The Structure of Crystalline Solids Atomic Arrangement Crystal














- Slides: 32

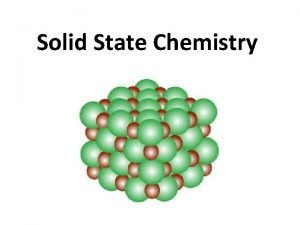
The Structure of Crystalline Solids

Atomic Arrangement Crystal structures have short-range order and long-range order (on a lattice - see CDROM) unit cell = smallest grouping of atoms that show the geometry of the structure and be repeated to form the structure Seven unit cell geometries possible

Atomic Arrangement When a=b=c and a=b=g=90 the crystal system is cubic lattice points = atom positions within a unit cell (e. g. , at the corners, center of the cube, center of the faces, etc. ) 14 types of unit cells (Bravis lattices) in 7 crystal systems are possible

Atomic Arrangement lattice parameter (for cubic crystals) = a 0 measured at room T and given in nm = 1 x 10 -9 m you should remember or these unit conversions Å = 1 x 10 -10 m

Why do we care? To study properties of crystalline materials (such as strength or electrical conductivity) we work with unit cells. Therefore we need to know how many atoms/unit cell there are for a particular material

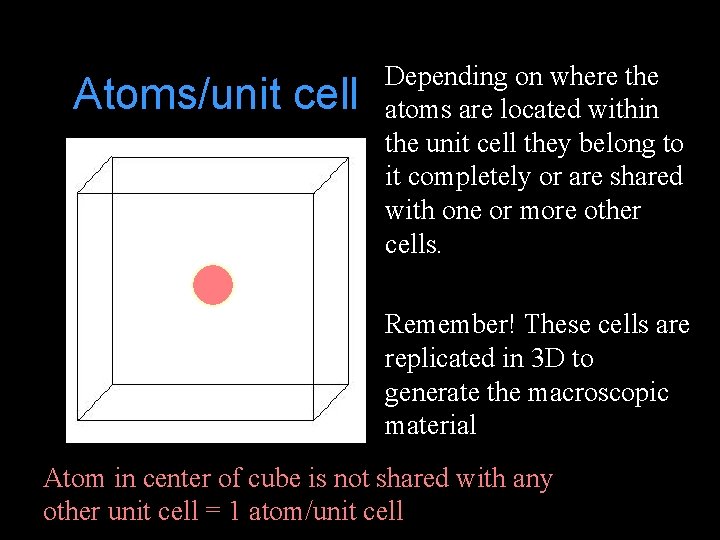
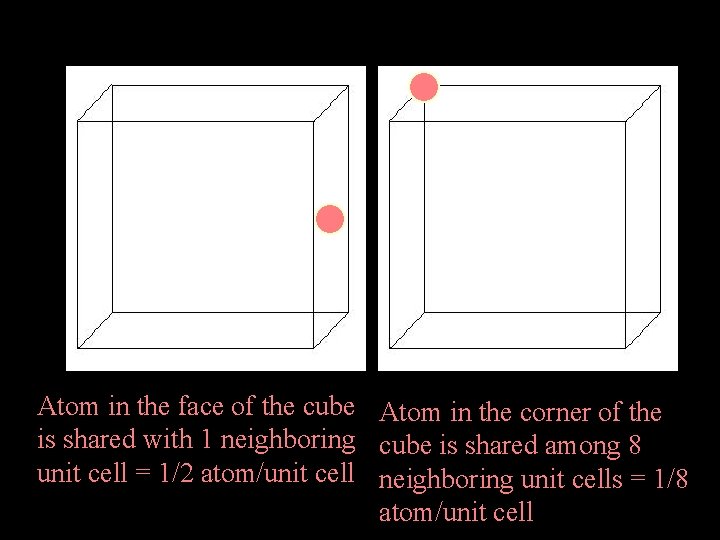
Atoms/unit cell Depending on where the atoms are located within the unit cell they belong to it completely or are shared with one or more other cells. Remember! These cells are replicated in 3 D to generate the macroscopic material Atom in center of cube is not shared with any other unit cell = 1 atom/unit cell

Atoms/unit cell Atom in the face of the cube Atom in the corner of the is shared with 1 neighboring cube is shared among 8 unit cell = 1/2 atom/unit cell neighboring unit cells = 1/8 atom/unit cell

