Chapter 15 Water and Aqueous Systems 15 1






























































































- Slides: 133

Chapter 15 Water and Aqueous Systems 15. 1 Water and Its Properties 15. 2 Homogeneous Aqueous Systems 15. 3 Heterogeneous Aqueous Systems Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU What properties of water make it essential to life on Earth? Water covers about three quarters of Earth’s surface. All known life forms are made mostly of water. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State What factor causes the high surface tension, low vapor pressure, and high boiling point of water? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

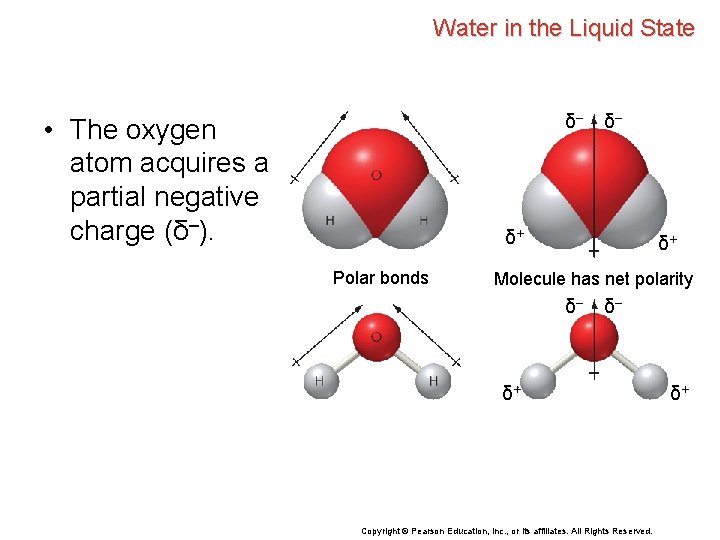
Water in the Liquid State Water, H 2 O, is a simple molecule consisting of three atoms. • The oxygen atom forms a covalent bond with each of the hydrogen atoms. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Water, H 2 O, is a simple molecule consisting of three atoms. • The oxygen atom forms a covalent bond with each of the hydrogen atoms. • Oxygen has a greater electronegativity than hydrogen, so the oxygen atom attracts the electron pair of the covalent O—H bond to a greater extent than the hydrogen atom. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Water, H 2 O, is a simple molecule consisting of three atoms. • The oxygen atom forms a covalent bond with each of the hydrogen atoms. • Oxygen has a greater electronegativity than hydrogen, so the oxygen atom attracts the electron pair of the covalent O—H bond to a greater extent than the hydrogen atom. • Thus, the O—H bond is highly polar. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

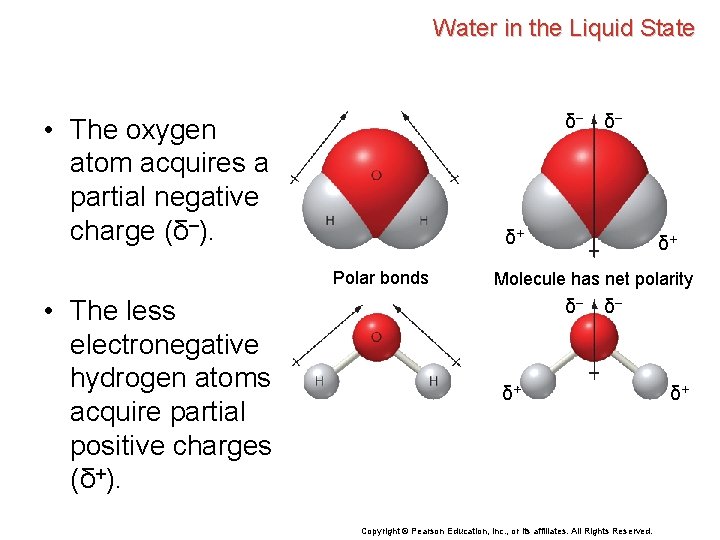
Water in the Liquid State δ– • The oxygen atom acquires a partial negative charge (δ–). δ– δ+ Polar bonds δ+ Molecule has net polarity δ– δ– δ+ Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. δ+

Water in the Liquid State δ– • The oxygen atom acquires a partial negative charge (δ–). δ+ Polar bonds • The less electronegative hydrogen atoms acquire partial positive charges (δ+). δ– δ+ Molecule has net polarity δ– δ– δ+ Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. δ+

