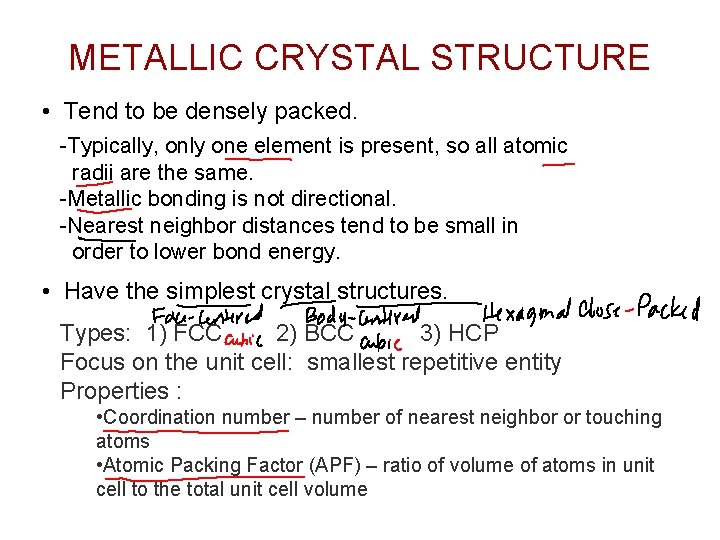
METALLIC CRYSTAL STRUCTURE • Tend to be densely packed. -Typically, only one element is present, so all atomic radii are the same. -Metallic bonding is not directional. -Nearest neighbor distances tend to be small in order to lower bond energy. • Have the simplest crystal structures. Types: 1) FCC 2) BCC 3) HCP Focus on the unit cell: smallest repetitive entity Properties : • Coordination number – number of nearest neighbor or touching atoms • Atomic Packing Factor (APF) – ratio of volume of atoms in unit cell to the total unit cell volume

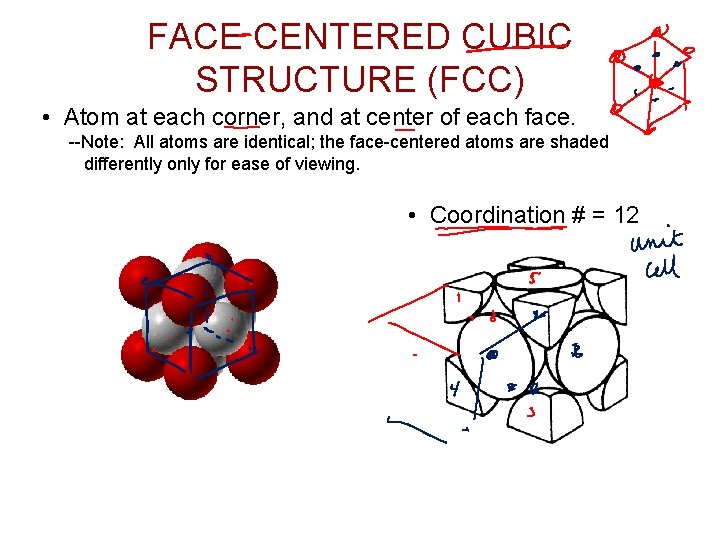
FACE CENTERED CUBIC STRUCTURE (FCC) • Atom at each corner, and at center of each face. --Note: All atoms are identical; the face-centered atoms are shaded differently only for ease of viewing. • Coordination # = 12

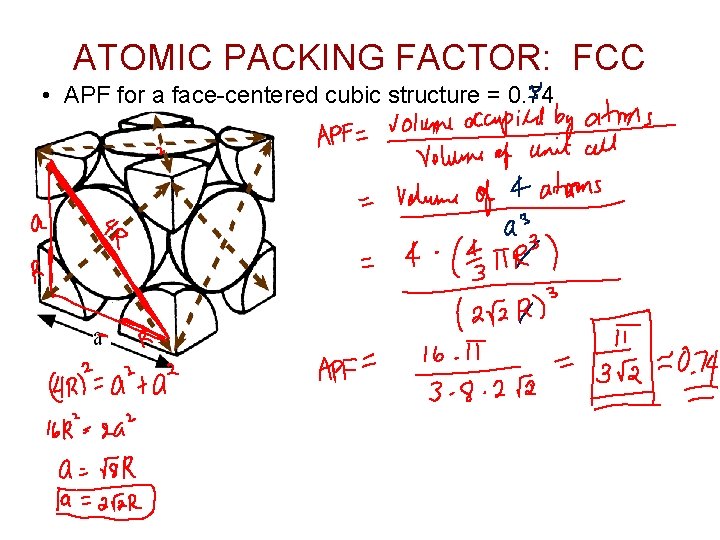
ATOMIC PACKING FACTOR: FCC • APF for a face-centered cubic structure = 0. 74

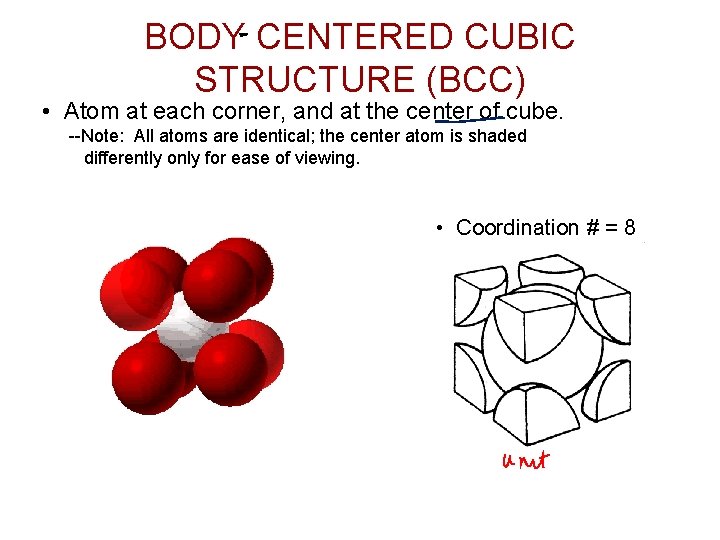
BODY CENTERED CUBIC STRUCTURE (BCC) • Atom at each corner, and at the center of cube. --Note: All atoms are identical; the center atom is shaded differently only for ease of viewing. • Coordination # = 8

