Qualitative Chemical Analysis Tro Chapter 17 Aqueous Ionic






- Slides: 9

Qualitative Chemical Analysis Tro Chapter 17 – Aqueous Ionic Equilibrium Section 17. 7 – Qualitative Chemical Analysis

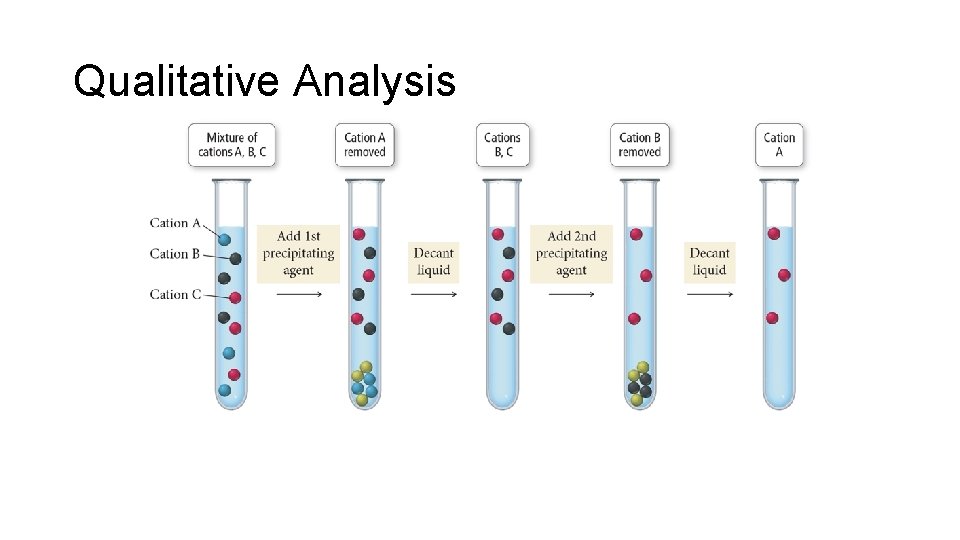
Qualitative Analysis • An analytical scheme that utilizes selective precipitation to identify the ions present in a solution is called qualitative analysis. • Wet chemistry • A sample containing several ions is subjected to the addition of several precipitating agents. • Addition of each reagent causes one of the ions present to precipitate out.

Qualitative Analysis

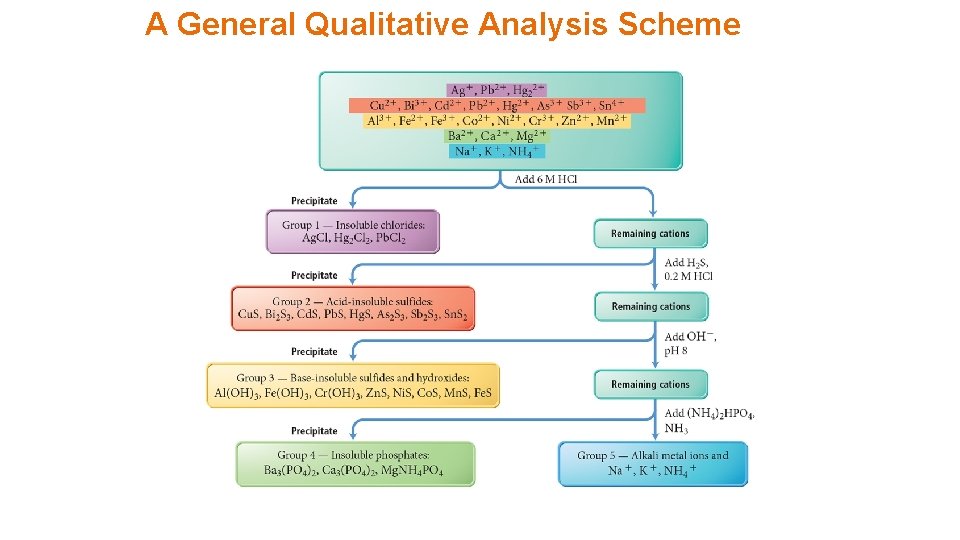
A General Qualitative Analysis Scheme

Group 1 • Group 1 cations are Ag+, Pb 2+, and Hg 22+. • All of these cations form compounds with Cl− that are insoluble in water. • As long as the concentration is large enough • Precipitated by the addition of HCl Pb. Cl 2

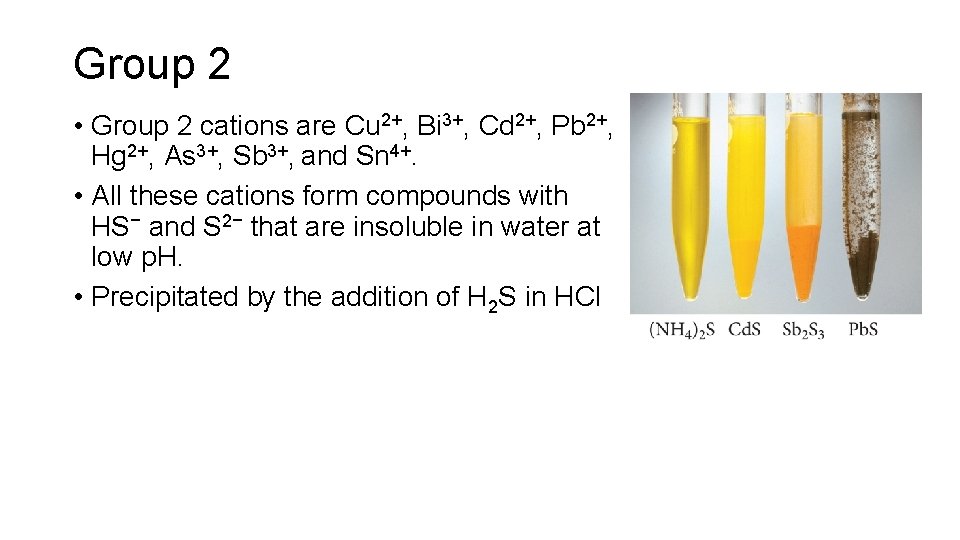
Group 2 • Group 2 cations are Cu 2+, Bi 3+, Cd 2+, Pb 2+, Hg 2+, As 3+, Sb 3+, and Sn 4+. • All these cations form compounds with HS− and S 2− that are insoluble in water at low p. H. • Precipitated by the addition of H 2 S in HCl

Group 3 • Group 3 cations are Fe 2+, Co 2+, Zn 2+, Mn 2+, and Ni 2+ precipitated as sulfides, as well as Cr 3+, Fe 3+, and Al 3+ precipitated as hydroxides. • All of these cations form compounds with S 2− that are insoluble in water at high p. H. • Precipitated by the addition of H 2 S in Na. OH. Fe(OH)2 Ni(OH)2 Mn(OH)2

Group 4 • Group 4 cations are Mg 2+, Ca 2+, and Ba 2+. • These cations form compounds with PO 43− that are insoluble in water at high p. H. • Precipitated by the addition of (NH 4)2 HPO 4 Calcium Phosphate Chromium Phosphate Magnesium Phosphate Cobalt Phosphate Used as a pigment for paint.

Group 5 • Group 5 cations are Na+, K+, and NH 4+. • All of these cations form compounds that are soluble in water; they do not precipitate. • They are identified by the color of their flame.