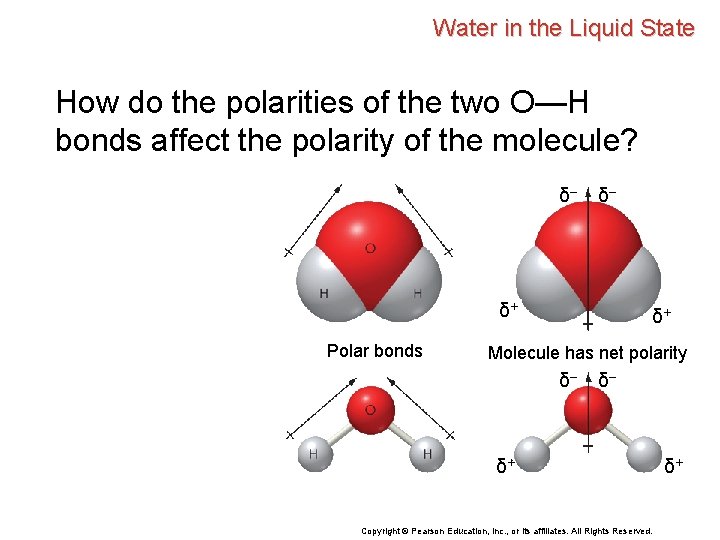
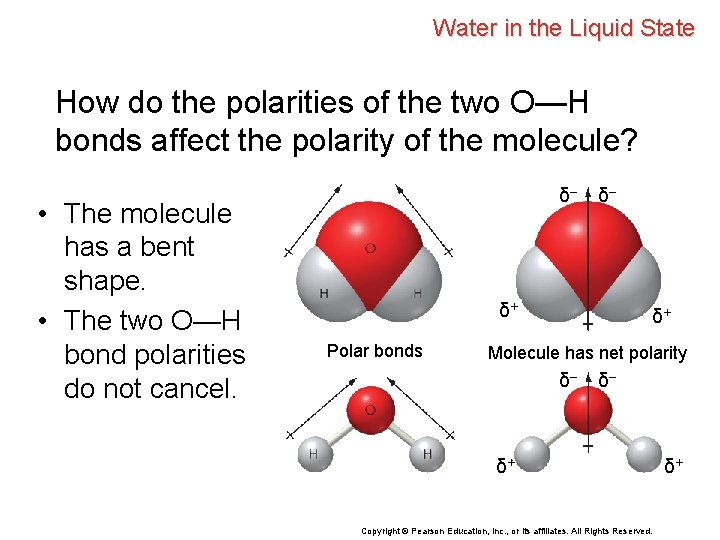
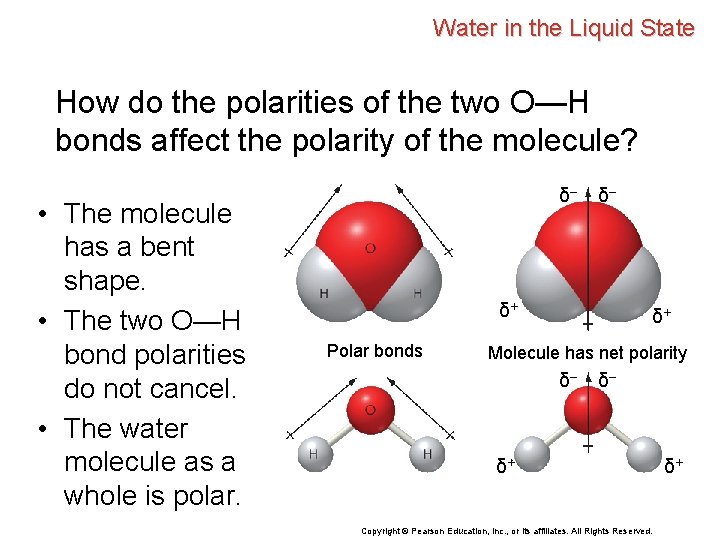
Water in the Liquid State How do the polarities of the two O—H bonds affect the polarity of the molecule? δ– δ– δ+ Polar bonds δ+ Molecule has net polarity δ– δ– δ+ Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. δ+