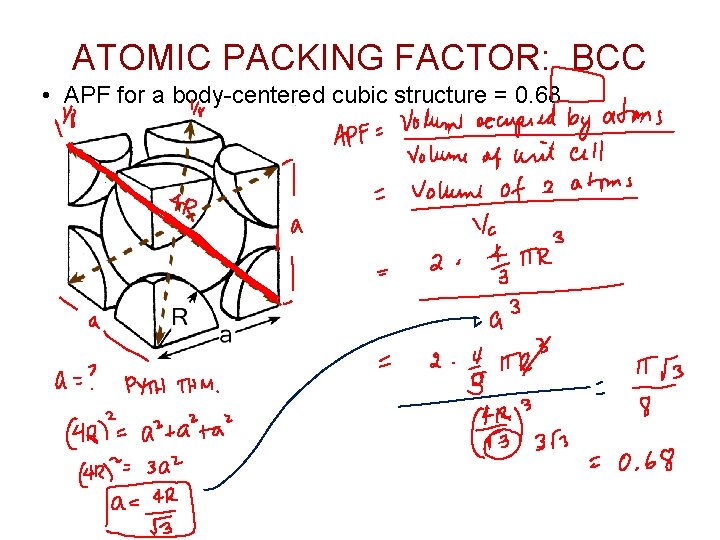
ATOMIC PACKING FACTOR: BCC • APF for a body-centered cubic structure = 0. 68

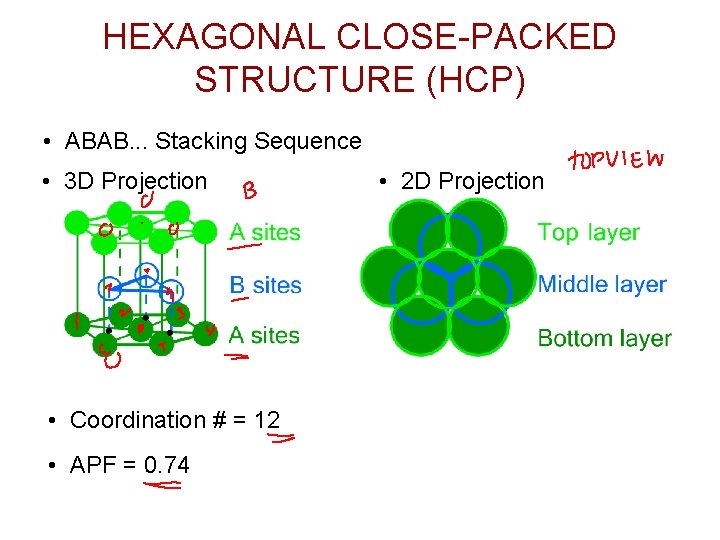
HEXAGONAL CLOSE-PACKED STRUCTURE (HCP) • ABAB. . . Stacking Sequence • 3 D Projection • Coordination # = 12 • APF = 0. 74 • 2 D Projection

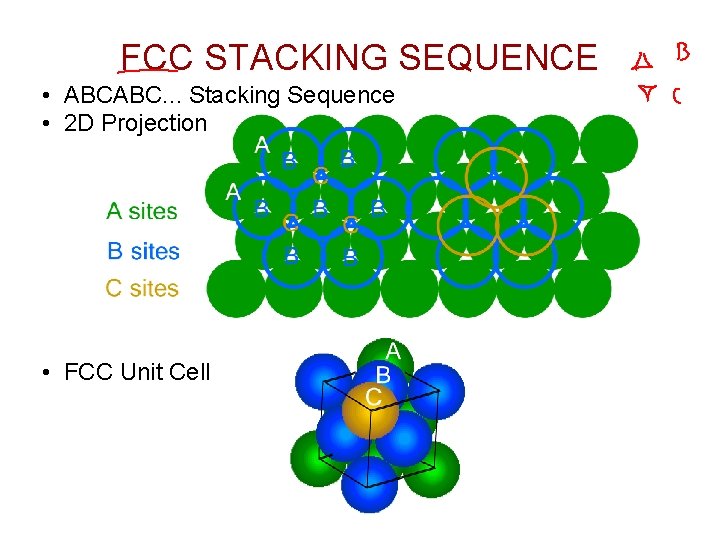
FCC STACKING SEQUENCE • ABCABC. . . Stacking Sequence • 2 D Projection • FCC Unit Cell

