Radius ratio In ionic solids cations try to

![Packing of spheres III Shape of interstitial voids B-layer C-layer A-layer tetrahedron [111] direction Packing of spheres III Shape of interstitial voids B-layer C-layer A-layer tetrahedron [111] direction](https://slidetodoc.com/presentation_image_h/b4f4c80543d5cb7ceeeb736b72c15cf5/image-19.jpg)
- Slides: 19


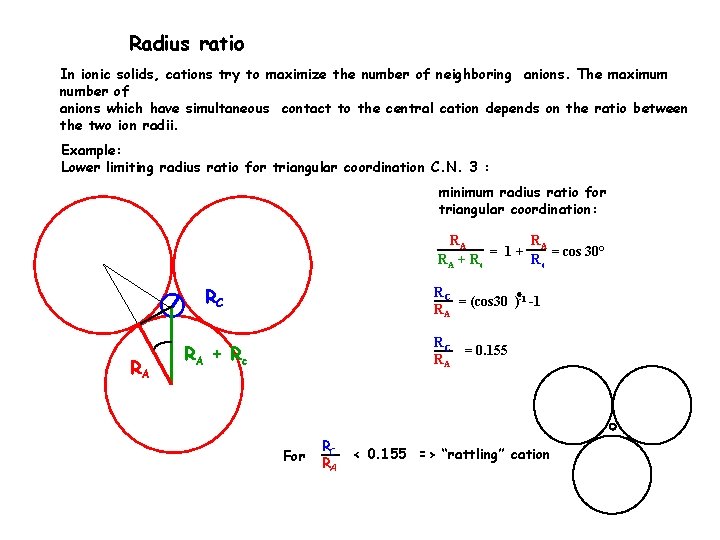
Radius ratio In ionic solids, cations try to maximize the number of neighboring anions. The maximum number of anions which have simultaneous contact to the central cation depends on the ratio between the two ion radii. Example: Lower limiting radius ratio for triangular coordination C. N. 3 : minimum radius ratio for triangular coordination: RA R = 1 + A = cos 30° RA + R c Rc RC RA RC = (cos 30 )-1 -1 RA RC = 0. 155 RA RA + R c For RC RA < 0. 155 => “rattling” cation

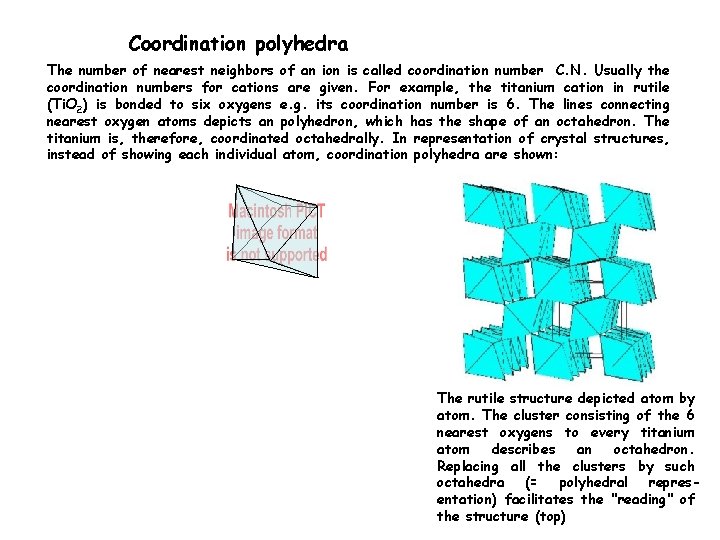
Coordination polyhedra The number of nearest neighbors of an ion is called coordination number C. N. Usually the coordination numbers for cations are given. For example, the titanium cation in rutile (Ti. O 2) is bonded to six oxygens e. g. its coordination number is 6. The lines connecting nearest oxygen atoms depicts an polyhedron, which has the shape of an octahedron. The titanium is, therefore, coordinated octahedrally. In representation of crystal structures, instead of showing each individual atom, coordination polyhedra are shown: The rutile structure depicted atom by atom. The cluster consisting of the 6 nearest oxygens to every titanium atom describes an octahedron. Replacing all the clusters by such octahedra (= polyhedral representation) facilitates the "reading" of the structure (top)