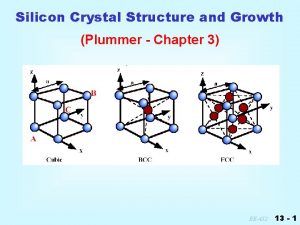
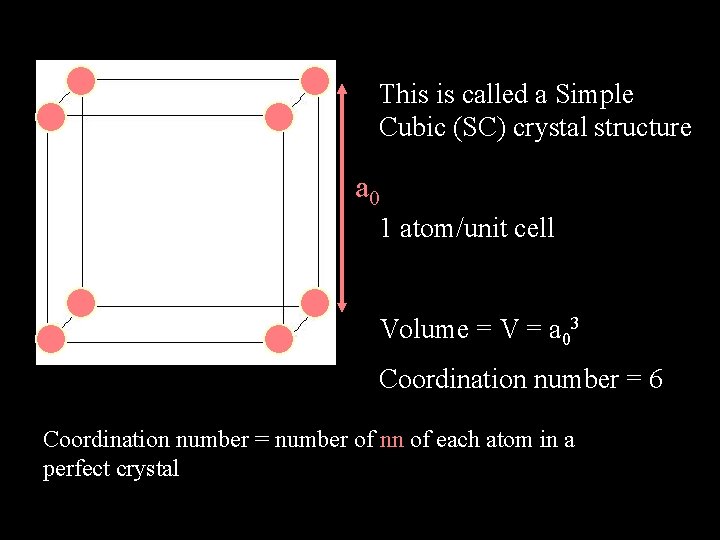
This is called a Simple Cubic (SC) crystal structure a 0 1 atom/unit cell Volume = V = a 03 Coordination number = 6 Coordination number = number of nn of each atom in a perfect crystal

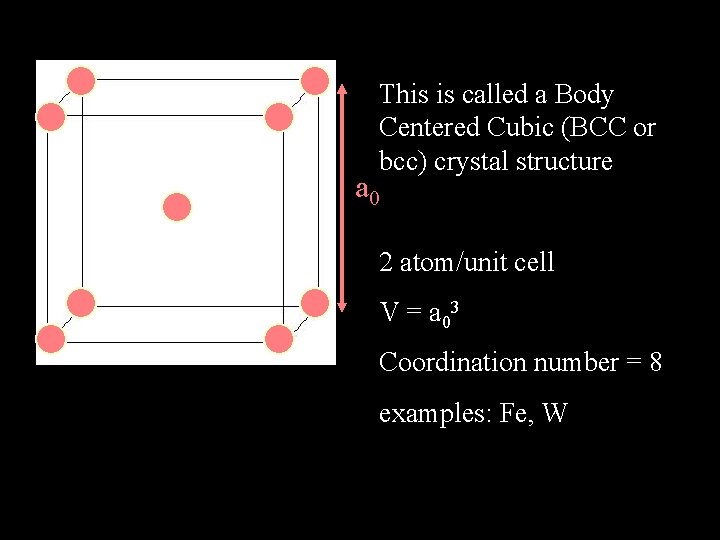
This is called a Body Centered Cubic (BCC or bcc) crystal structure a 0 2 atom/unit cell V = a 03 Coordination number = 8 examples: Fe, W

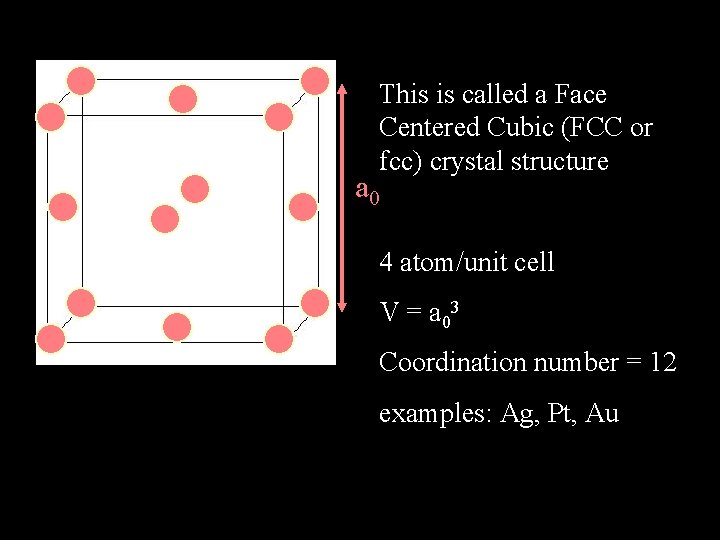
This is called a Face Centered Cubic (FCC or fcc) crystal structure a 0 4 atom/unit cell V = a 03 Coordination number = 12 examples: Ag, Pt, Au