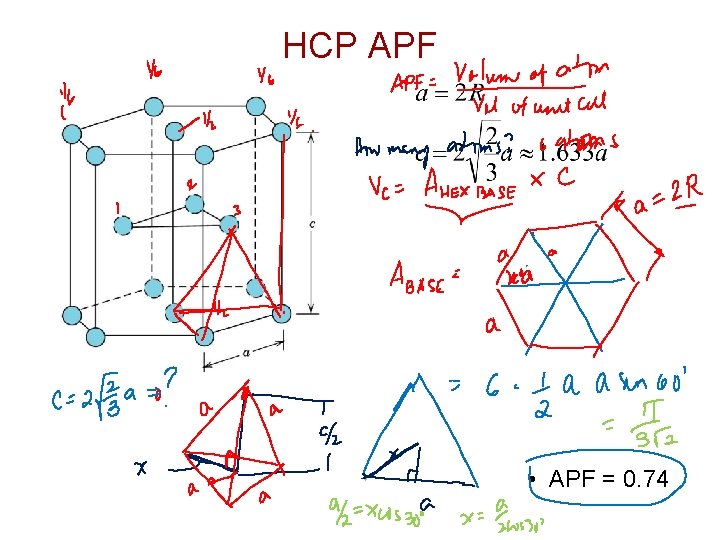
HCP APF • APF = 0. 74

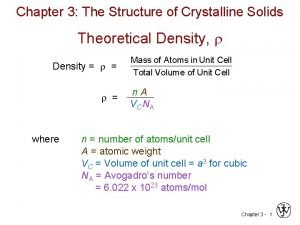
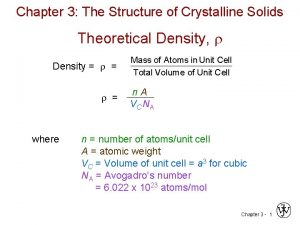
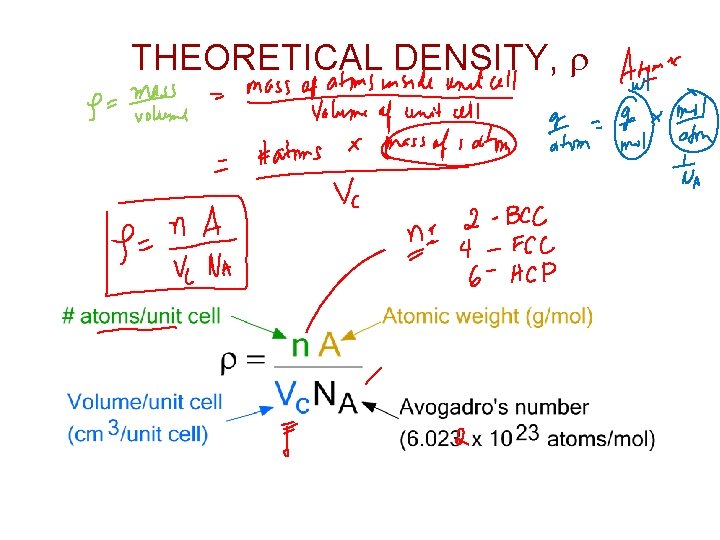
THEORETICAL DENSITY, r

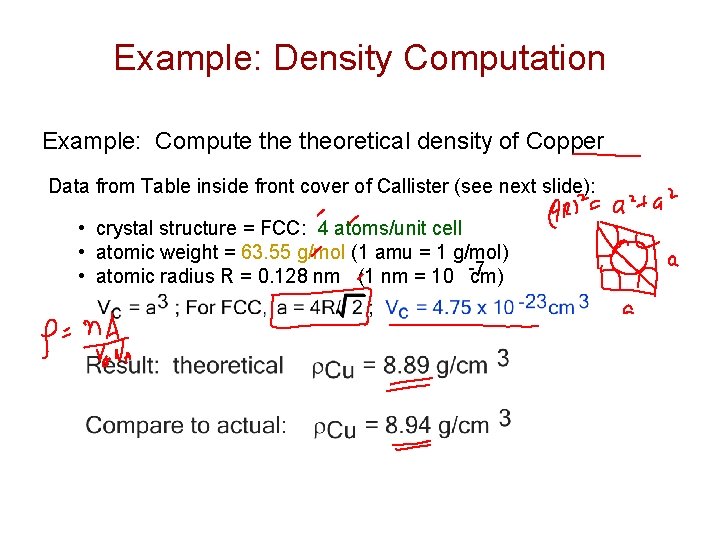
Example: Density Computation Example: Compute theoretical density of Copper Data from Table inside front cover of Callister (see next slide): • crystal structure = FCC: 4 atoms/unit cell • atomic weight = 63. 55 g/mol (1 amu = 1 g/mol) -7 • atomic radius R = 0. 128 nm (1 nm = 10 cm)

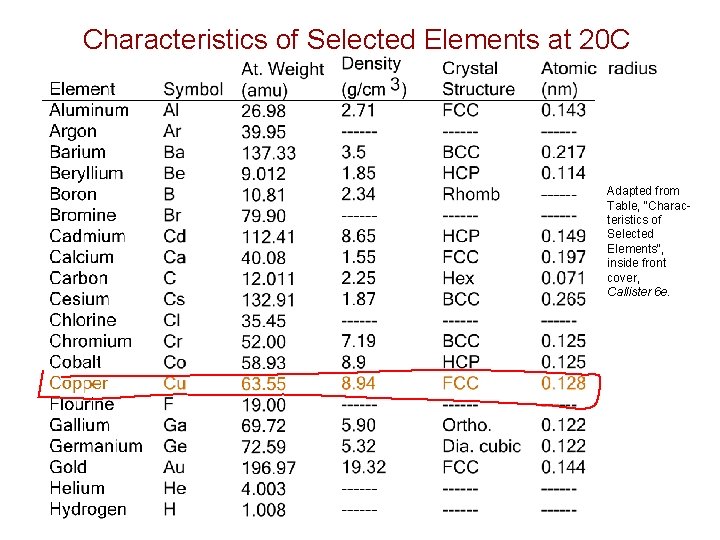
Characteristics of Selected Elements at 20 C Adapted from Table, "Characteristics of Selected Elements", inside front cover, Callister 6 e.