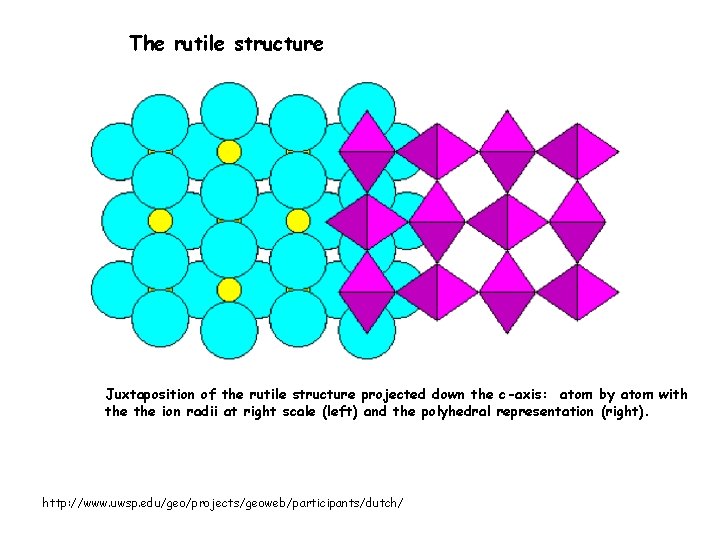
The rutile structure Juxtaposition of the rutile structure projected down the c-axis: atom by atom with the ion radii at right scale (left) and the polyhedral representation (right). http: //www. uwsp. edu/geo/projects/geoweb/participants/dutch/

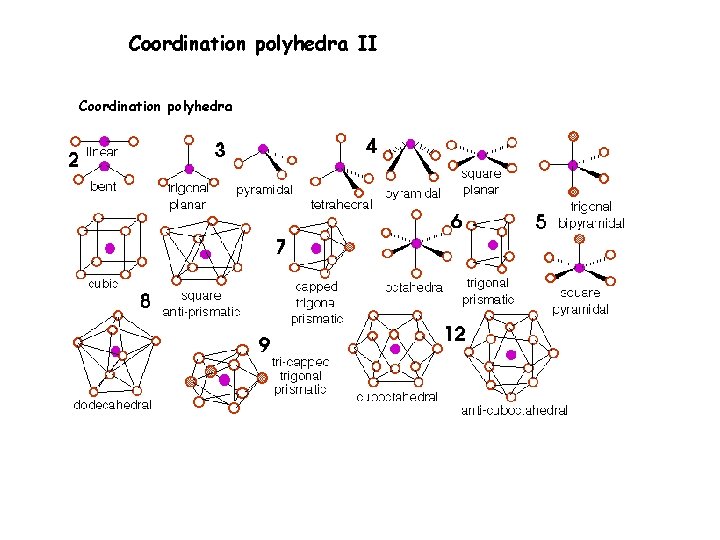
Coordination polyhedra II Coordination polyhedra

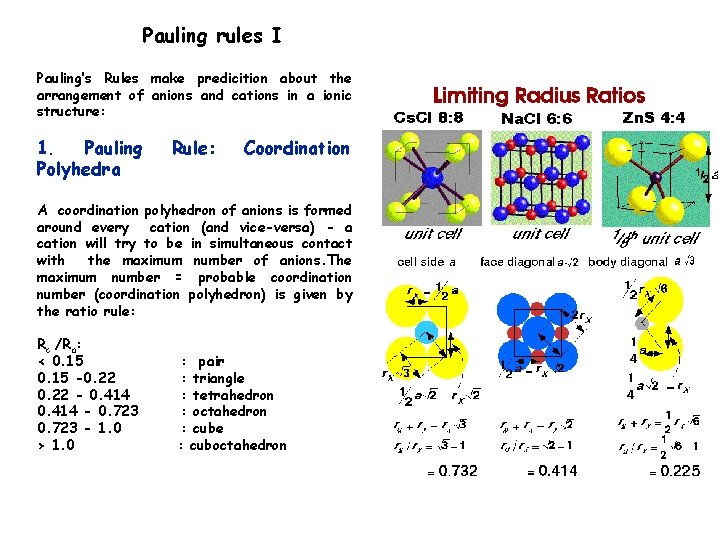
Pauling rules I Pauling’s Rules make predicition about the arrangement of anions and cations in a ionic structure: 1. Pauling Polyhedra Rule: Coordination A coordination polyhedron of anions is formed around every cation (and vice-versa) - a cation will try to be in simultaneous contact with the maximum number of anions. The maximum number = probable coordination number (coordination polyhedron) is given by the ratio rule: Rc /Ra: < 0. 15 -0. 22 - 0. 414 - 0. 723 - 1. 0 > 1. 0 : pair : triangle : tetrahedron : octahedron : cube : cuboctahedron

