CCl 4 Sorption and Degradation on Feoxide surfaces



- Slides: 13

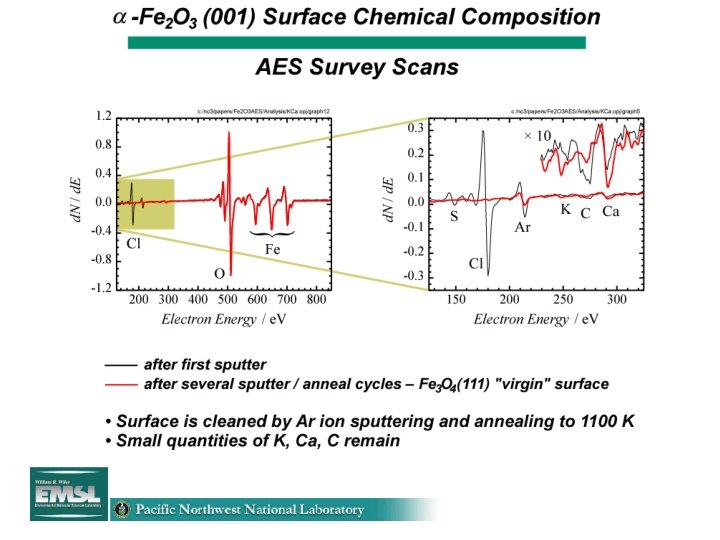
CCl 4 Sorption and Degradation on Fe-oxide surfaces Jeff Fitts, Kwang Rim 1, George Flynn 1, Kaveh Adib 2, Nick Camillone III 2, Richard Osgood 2, Peter Schlosser 3, Steve Joyce 4 Environmental Molecular Sciences Institute Columbia University, New York, NY Electrical Engineering 2 Chemistry 1 Environmental Engineering 3 EMSL @ PNNL 4

C-Cl + Me. Osurf = Me. Cl + CO Organochlorine compounds: PCBs, DDT, CCl 4. . . • Extensive point source contamination and elevated global background levels. • Compounds are hormonally active, toxic and carcinogenic. • High levels observed in human tissue, blood and breast milk. Compounds readily react on certain metal oxide surfaces. • CCl 4: oxides of V, Cr, Ti and Fe

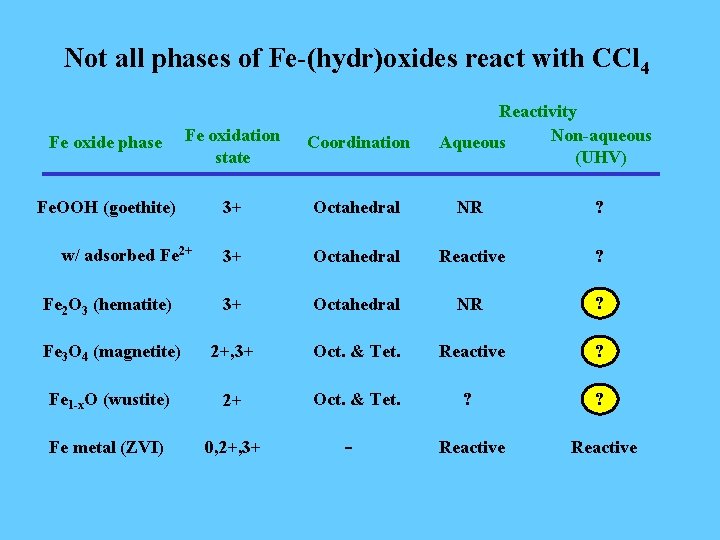
Not all phases of Fe-(hydr)oxides react with CCl 4 Reactivity Non-aqueous Aqueous (UHV) Fe oxide phase Fe oxidation state Coordination Fe. OOH (goethite) 3+ Octahedral NR ? 3+ Octahedral Reactive ? Fe 2 O 3 (hematite) 3+ Octahedral NR ? Fe 3 O 4 (magnetite) 2+, 3+ Oct. & Tet. Reactive ? Fe 1 -x. O (wustite) 2+ Oct. & Tet. ? ? Fe metal (ZVI) 0, 2+, 3+ Reactive w/ adsorbed Fe 2+ -

Project goals • Determine whether CCl 4 reacts at hematite, magnetite and wustite surfaces in UHV. • Identify unique characteristics of Fe-oxides surfaces responsible for observed reactivity.