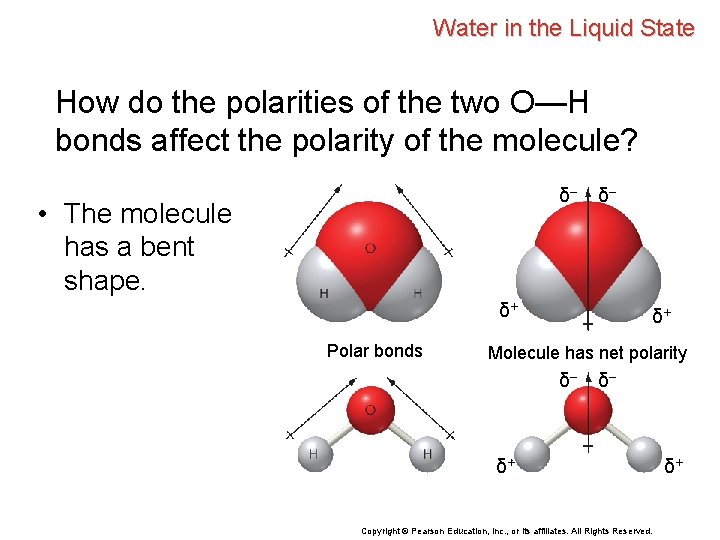
Water in the Liquid State How do the polarities of the two O—H bonds affect the polarity of the molecule? δ– • The molecule has a bent shape. δ– δ+ Polar bonds δ+ Molecule has net polarity δ– δ– δ+ Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. δ+

Water in the Liquid State How do the polarities of the two O—H bonds affect the polarity of the molecule? • The molecule has a bent shape. • The two O—H bond polarities do not cancel. δ– δ– δ+ Polar bonds δ+ Molecule has net polarity δ– δ– δ+ Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. δ+

Water in the Liquid State How do the polarities of the two O—H bonds affect the polarity of the molecule? • The molecule has a bent shape. • The two O—H bond polarities do not cancel. • The water molecule as a whole is polar. δ– δ– δ+ Polar bonds δ+ Molecule has net polarity δ– δ– δ+ Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved. δ+

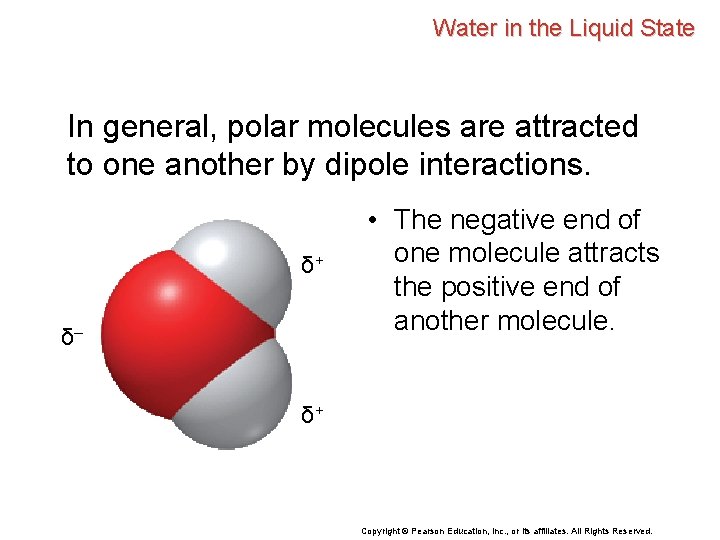
Water in the Liquid State In general, polar molecules are attracted to one another by dipole interactions. δ+ δ– • The negative end of one molecule attracts the positive end of another molecule. δ+ Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

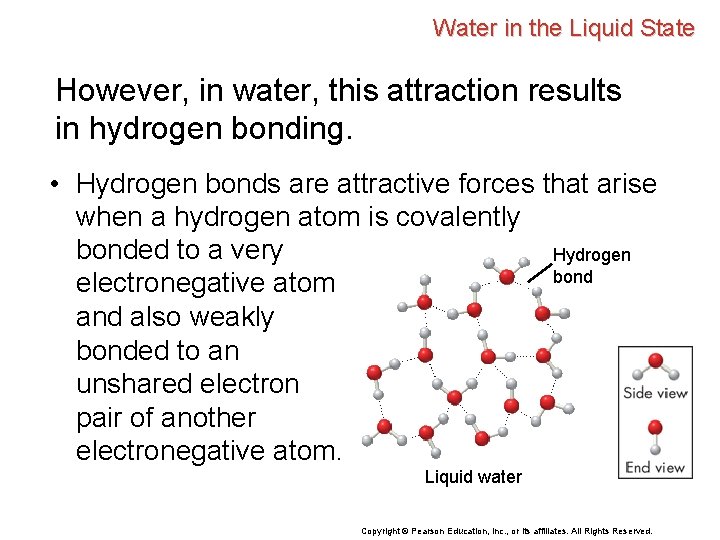
Water in the Liquid State However, in water, this attraction results in hydrogen bonding. • Hydrogen bonds are attractive forces that arise when a hydrogen atom is covalently bonded to a very Hydrogen bond electronegative atom and also weakly bonded to an unshared electron pair of another electronegative atom. Liquid water Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Many unique and important properties of water—including its high surface tension, low vapor pressure, and high boiling point—result from hydrogen bonding. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Surface Tension Have you ever noticed that water forms nearly spherical droplets on a leaf? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