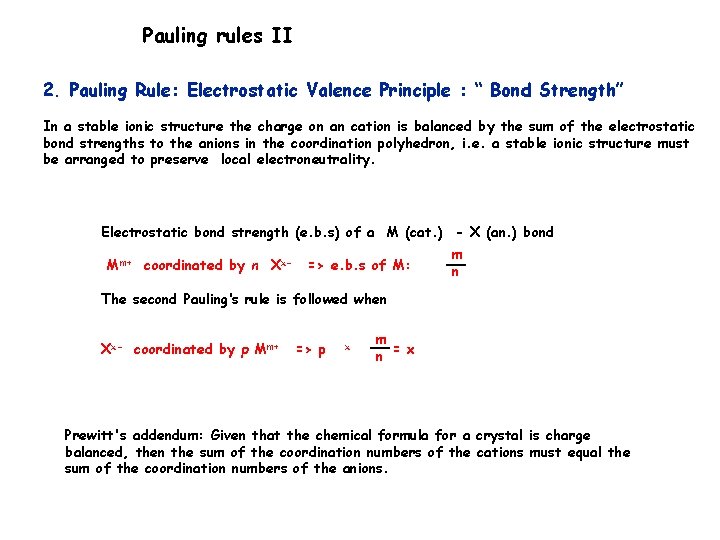
Pauling rules II 2. Pauling Rule: Electrostatic Valence Principle : “ Bond Strength” In a stable ionic structure the charge on an cation is balanced by the sum of the electrostatic bond strengths to the anions in the coordination polyhedron, i. e. a stable ionic structure must be arranged to preserve local electroneutrality. Electrostatic bond strength (e. b. s) of a M (cat. ) - X (an. ) bond Mm+ coordinated by n Xx- => e. b. s of M: m n The second Pauling‘s rule is followed when Xx- coordinated by p Mm+ => p x m n = x Prewitt's addendum: Given that the chemical formula for a crystal is charge balanced, then the sum of the coordination numbers of the cations must equal the sum of the coordination numbers of the anions.

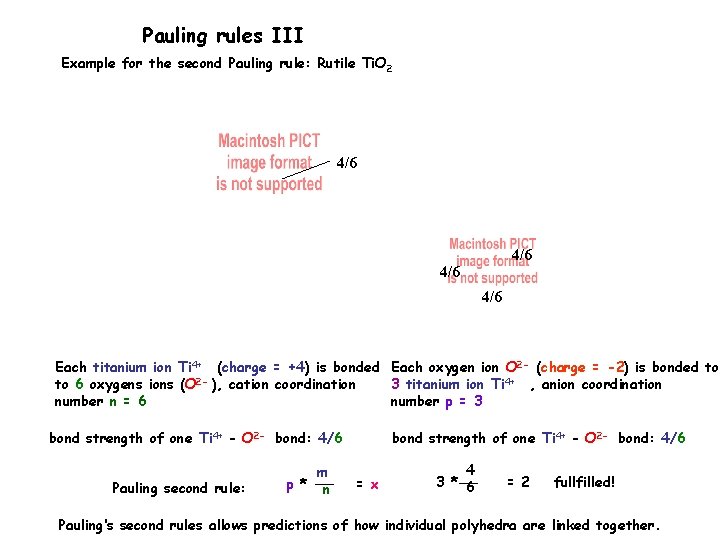
Pauling rules III Example for the second Pauling rule: Rutile Ti. O 2 4/6 4/6 Each titanium ion Ti 4+ (charge = +4) is bonded Each oxygen ion O 2 - (charge = -2) is bonded to to 6 oxygens ions (O 2 - ), cation coordination 3 titanium ion Ti 4+ , anion coordination number n = 6 number p = 3 bond strength of one Ti 4+ - O 2 - bond: 4/6 Pauling second rule: m p * n bond strength of one Ti 4+ - O 2 - bond: 4/6 = x 4 3 * 6 = 2 fullfilled! Pauling‘s second rules allows predictions of how individual polyhedra are linked together.