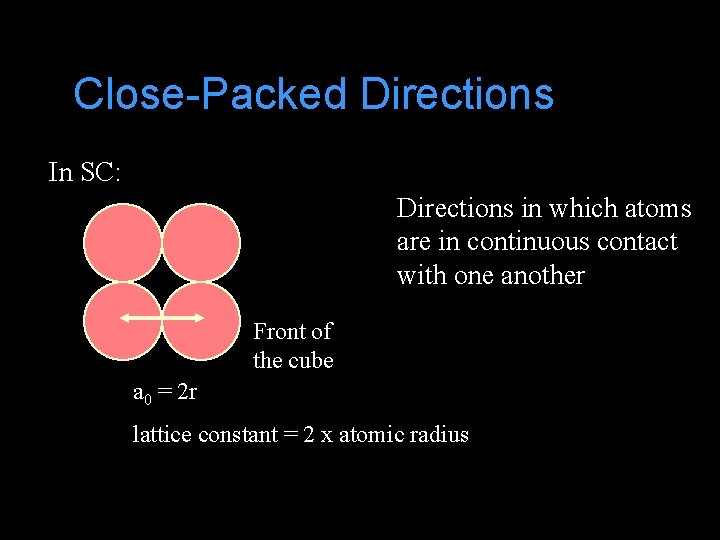
Close-Packed Directions In SC: Directions in which atoms are in continuous contact with one another Front of the cube a 0 = 2 r lattice constant = 2 x atomic radius

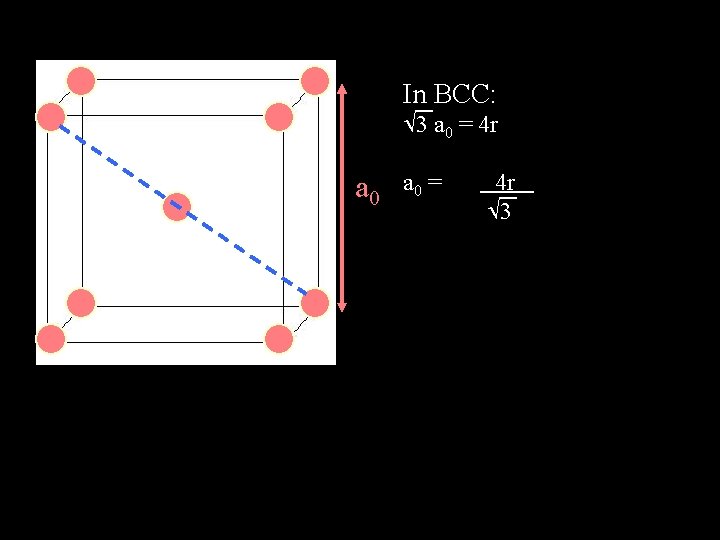
In BCC: 3 a 0 = 4 r 3

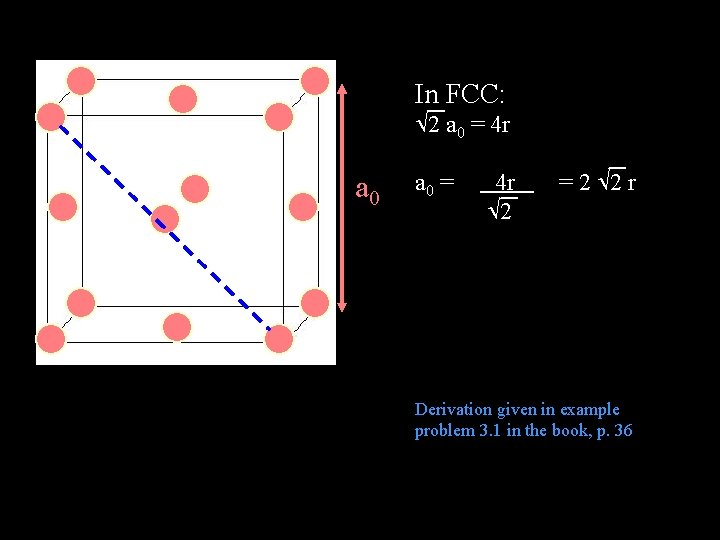
In FCC: 2 a 0 = 4 r 2 = 2 2 r Derivation given in example problem 3. 1 in the book, p. 36