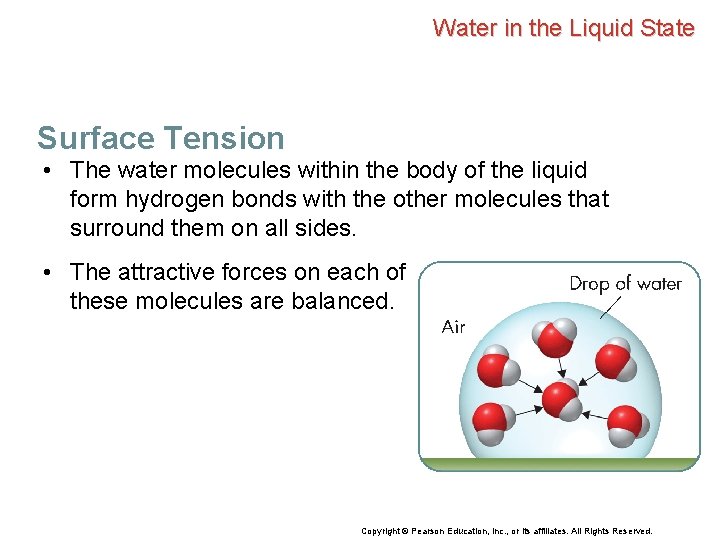
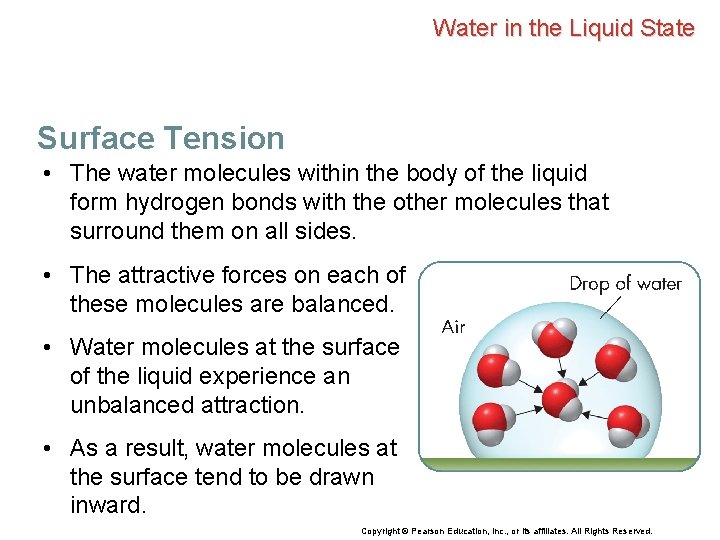
Water in the Liquid State Surface Tension • The water molecules within the body of the liquid form hydrogen bonds with the other molecules that surround them on all sides. • The attractive forces on each of these molecules are balanced. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Surface Tension • The water molecules within the body of the liquid form hydrogen bonds with the other molecules that surround them on all sides. • The attractive forces on each of these molecules are balanced. • Water molecules at the surface of the liquid experience an unbalanced attraction. • As a result, water molecules at the surface tend to be drawn inward. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Surface Tension The inward force, or pull, that tends to minimize the surface area of a liquid is called surface tension. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Surface Tension The inward force, or pull, that tends to minimize the surface area of a liquid is called surface tension. • All liquids have a surface tension, but water’s surface tension is higher than most. • The surface tension of water tends to hold a drop of liquid in a spherical shape. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Surface Tension It is possible to decrease the surface tension of water by adding a surfactant. • A surfactant is any substance that interferes with the hydrogen bonding between water molecules and thereby reduces surface tension. • Soaps and detergents are surfactants. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Vapor Pressure Hydrogen bonding between water molecules also explains water’s unusually low vapor pressure. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Vapor Pressure Hydrogen bonding between water molecules also explains water’s unusually low vapor pressure. • An extensive network of hydrogen bonds holds the molecules in liquid water to one another. • These hydrogen bonds must be broken before water changes from the liquid to the vapor state, so the tendency of these molecules to escape is low and evaporation is slow. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Boiling Point Molecular compounds of low molecular mass are usually gases or liquids with low boiling points at normal atmospheric pressure. • Ammonia (NH 3) has a molar mass of 17. 0 g/mol and boils at about – 33˚C. • Water has a molar mass of 18. 0 g/mol, but it has a boiling point of 100˚C. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Liquid State Boiling Point The difference between the boiling points of ammonia and water is due to hydrogen bonding, which is more extensive in water than in ammonia. • It takes much more heat to disrupt the attractions between water molecules than those between ammonia molecules. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Some insects are able to walk across water. How do the properties of water explain their ability? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Some insects are able to walk across water. How do the properties of water explain their ability? The surface tension of water is relatively high. As long as the forces holding the surface water molecules together are stronger than the forces exerted down on the water by the insect, the insect will not sink. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Solid State How can you describe the structure of ice? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Solid State Ice cubes float in your glass of iced tea because solid water has a lower density than liquid water. • This situation is not usual for liquids. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