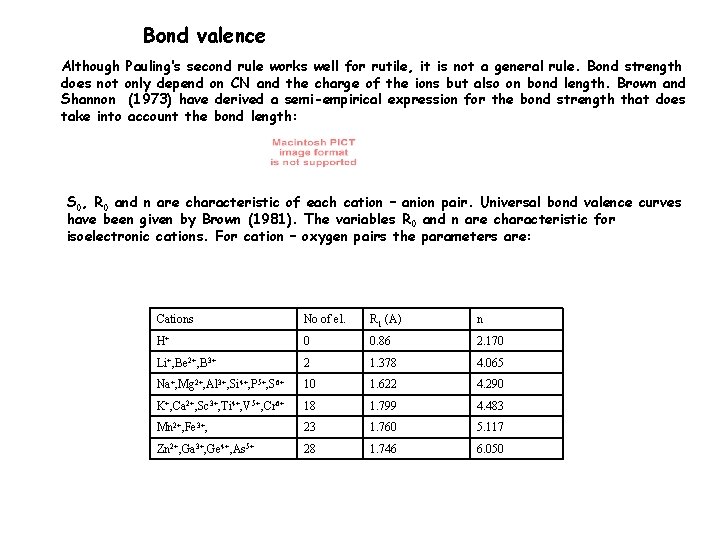
Bond valence Although Pauling‘s second rule works well for rutile, it is not a general rule. Bond strength does not only depend on CN and the charge of the ions but also on bond length. Brown and Shannon (1973) have derived a semi-empirical expression for the bond strength that does take into account the bond length: S 0, R 0 and n are characteristic of each cation – anion pair. Universal bond valence curves have been given by Brown (1981). The variables R 0 and n are characteristic for isoelectronic cations. For cation – oxygen pairs the parameters are: Cations No of el. R 1 (A) n H+ 0 0. 86 2. 170 Li+, Be 2+, B 3+ 2 1. 378 4. 065 Na+, Mg 2+, Al 3+, Si 4+, P 5+, S 6+ 10 1. 622 4. 290 K+, Ca 2+, Sc 3+, Ti 4+, V 5+, Cr 6+ 18 1. 799 4. 483 Mn 2+, Fe 3+, 23 1. 760 5. 117 Zn 2+, Ga 3+, Ge 4+, As 5+ 28 1. 746 6. 050

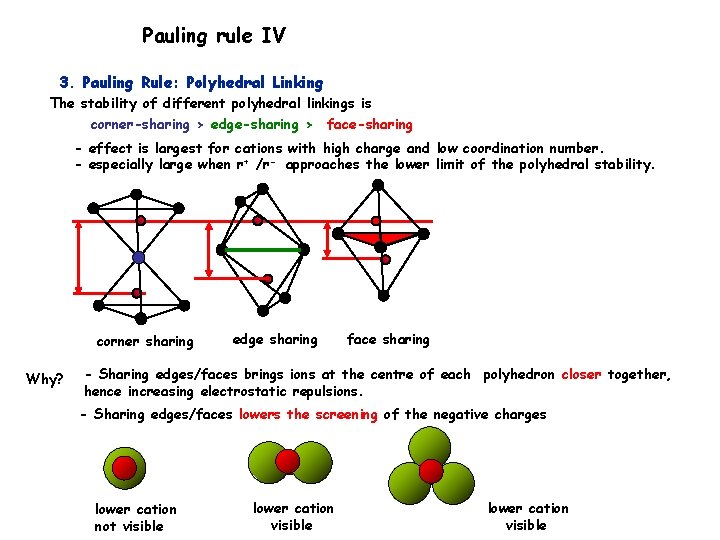
Pauling rule IV 3. Pauling Rule: Polyhedral Linking The stability of different polyhedral linkings is corner-sharing > edge-sharing > face-sharing - effect is largest for cations with high charge and low coordination number. - especially large when r+ /r- approaches the lower limit of the polyhedral stability. corner sharing Why? edge sharing face sharing - Sharing edges/faces brings ions at the centre of each polyhedron closer together, hence increasing electrostatic repulsions. - Sharing edges/faces lowers the screening of the negative charges lower cation not visible lower cation visible