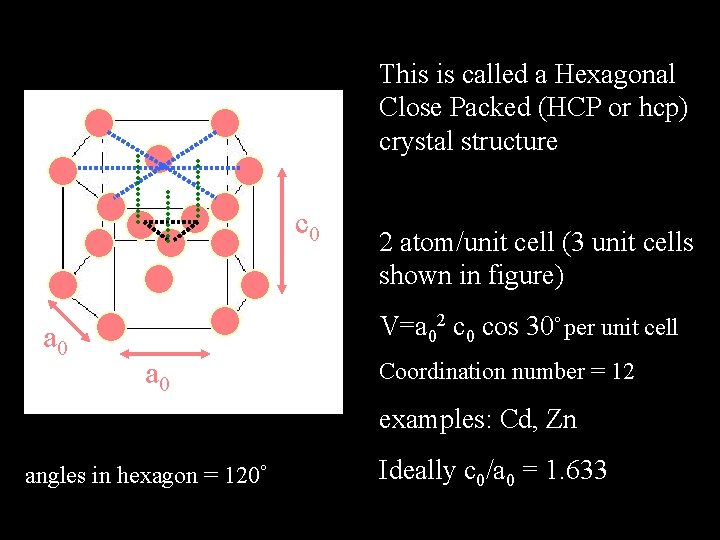
This is called a Hexagonal Close Packed (HCP or hcp) crystal structure c 0 a 0 2 atom/unit cell (3 unit cells shown in figure) V=a 02 c 0 cos 30 per unit cell a 0 Coordination number = 12 examples: Cd, Zn angles in hexagon = 120 Ideally c 0/a 0 = 1. 633

Atomic Packing Factor If we assume each atom is a hard sphere the atomic packing factor (APF) is the fraction of the unit cell occupied by atoms APF = Vatoms/Vcell = (#atoms)(Vatom)/(Vcell) Vatom = 4 pr 3 where r = radius of atom 3 Vcell given for each type of unit cell; r and a are related to each other by close-packed directions (examples)

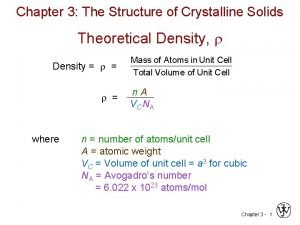
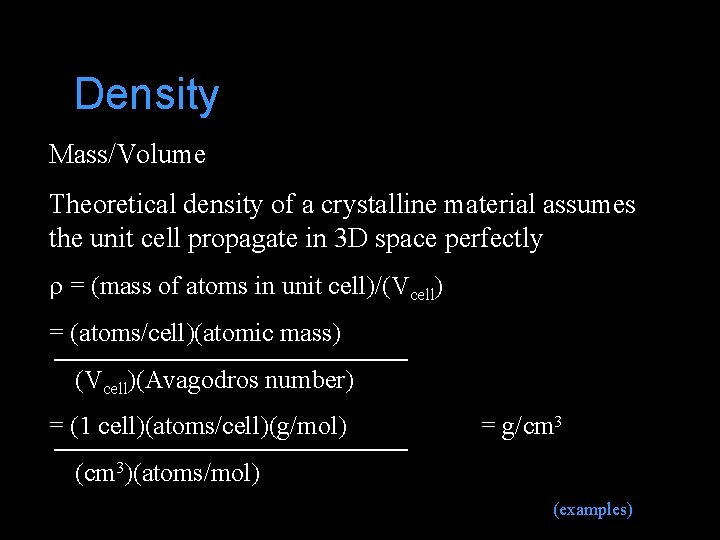
Density Mass/Volume Theoretical density of a crystalline material assumes the unit cell propagate in 3 D space perfectly r = (mass of atoms in unit cell)/(Vcell) = (atoms/cell)(atomic mass) (Vcell)(Avagodros number) = (1 cell)(atoms/cell)(g/mol) = g/cm 3 (cm 3)(atoms/mol) (examples)

Polymorphic Structures Same material, more than one crystal structure possible. When an elemental material is polymorphic, this is called allotropy.