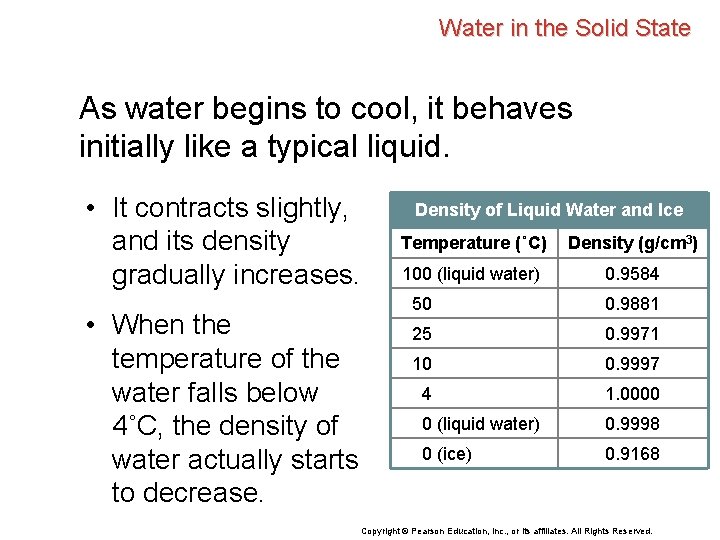
Water in the Solid State As water begins to cool, it behaves initially like a typical liquid. • It contracts slightly, and its density gradually increases. • When the temperature of the water falls below 4˚C, the density of water actually starts to decrease. Density of Liquid Water and Ice Temperature (˚C) Density (g/cm 3) 100 (liquid water) 0. 9584 50 0. 9881 25 0. 9971 10 0. 9997 4 1. 0000 0 (liquid water) 0. 9998 0 (ice) 0. 9168 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

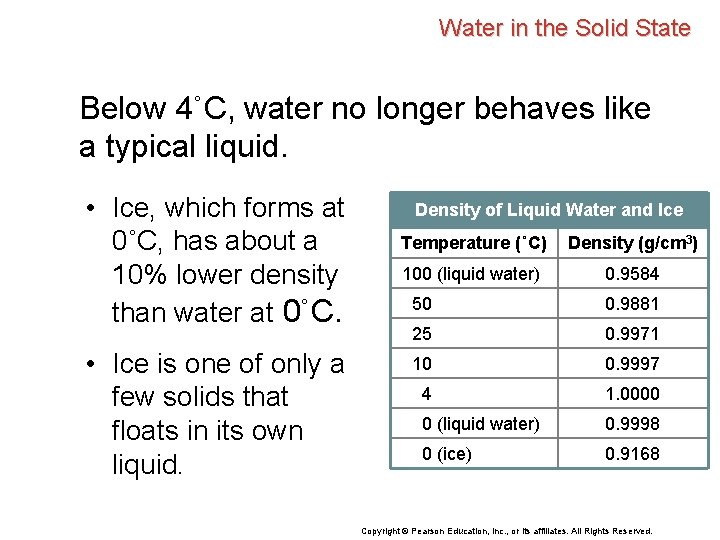
Water in the Solid State Below 4˚C, water no longer behaves like a typical liquid. • Ice, which forms at 0˚C, has about a 10% lower density than water at 0˚C. • Ice is one of only a few solids that floats in its own liquid. Density of Liquid Water and Ice Temperature (˚C) Density (g/cm 3) 100 (liquid water) 0. 9584 50 0. 9881 25 0. 9971 10 0. 9997 4 1. 0000 0 (liquid water) 0. 9998 0 (ice) 0. 9168 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