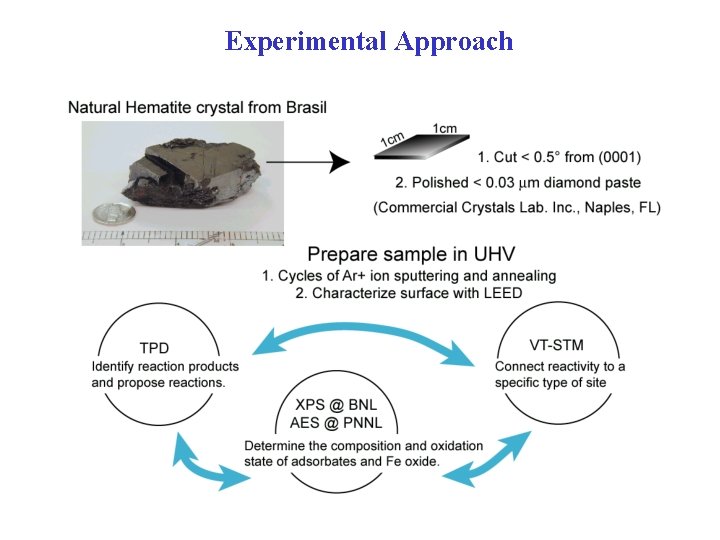
Experimental Approach

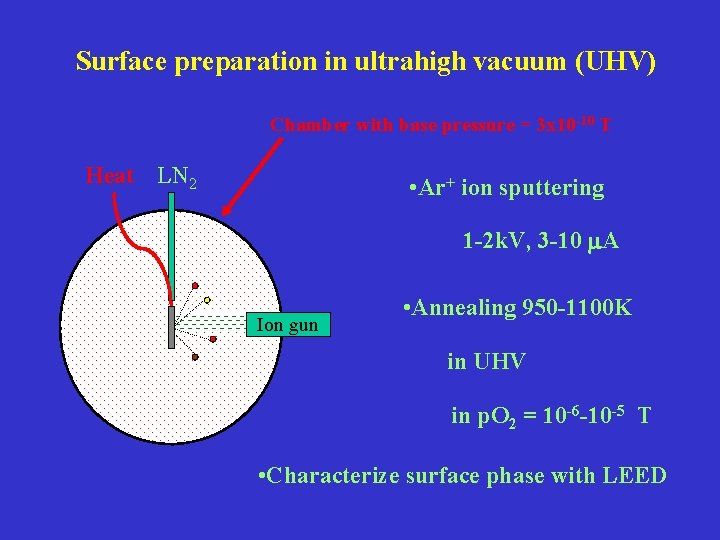
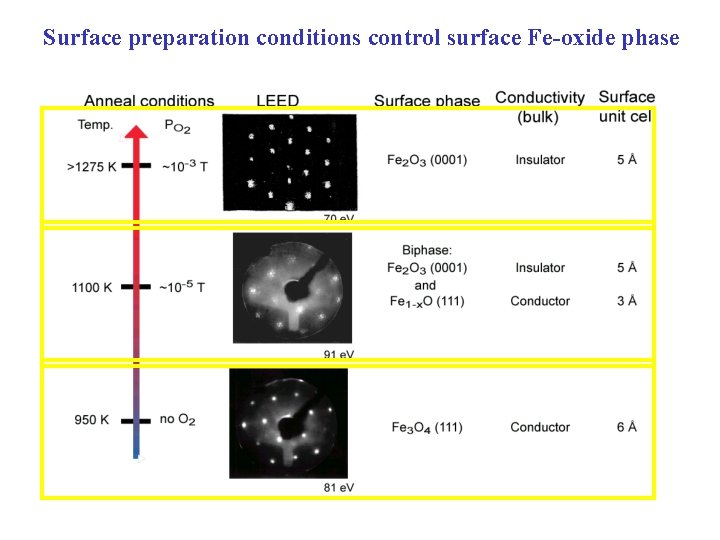
Surface preparation in ultrahigh vacuum (UHV) Chamber with base pressure = 3 x 10 -10 T Heat LN 2 • Ar+ ion sputtering 1 -2 k. V, 3 -10 m. A Ion gun • Annealing 950 -1100 K in UHV in p. O 2 = 10 -6 -10 -5 T • Characterize surface phase with LEED