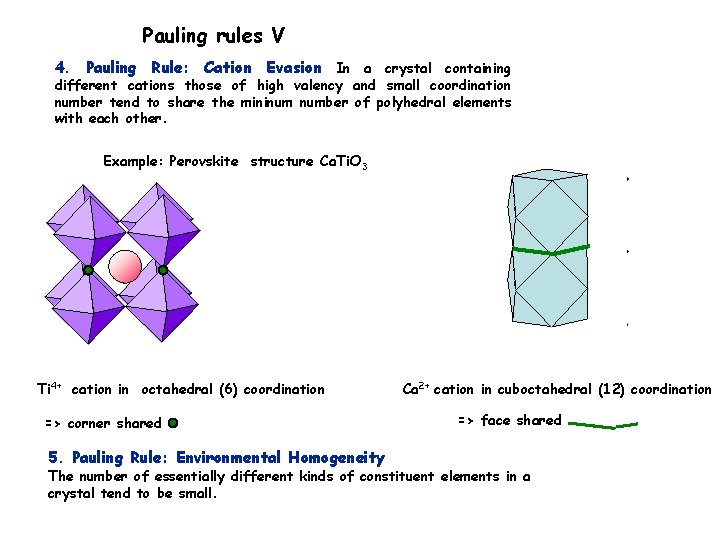
Pauling rules V 4. Pauling Rule: Cation Evasion In a crystal containing different cations those of high valency and small coordination number tend to share the mininum number of polyhedral elements with each other. Example: Perovskite structure Ca. Ti. O 3 Ti 4+ cation in octahedral (6) coordination => corner shared 5. Pauling Rule: Environmental Homogeneity Ca 2+ cation in cuboctahedral (12) coordination => face shared The number of essentially different kinds of constituent elements in a crystal tend to be small.

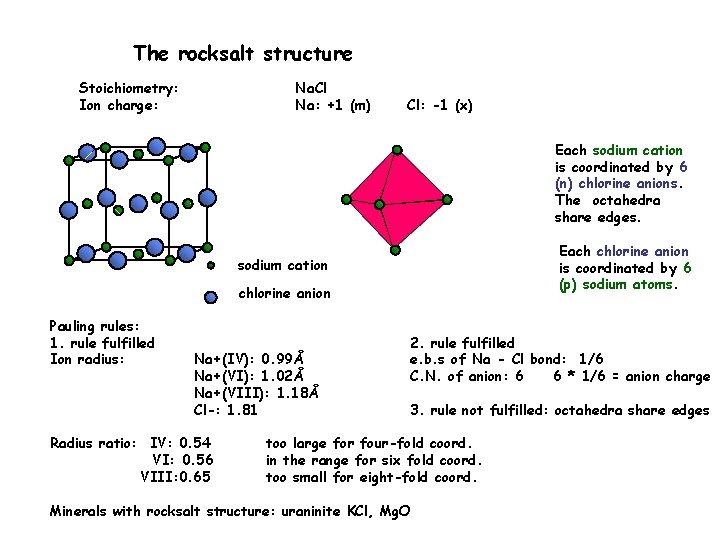
The rocksalt structure Stoichiometry: Ion charge: Na. Cl Na: +1 (m) Cl: -1 (x) Each sodium cation is coordinated by 6 (n) chlorine anions. The octahedra share edges. Each chlorine anion is coordinated by 6 (p) sodium atoms. sodium cation chlorine anion Pauling rules: 1. rule fulfilled Ion radius: Na+(IV): 0. 99Å Na+(VI): 1. 02Å Na+(VIII): 1. 18Å Cl-: 1. 81 Radius ratio: IV: 0. 54 VI: 0. 56 VIII: 0. 65 2. rule fulfilled e. b. s of Na - Cl bond: 1/6 C. N. of anion: 6 6 * 1/6 = anion charge 3. rule not fulfilled: octahedra share edges too large for four-fold coord. in the range for six fold coord. too small for eight-fold coord. Minerals with rocksalt structure: uraninite KCl, Mg. O