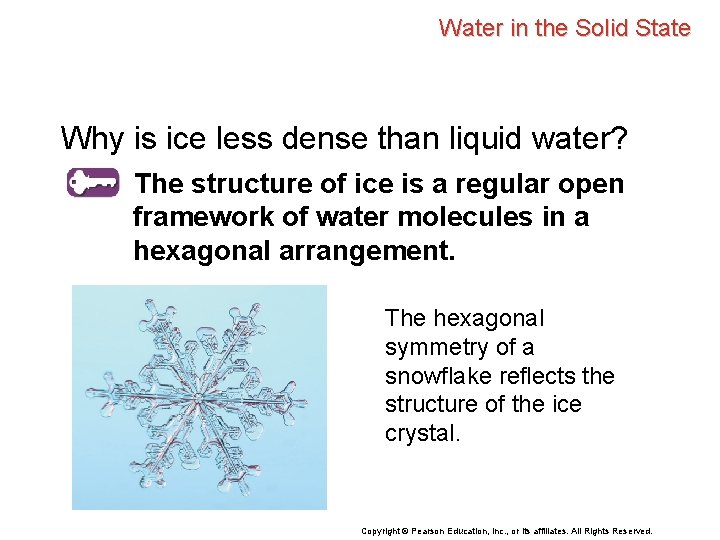
Water in the Solid State Why is ice less dense than liquid water? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Solid State Why is ice less dense than liquid water? The structure of ice is a regular open framework of water molecules in a hexagonal arrangement. The hexagonal symmetry of a snowflake reflects the structure of the ice crystal. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

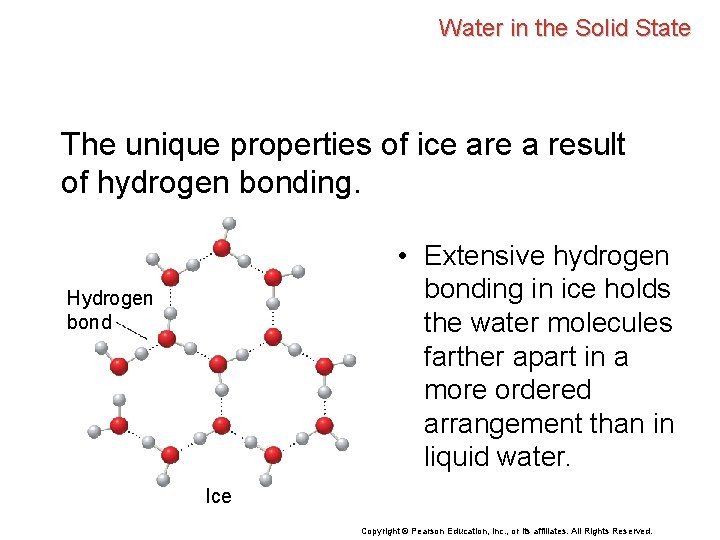
Water in the Solid State The unique properties of ice are a result of hydrogen bonding. • Extensive hydrogen bonding in ice holds the water molecules farther apart in a more ordered arrangement than in liquid water. Hydrogen bond Ice Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Water in the Solid State The fact that ice floats has important consequences for all organisms. • The liquid water at the bottom of an otherwise frozen body of water is warmer than 0˚C, so fish and other aquatic life are better able to survive. • If ice were denser than liquid water, bodies of water would tend to freeze solid during the winter months, destroying many types of organisms. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU What properties of water that result from hydrogen bonding make it essential to life on Earth? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU What properties of water that result from hydrogen bonding make it essential to life on Earth? • The low vapor pressure of water keeps the liquid water in all of Earth’s lakes and oceans from evaporating rapidly. • If water did not have such a high boiling point, it would be a vapor at the usual temperatures found on Earth. • The fact that ice floats allows fish and other aquatic life to survive the winter months. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