Surface preparation conditions control surface Fe-oxide phase

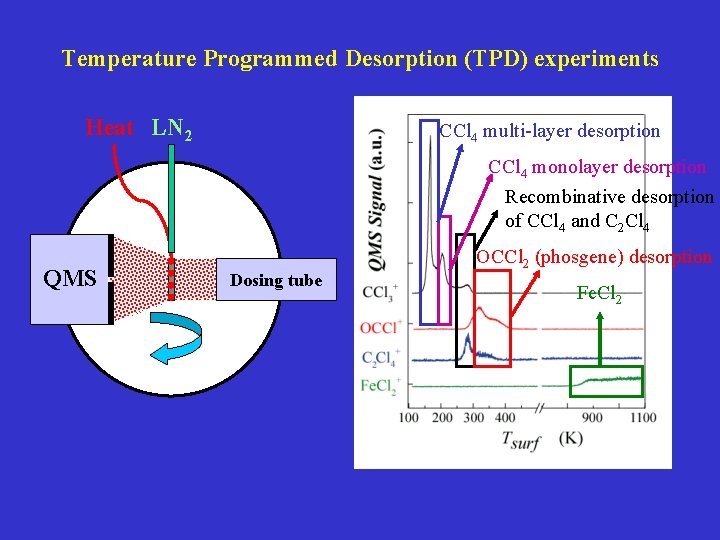
Temperature Programmed Desorption (TPD) experiments Heat LN 2 CCl 4 multi-layer desorption CCl 4 monolayer desorption Recombinative desorption of CCl 4 and C 2 Cl 4 QMS Dosing tube OCCl 2 (phosgene) desorption Fe. Cl 2

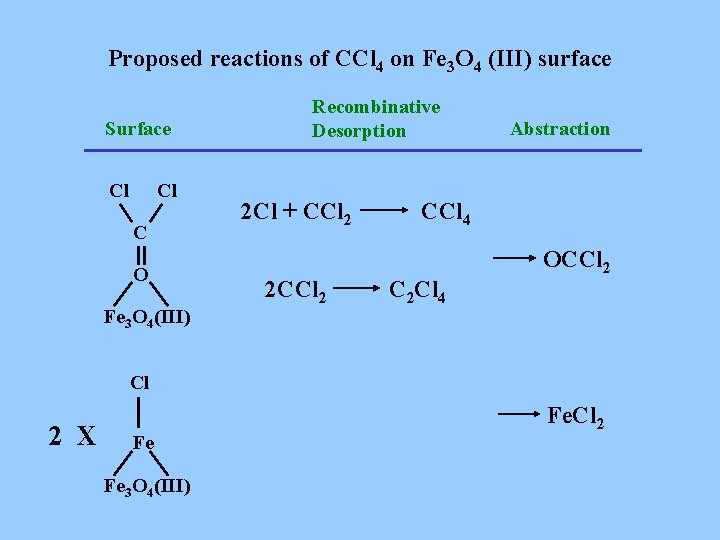
Proposed reactions of CCl 4 on Fe 3 O 4 (III) surface Surface Cl Cl C O Fe 3 O 4(III) Recombinative Desorption 2 Cl + CCl 2 2 CCl 2 Abstraction CCl 4 C 2 Cl 4 OCCl 2 X Fe Fe 3 O 4(III) Fe. Cl 2