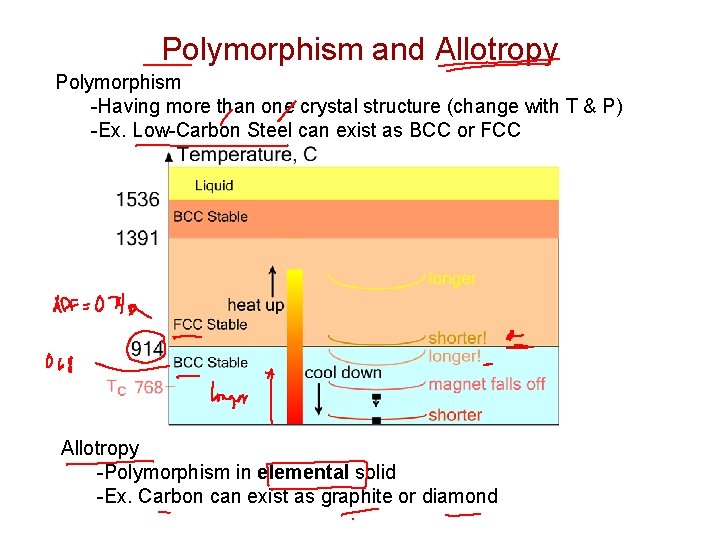
Polymorphism and Allotropy Polymorphism -Having more than one crystal structure (change with T & P) -Ex. Low-Carbon Steel can exist as BCC or FCC Allotropy -Polymorphism in elemental solid -Ex. Carbon can exist as graphite or diamond

SUMMARY • Atoms may have crystalline or amorphous structures. • We can predict the density of a material from the atomic weight, atomic radius, and crystal structure (e. g. , FCC, BCC, HCP). • Anisotropic material - properties vary with direction – true for a single crystal orientation. • Isotropic material - properties are non directional – true for polycrystals with randomly oriented grains.

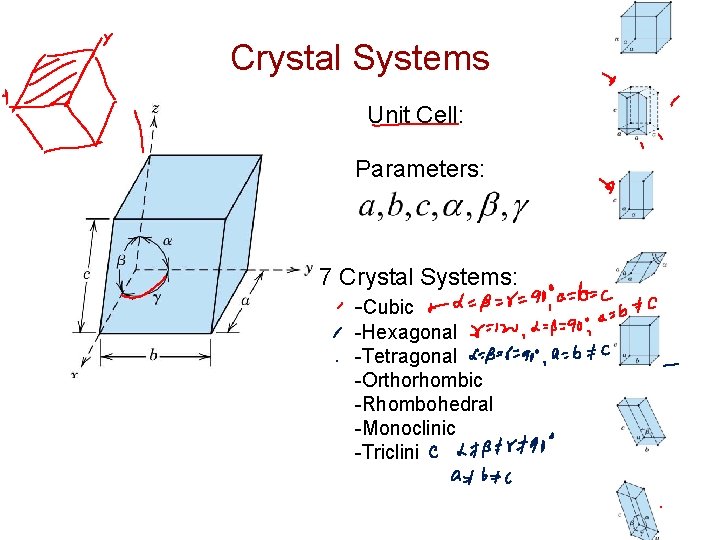
Crystal Systems Unit Cell: Parameters: 7 Crystal Systems: -Cubic -Hexagonal -Tetragonal -Orthorhombic -Rhombohedral -Monoclinic -Triclini

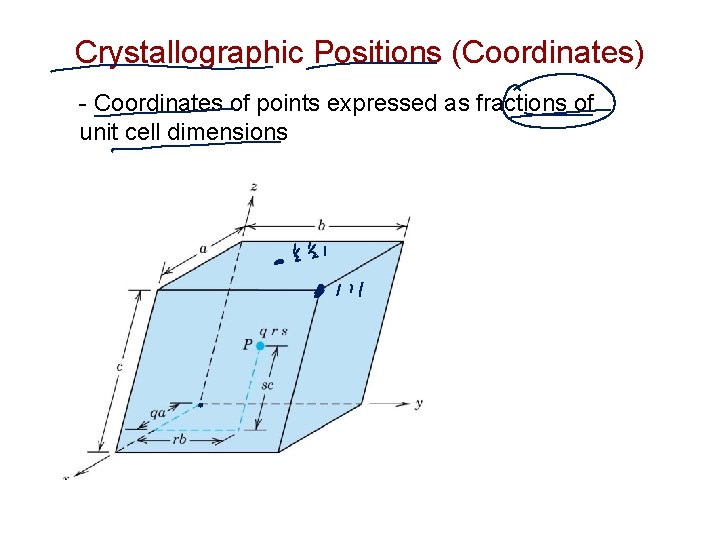
Crystallographic Positions (Coordinates) - Coordinates of points expressed as fractions of unit cell dimensions

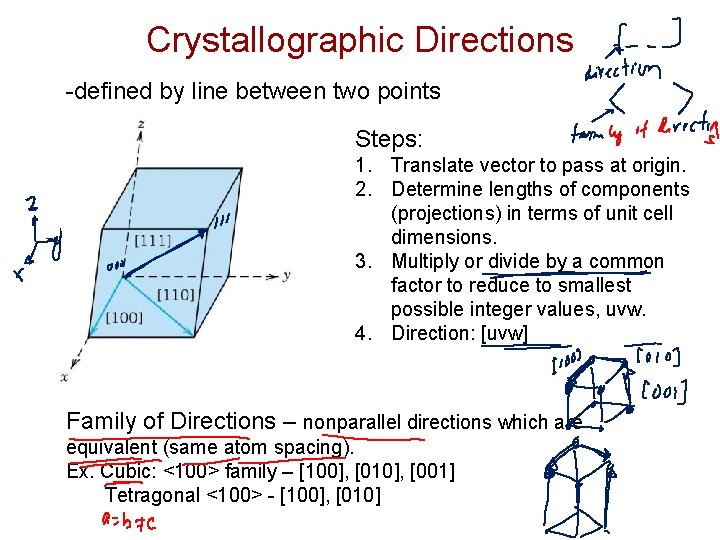
Crystallographic Directions -defined by line between two points Steps: 1. Translate vector to pass at origin. 2. Determine lengths of components (projections) in terms of unit cell dimensions. 3. Multiply or divide by a common factor to reduce to smallest possible integer values, uvw. 4. Direction: [uvw] Family of Directions – nonparallel directions which are equivalent (same atom spacing). Ex. Cubic: <100> family – [100], [010], [001] Tetragonal <100> - [100], [010]