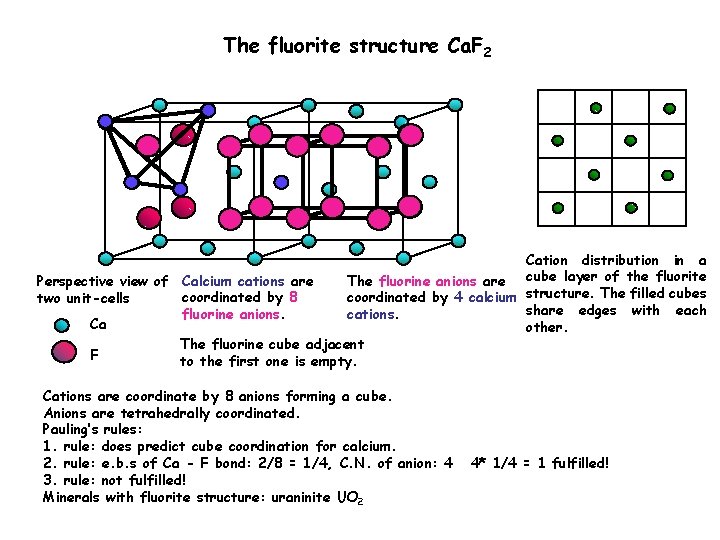
The fluorite structure Ca. F 2 Cation distribution in a The fluorine anions are cube layer of the fluorite Perspective view of Calcium cations are coordinated by 8 coordinated by 4 calcium structure. The filled cubes two unit-cells share edges with each fluorine anions. cations. Ca other. The fluorine cube adjacent F to the first one is empty. Cations are coordinate by 8 anions forming a cube. Anions are tetrahedrally coordinated. Pauling’s rules: 1. rule: does predict cube coordination for calcium. 2. rule: e. b. s of Ca - F bond: 2/8 = 1/4, C. N. of anion: 4 3. rule: not fulfilled! Minerals with fluorite structure: uraninite UO 2 4* 1/4 = 1 fulfilled!

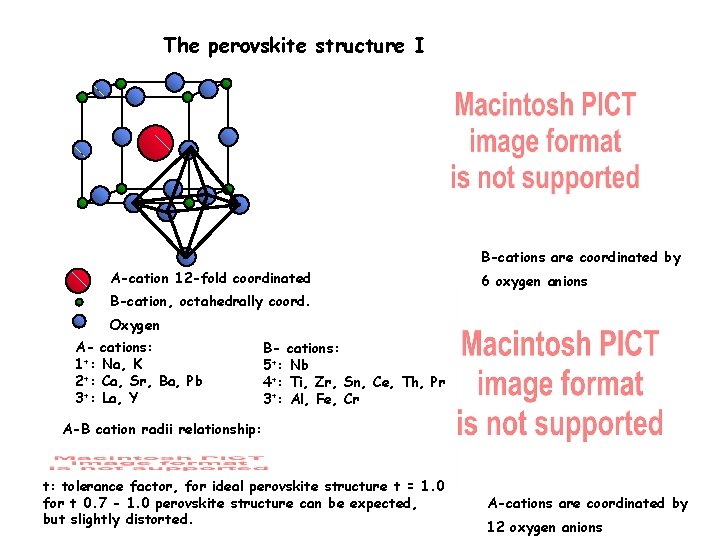
The perovskite structure I B-cations are coordinated by A-cation 12 -fold coordinated 6 oxygen anions B-cation, octahedrally coord. Oxygen A 1+: 2+: 3+: cations: Na, K Ca, Sr, Ba, Pb La, Y B- cations: 5+: Nb 4+: Ti, Zr, Sn, Ce, Th, Pr 3+: Al, Fe, Cr A-B cation radii relationship: t: tolerance factor, for ideal perovskite structure t = 1. 0 for t 0. 7 - 1. 0 perovskite structure can be expected, but slightly distorted. A-cations are coordinated by 12 oxygen anions