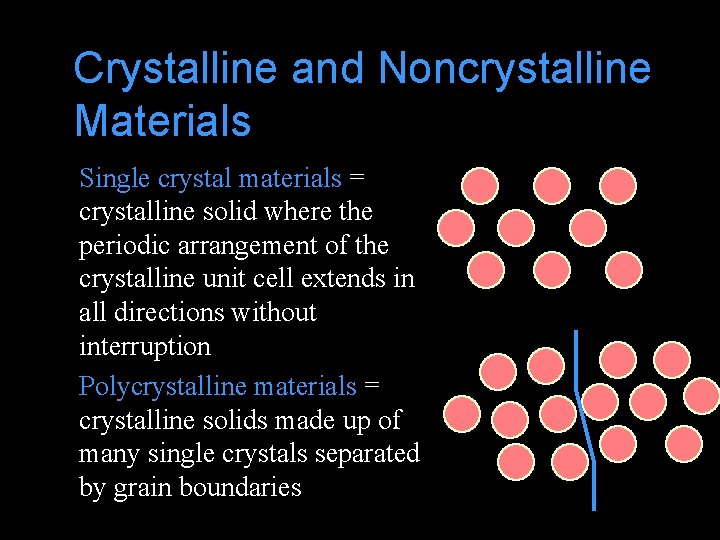
Crystalline and Noncrystalline Materials Single crystal materials = crystalline solid where the periodic arrangement of the crystalline unit cell extends in all directions without interruption Polycrystalline materials = crystalline solids made up of many single crystals separated by grain boundaries

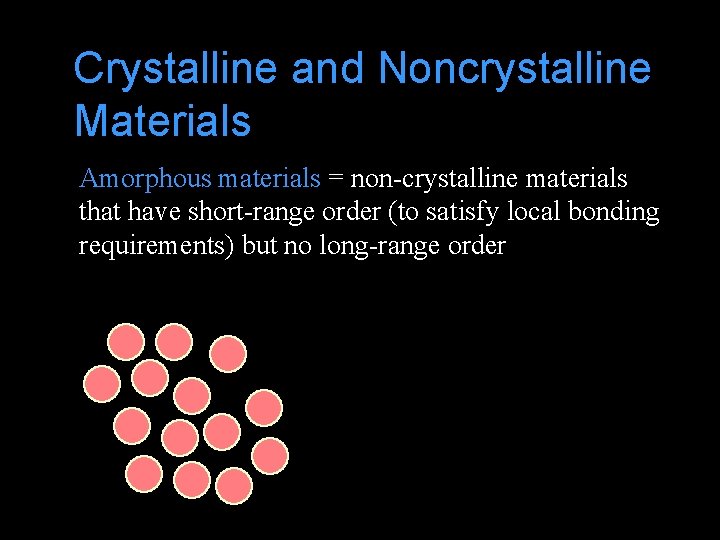
Crystalline and Noncrystalline Materials Amorphous materials = non-crystalline materials that have short-range order (to satisfy local bonding requirements) but no long-range order

Crystalline and Noncrystalline Materials Isotropic materials => measured properties are independent of direction of measurement (e. g. , cubic single crystals, polycrystalline materials) Anisotropic materials => measured properties depend on direction of measurement (e. g. , HCP single crystals)

Points, Directions, and Planes We have already discussed close-packed directions in some unit cells. Materials cleave, deform, etc. preferentially along certain planes or in certain directions. Therefore, we need clear, unambiguous language to discuss directions and planes in crystalline unit cells.