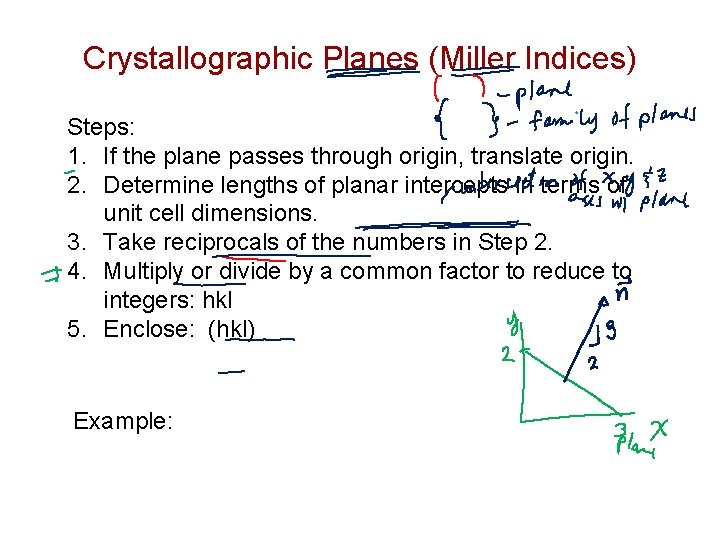
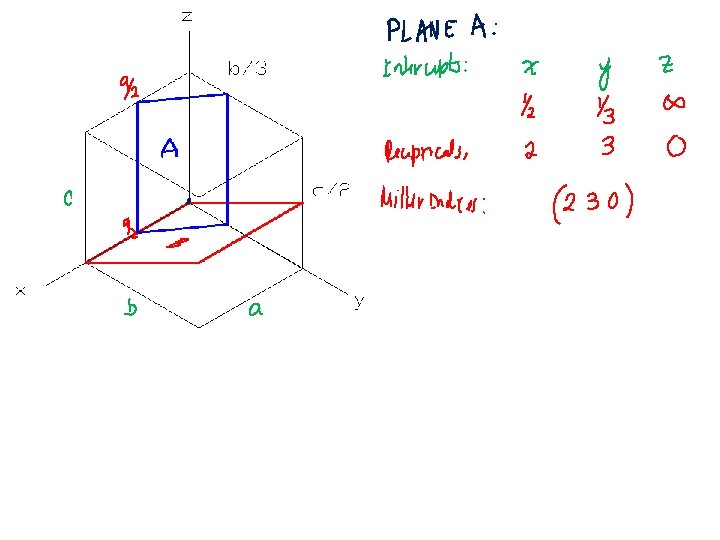
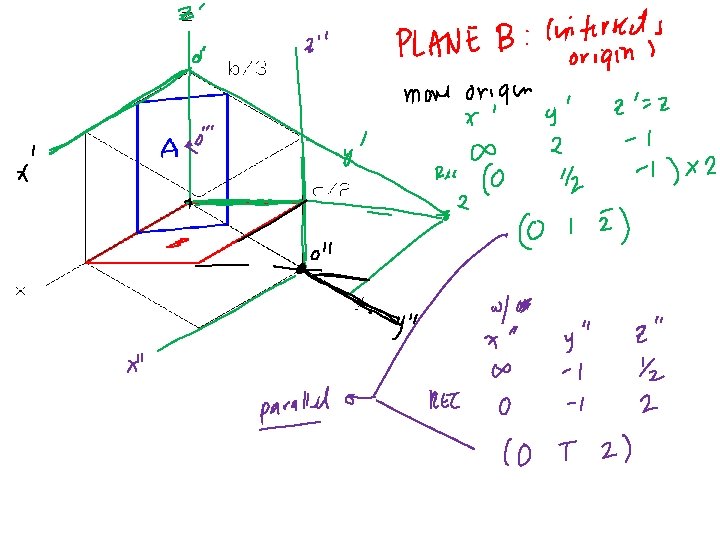
Crystallographic Planes (Miller Indices) Steps: 1. If the plane passes through origin, translate origin. 2. Determine lengths of planar intercepts in terms of unit cell dimensions. 3. Take reciprocals of the numbers in Step 2. 4. Multiply or divide by a common factor to reduce to integers: hkl 5. Enclose: (hkl) Example:



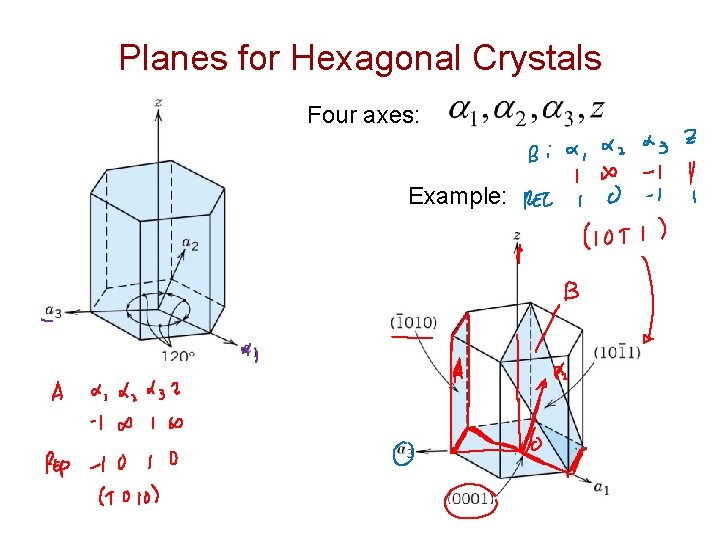
Planes for Hexagonal Crystals Four axes: Example:

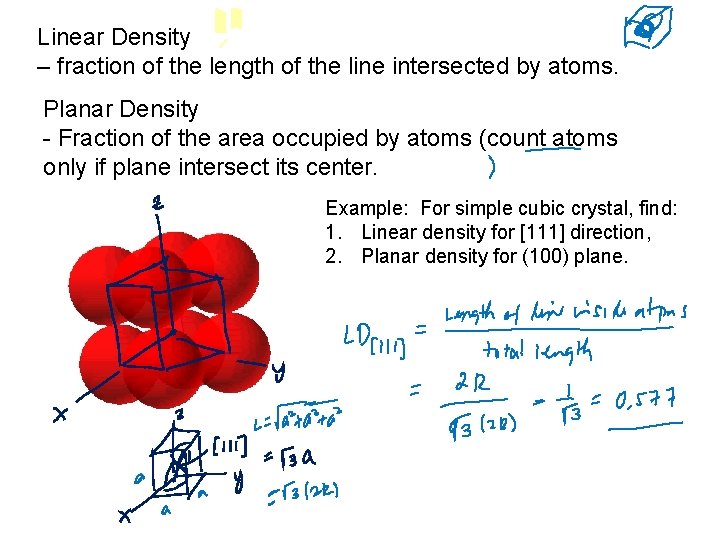
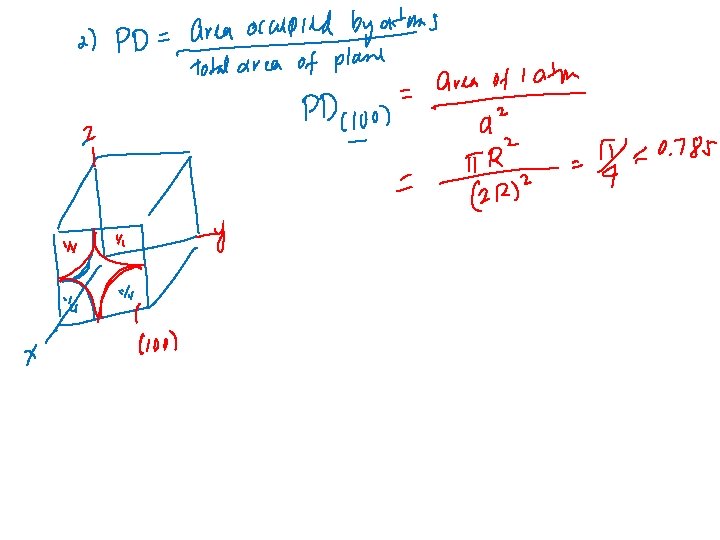
Linear Density – fraction of the length of the line intersected by atoms. Planar Density - Fraction of the area occupied by atoms (count atoms only if plane intersect its center. Example: For simple cubic crystal, find: 1. Linear density for [111] direction, 2. Planar density for (100) plane.


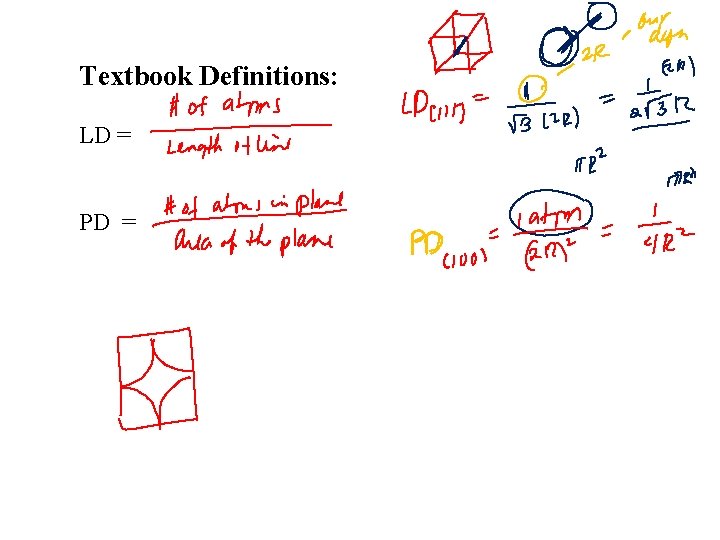
Textbook Definitions: LD = PD =

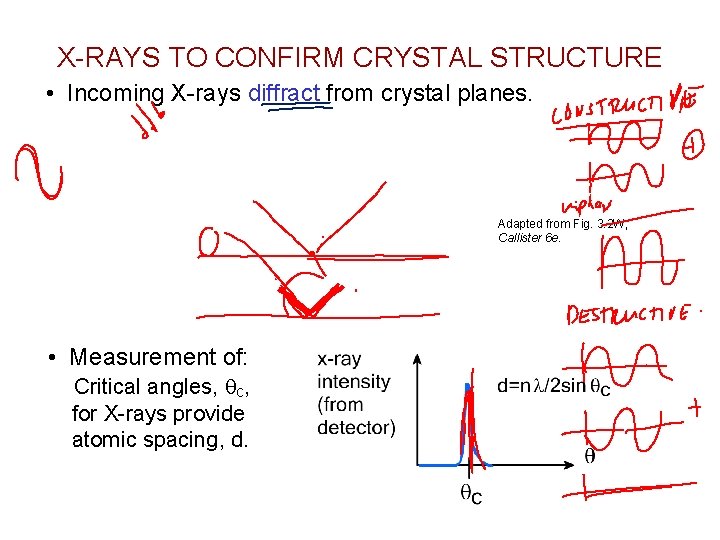
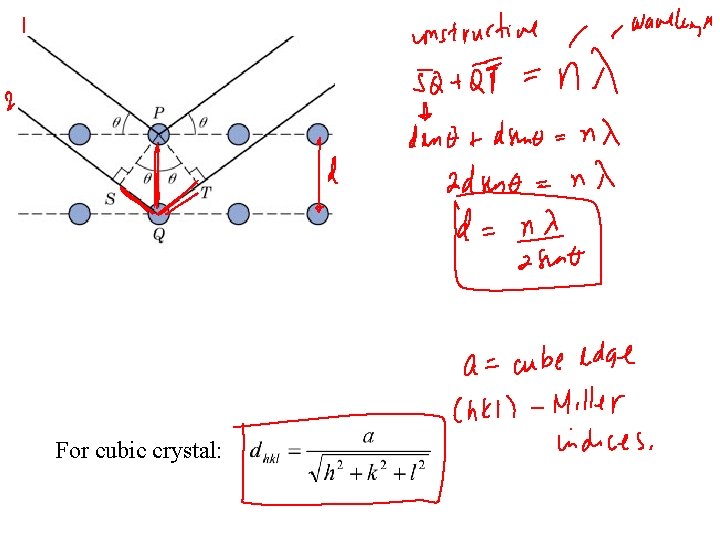
X-RAYS TO CONFIRM CRYSTAL STRUCTURE • Incoming X-rays diffract from crystal planes. Adapted from Fig. 3. 2 W, Callister 6 e. • Measurement of: Critical angles, qc, for X-rays provide atomic spacing, d.