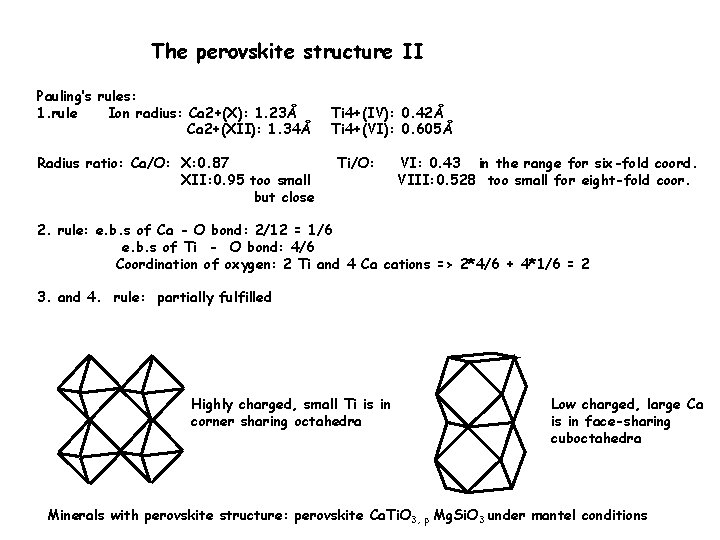
The perovskite structure II Pauling’s rules: 1. rule Ion radius: Ca 2+(X): 1. 23Å Ca 2+(XII): 1. 34Å Radius ratio: Ca/O: X: 0. 87 XII: 0. 95 too small but close Ti 4+(IV): 0. 42Å Ti 4+(VI): 0. 605Å Ti/O: VI: 0. 43 in the range for six-fold coord. VIII: 0. 528 too small for eight-fold coor. 2. rule: e. b. s of Ca - O bond: 2/12 = 1/6 e. b. s of Ti - O bond: 4/6 Coordination of oxygen: 2 Ti and 4 Ca cations => 2*4/6 + 4*1/6 = 2 3. and 4. rule: partially fulfilled Highly charged, small Ti is in corner sharing octahedra Low charged, large Ca is in face-sharing cuboctahedra Minerals with perovskite structure: perovskite Ca. Ti. O 3, p Mg. Si. O 3 under mantel conditions

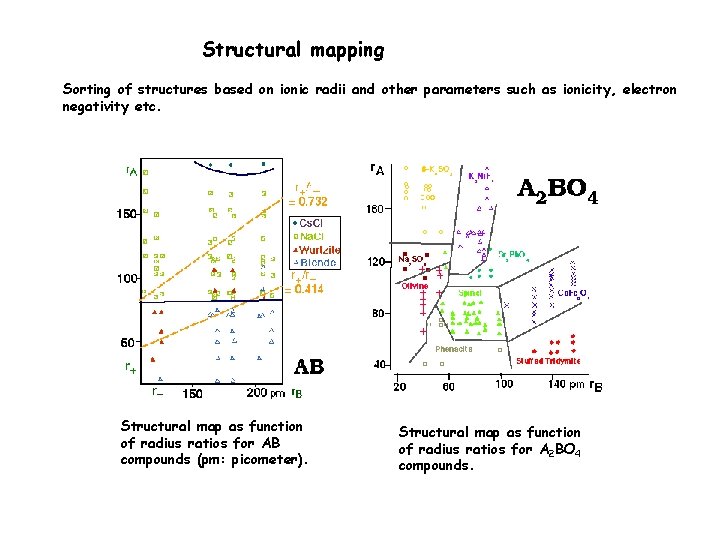
Structural mapping Sorting of structures based on ionic radii and other parameters such as ionicity, electron negativity etc. Structural map as function of radius ratios for AB compounds (pm: picometer). Structural map as function of radius ratios for A 2 BO 4 compounds.