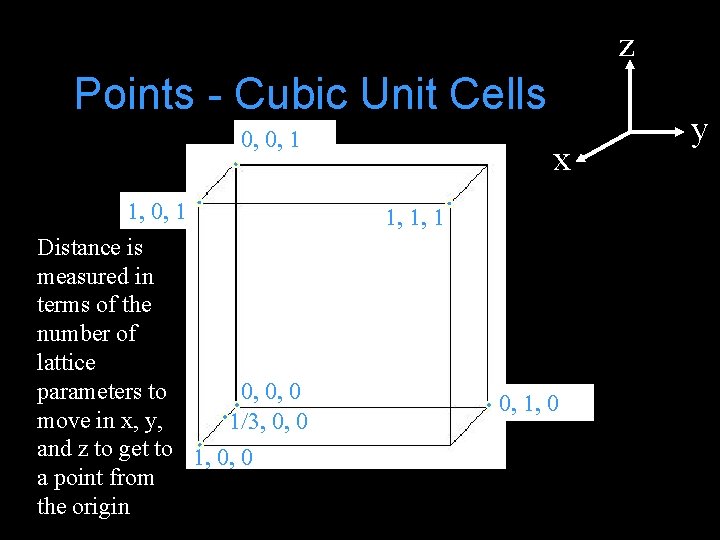
z Points - Cubic Unit Cells 0, 0, 1 1, 0, 1 Distance is measured in terms of the number of lattice parameters to 0, 0, 0 move in x, y, 1/3, 0, 0 and z to get to 1, 0, 0 a point from the origin x 1, 1, 1 0, 1, 0 y

Miller Indices Short-hand notation to unambiguously describe directions and planes in crystalline materials There is a specific procedure for finding miller indices for Directions

Directions - Cubic Unit Cells Determine coordinates of 2 points that form a line in the direction of interest w Subtract the coordinates of the tail point from the coordinates of the head point = # lattice parameters traveled along each axis w Reduce to smallest integers w Put final numbers in [uvw] with any negative numbers shown as “bar” numbers (e. g. , -2 is written as 2) (examples) w

Notes Because directions are vectors, a direction and its negative are not identical. Therefore, [100] is not the same direction as [100]. w A direction and its multiple are identical. [100] is the same direction as [200]; it has just not been reduced to the smallest integers. w (examples)

More Notes w Certain groups of directions are equivalent; they are only different because of the way we constructed the coordinates. In cubic lattices, [100] is equivalent to [010] if we redefine the coordinate system (or rotate the unit cell). Equivalent groups of equivalent directions are written in <uvw> (examples)

Planes - Cubic Unit Cells Find the x, y, z intercepts of the plane within the unit cell in terms of the number of lattice parameters. Note: if the plane passes through the point you have designated as the origin, the origin must be moved. w Take the reciprocals of the intercepts w Clear all fractions but do not reduce to lowest integers w Write inside (uvw); negative numbers written as “bar” numbers (examples) w

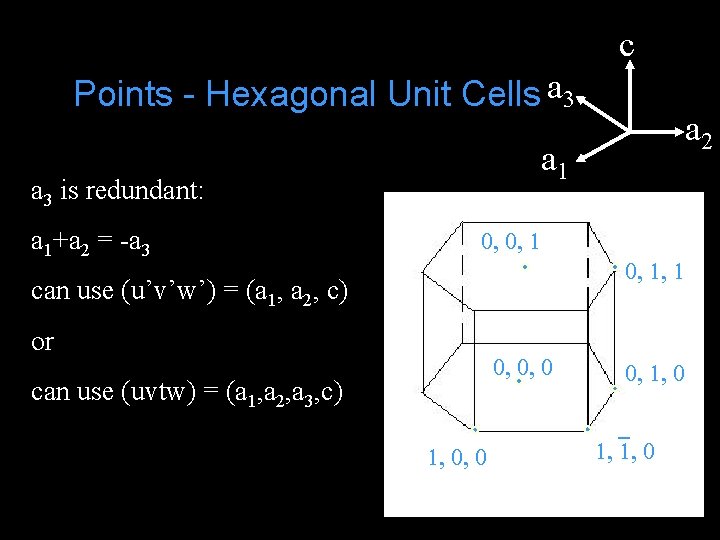
c Points - Hexagonal Unit Cells a 3 a 1 a 3 is redundant: a 1+a 2 = -a 3 a 2 0, 0, 1, 1 can use (u’v’w’) = (a 1, a 2, c) or 0, 0, 0 can use (uvtw) = (a 1, a 2, a 3, c) 1, 0, 0 0, 1, 0 1, 1, 0