For cubic crystal:

Quiz Review 1. At the position equal to the equilibrium spacing of two atoms, which of the following is/are true? A. The net force is equal to zero. B. The net energy is maximum. C. The net energy is zero. D. A and B above.

2. Two atoms are at an interatomic spacing less than the equilibrium spacing. Which of the following is true regarding the atoms? A. The net energy is minimum. B. The new force is zero. C. The attractive force is greater than the repulsive force. D. The attractive force is less than the repulsive force.

3. The interatomic bond that is formed by the transfer of one or more electrons from one atom to the other is called A. Covalent bond. B. Ionic bond. C. Metallic bond. D. Secondary bond.

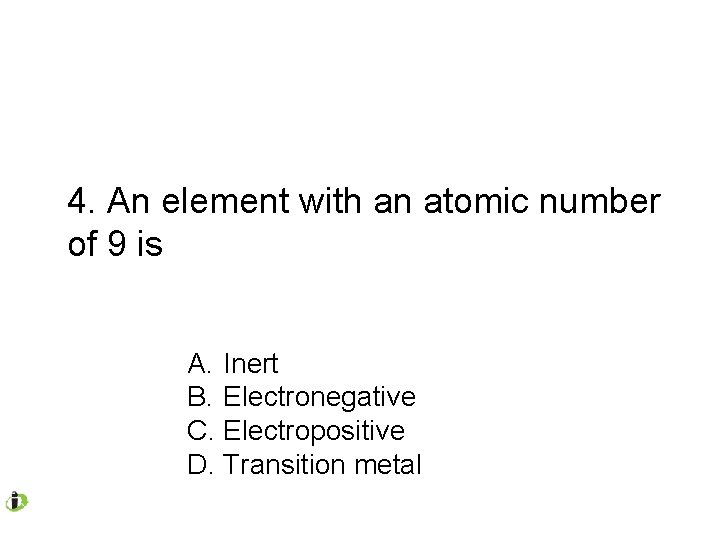
4. An element with an atomic number of 9 is A. Inert B. Electronegative C. Electropositive D. Transition metal

5. Two atoms of different metals have the same crystal structure. If Atom A has a bigger atomic radius than atom B, which has the higher atomic packing factor? A. Atom A B. Atom B C. Depends on atomic weights D. None of the above.

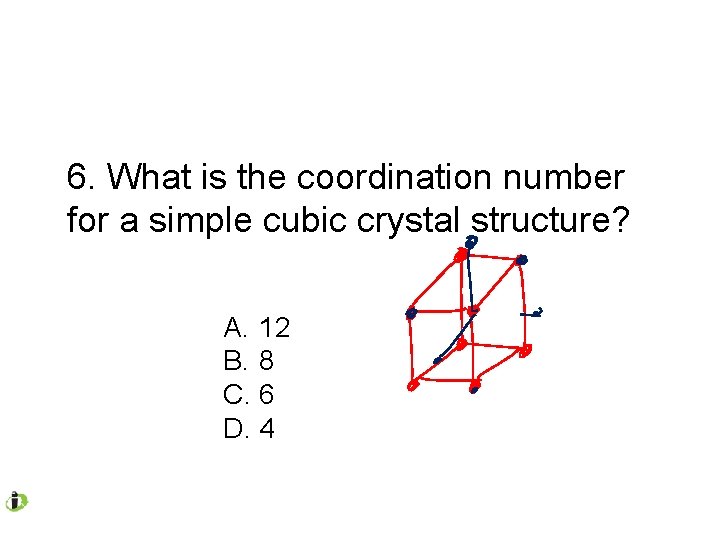
6. What is the coordination number for a simple cubic crystal structure? A. 12 B. 8 C. 6 D. 4

7. For which crystal system is are the angles between the three axes equal to 90 o and two of the unit cell dimensions are equal to each other? A. Cubic B. Tetragonal C. Orthorhombic D. Triclinic
![8 Identify the crystallographic direction of the vector A 1 1 1 B 1 8. Identify the crystallographic direction of the vector: A. [-1 1 1] B. [1](https://slidetodoc.com/presentation_image/229b31d6f2e8b9d3c62addb8d13f7dc8/image-35.jpg)
8. Identify the crystallographic direction of the vector: A. [-1 1 1] B. [1 1 -1] C. [1 -1 1] D. [1 1 1]
![9 Identify the crystallographic direction of the vector A 2 1 2 B 2 9. Identify the crystallographic direction of the vector: A. [-2 1 2] B. [-2](https://slidetodoc.com/presentation_image/229b31d6f2e8b9d3c62addb8d13f7dc8/image-36.jpg)
9. Identify the crystallographic direction of the vector: A. [-2 1 2] B. [-2 1 1] C. [1 -2 2] D. [-1 2 1]