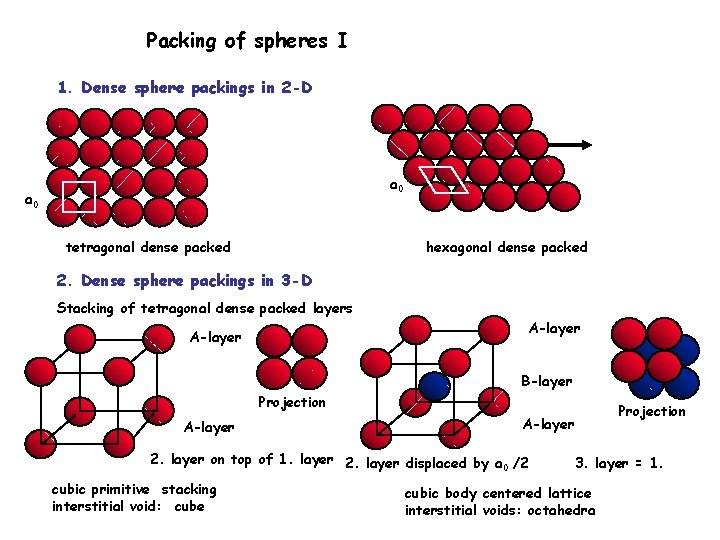
Packing of spheres I 1. Dense sphere packings in 2 -D a 0 tetragonal dense packed hexagonal dense packed 2. Dense sphere packings in 3 -D Stacking of tetragonal dense packed layers A-layer B-layer Projection A-layer 2. layer on top of 1. layer 2. layer displaced by a /2 0 cubic primitive stacking interstitial void: cube Projection A-layer 3. layer = 1. cubic body centered lattice interstitial voids: octahedra

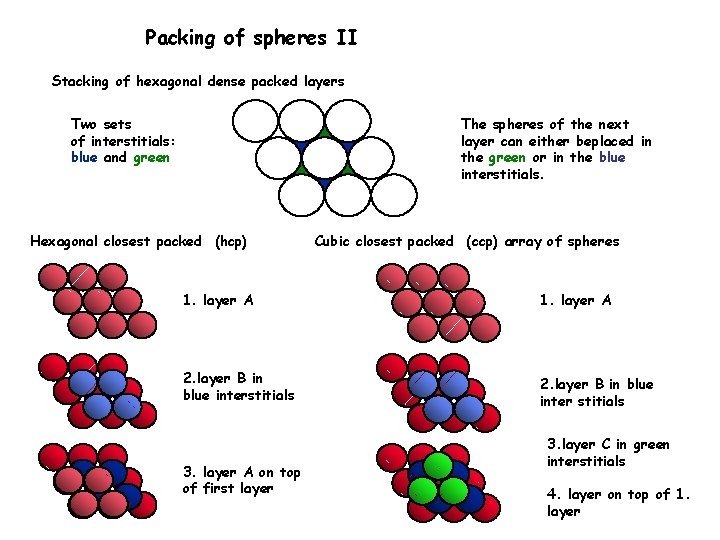
Packing of spheres II Stacking of hexagonal dense packed layers Two sets of interstitials: blue and green The spheres of the next layer can either beplaced in the green or in the blue interstitials. Hexagonal closest packed (hcp) Cubic closest packed (ccp) array of spheres 1. layer A 2. layer B in blue interstitials 2. layer B in blue inter stitials 3. layer A on top of first layer 3. layer C in green interstitials 4. layer on top of 1. layer
![Packing of spheres III Shape of interstitial voids Blayer Clayer Alayer tetrahedron 111 direction Packing of spheres III Shape of interstitial voids B-layer C-layer A-layer tetrahedron [111] direction](https://slidetodoc.com/presentation_image_h/b4f4c80543d5cb7ceeeb736b72c15cf5/image-19.jpg)
Packing of spheres III Shape of interstitial voids B-layer C-layer A-layer tetrahedron [111] direction is perpendicular to closed packed chlorine layers The rocksalt structure: Chlorine forms a ccp array and sodium (black circles) fills all octahedral voids octahedron