Directions - Hexagonal Unit Cells w. Determine the number of lattice parameters to move in each direction to get from tail to head and keep u + v = -t w. Can be expressed in terms of [u’v’w’] or [uvtw] w. To convert between 3 -coordinate and 4 -coordinate system, follow these rules: u = 1/3 (2 u’-v’) Then clear fractions and reduce v = 1/3 (2 v’-u’) to the smallest integers t = -1/3(u’+v’) = -(u+v) (examples) w = w’

Planes - Hexagonal Unit Cells Express in 3 -coordinate system or 4 -coordinate system w Use u + v = -t to convert back and forth between the two w Otherwise, no different from planes in cubic crystals w (examples)

Linear Density Number of atoms along a particular directions. 1 D version of the packing factor Fraction of the line length intersected by atoms (examples)

Planar Density Number of atoms per unit area in the plane. 2 D version of the packing factor Fraction of area of the plane covered by atoms (examples)
 Crystallography types
Crystallography types Crystalline and non crystalline materials
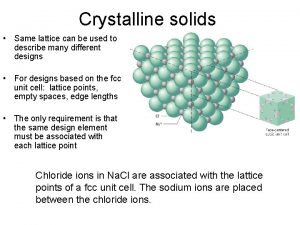
Crystalline and non crystalline materials Crystalline solids
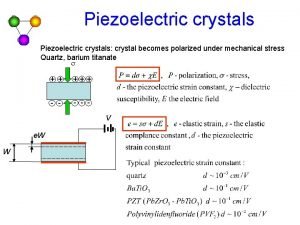
Crystalline solids Piezoelectric crystal atomic structure
Piezoelectric crystal atomic structure Relative formula mass of hcl
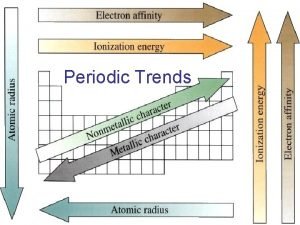
Relative formula mass of hcl Periodic trebds
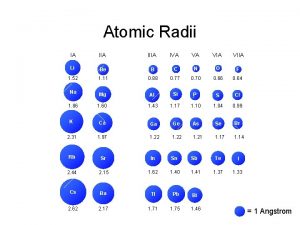
Periodic trebds Atomic radius increases from left to right
Atomic radius increases from left to right Atomic weight of oxygen
Atomic weight of oxygen Atomic mass and atomic number difference
Atomic mass and atomic number difference Atomic number vs atomic radius
Atomic number vs atomic radius Amorphous vs crystalline
Amorphous vs crystalline Anisotropic
Anisotropic Crystalline substances
Crystalline substances Crystalline candy
Crystalline candy Crystalline solid
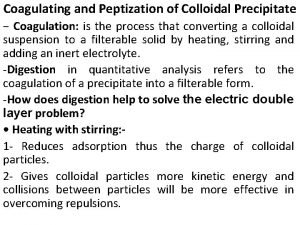
Crystalline solid Difference between colloidal and crystalline precipitate
Difference between colloidal and crystalline precipitate Peptization
Peptization Difference between colloidal and crystalline precipitate
Difference between colloidal and crystalline precipitate Difference between occlusion and mixed-crystal formation
Difference between occlusion and mixed-crystal formation Snap head rivet application
Snap head rivet application Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Crystalline silicate clays
Crystalline silicate clays Crystal state
Crystal state Crystalline solid
Crystalline solid Destiny 2 crystalline formations
Destiny 2 crystalline formations Ccl
Ccl Diamond zinc blende structure
Diamond zinc blende structure Silicon crystal lattice
Silicon crystal lattice Titanium crystal structure
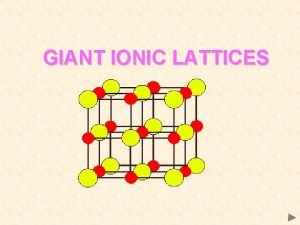
Titanium crystal structure Giant ionic structure
Giant ionic structure Simple cubic structure coordination number
Simple cubic structure coordination number Basis in crystal structure
Basis in crystal structure Crystal structure of ceramics
Crystal structure of ceramics