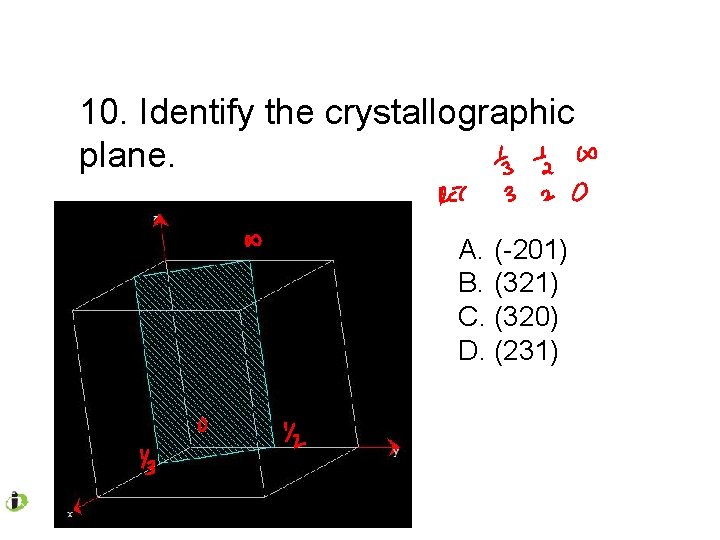
10. Identify the crystallographic plane. A. (-201) B. (321) C. (320) D. (231)

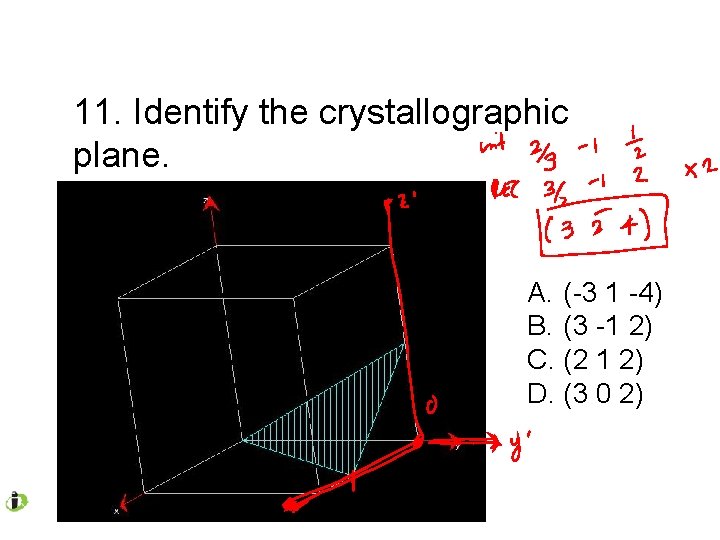
11. Identify the crystallographic plane. A. (-3 1 -4) B. (3 -1 2) C. (2 1 2) D. (3 0 2)

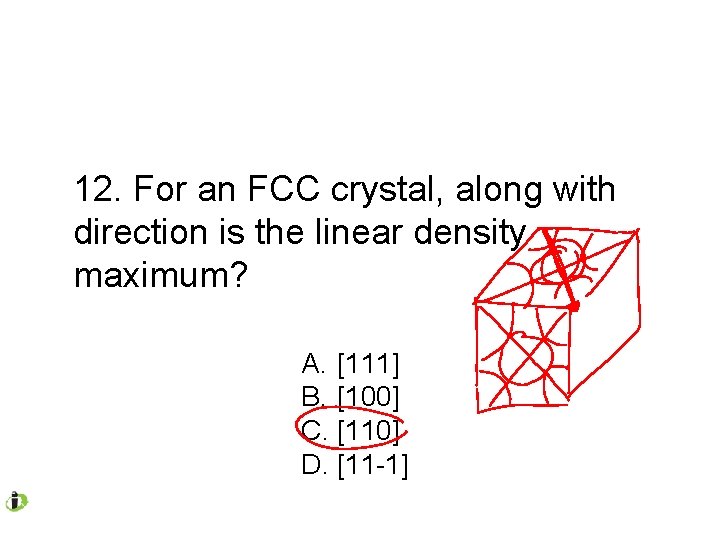
12. For an FCC crystal, along with direction is the linear density maximum? A. [111] B. [100] C. [110] D. [11 -1]
 Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Volume to atoms
Volume to atoms Crystalline solids
Crystalline solids Cortilage
Cortilage Repetitive nearest neighbor algorithm
Repetitive nearest neighbor algorithm It is a repetitive process in which algorithm calls itself.
It is a repetitive process in which algorithm calls itself. Process-oriented layout example
Process-oriented layout example Repetitive signal
Repetitive signal Example of repetitive process
Example of repetitive process Automate repetitive tasks in excel
Automate repetitive tasks in excel Janice eng
Janice eng Drafting lines
Drafting lines Metode pengulangan
Metode pengulangan Rhythm is the of visual movement colors shapes or lines
Rhythm is the of visual movement colors shapes or lines Repetitive process in operations management
Repetitive process in operations management How long does it take to make a car
How long does it take to make a car Contoh repetitive focus
Contoh repetitive focus Ocd obsessive thoughts
Ocd obsessive thoughts Structure of metallic solids
Structure of metallic solids Crystalline solid and amorphous solid
Crystalline solid and amorphous solid State of matter
State of matter Crystalline substances
Crystalline substances Is fudge a crystalline candy
Is fudge a crystalline candy Polycrystalline solids
Polycrystalline solids Coprecipitation errors
Coprecipitation errors Difference between occlusion and mixed-crystal formation
Difference between occlusion and mixed-crystal formation Difference between colloidal and crystalline precipitate
Difference between colloidal and crystalline precipitate Volatilization gravimetry
Volatilization gravimetry Snap head rivet application
Snap head rivet application Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Crystalline silicate clays
Crystalline silicate clays Crystal state
Crystal state Metallic crystal structure
Metallic crystal structure Destiny 2 crystalline formations
Destiny 2 crystalline formations Ccl
Ccl Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Surface area of solids
Surface area of solids Cyanmethemoglobin method
Cyanmethemoglobin method