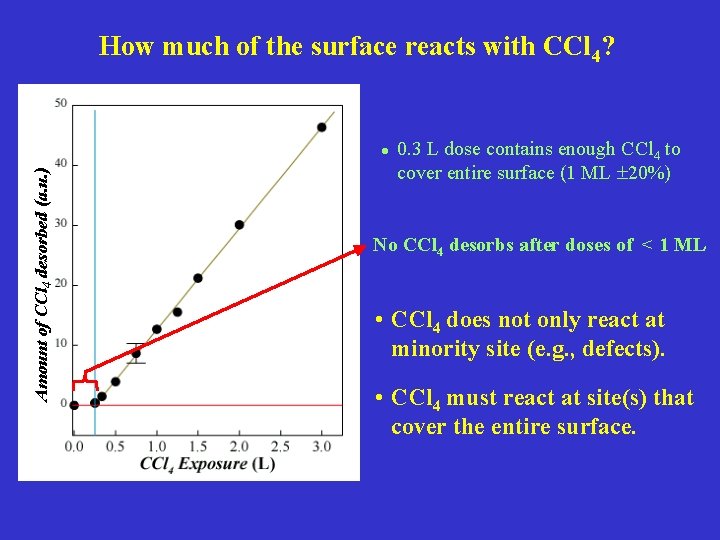
How much of the surface reacts with CCl 4? Amount of CCl 4 desorbed (a. u. ) • 0. 3 L dose contains enough CCl 4 to cover entire surface (1 ML 20%) No CCl 4 desorbs after doses of < 1 ML • CCl 4 does not only react at minority site (e. g. , defects). • CCl 4 must react at site(s) that cover the entire surface.

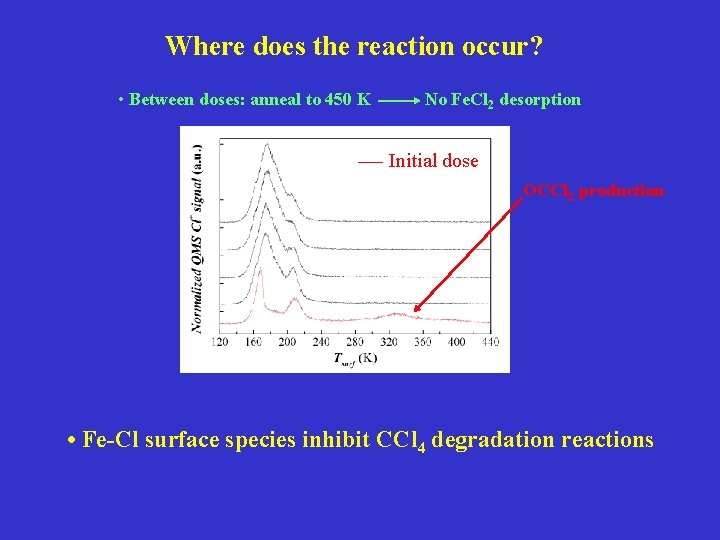
Where does the reaction occur? • Between doses: anneal to 450 K No Fe. Cl 2 desorption Initial dose OCCl 2 production • Fe-Cl surface species inhibit CCl 4 degradation reactions

What did we learn from the TPD studies? • CCl 4 readily reacts with the Fe 3 O 4(111) surface to predominantly form OCCl 2 • This reaction likely occurs at regular surface sites • Fe-Cl surface species inhibit further reaction What do we want to know about these reactions? • What type of surface site reacts with CCl 4? • How do Fe-Cl species inhibit CCl 4 degradation?