In ice, how many hydrogen bonds can be formed between one hydrogen atom of a water molecule and the oxygen in surrounding water molecules? A. 0 B. 1 C. 2 D. 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

In ice, how many hydrogen bonds can be formed between one hydrogen atom of a water molecule and the oxygen in surrounding water molecules? A. 0 B. 1 C. 2 D. 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Key Concepts Many unique and important properties of water—including its high surface tension, low vapor pressure, and high boiling point—result from hydrogen bonding. The structure of ice is a regular open framework of water molecules in a hexagonal arrangement. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • surface tension: an inward force that tends to minimize the surface area of a liquid; it causes the surface to behave as if it were a thin skin • surfactant: any substance that interferes with the hydrogen bonding between water molecules and thereby reduces surface tension; soaps and detergents are surfactants Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

BIG IDEA Bonding and Interactions • Water molecules are held together through hydrogen bonds. • The hydrogen bonding interactions between water molecules account for the unique properties of water, including its high surface tension, low vapor pressure, and high boiling point. • Hydrogen bonding also accounts for the fact that ice is less dense than liquid water. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Chapter 15 Water and Aqueous Systems 15. 1 Water and Its Properties 15. 2 Homogeneous Aqueous Systems 15. 3 Heterogeneous Aqueous Systems Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU How can you make a pickle glow? Although it sounds absurd, an ordinary dill pickle from the deli can be a source of light when connected to an electric current! Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Solutions What types of substances dissolve most readily in water? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Solutions What types of substances dissolve most readily in water? • An aqueous solution is water that contains dissolved substances. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Solutions Solvents and Solutes • In a solution, the dissolving medium is the solvent. • The dissolved particles in a solution are the solute. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Solutions Solvents and Solutes • A solvent dissolves the solute. • The solute becomes dispersed in the solvent. • Solvents and solutes may be gases, liquids, or solids. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Solutions Solvents and Solutes • Solutions are homogeneous mixtures. • Solute particles can be atoms, ions, or molecules. • If you filter a solution through filter paper, both the solute and solvent pass through the filter. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Solutions Solvents and Solutes Substances that dissolve most readily in water include ionic compounds and polar covalent compounds. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Solutions Solvents and Solutes Substances that dissolve most readily in water include ionic compounds and polar covalent compounds. • Nonpolar covalent compounds, such as methane, and compounds found in oil, grease, and gasoline, do not dissolve in water. • However, oil and grease will dissolve in gasoline. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

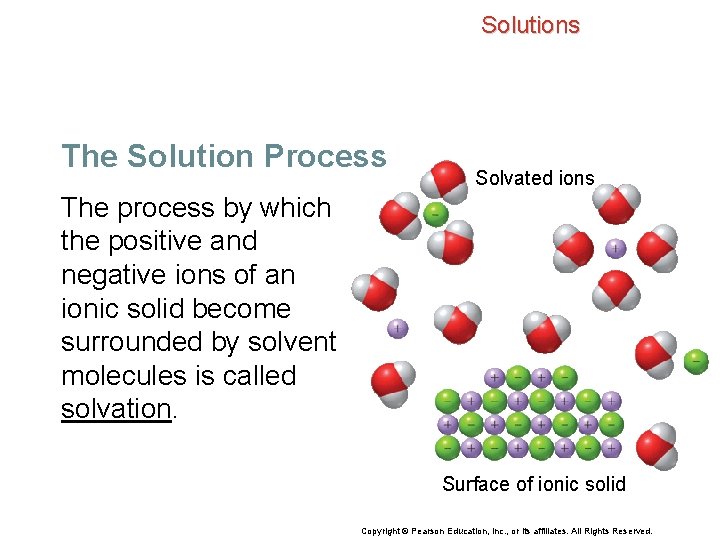
Solutions The Solution Process • A water molecule is polar, with a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. • As individual solute ions break away from the crystal, the negatively and positively charged ions become surrounded by solvent molecules and the ionic crystal dissolves. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Solutions The Solution Process Solvated ions The process by which the positive and negative ions of an ionic solid become surrounded by solvent molecules is called solvation. Surface of ionic solid Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Solutions The Solution Process • Polar solvents such as water dissolve ionic compounds and polar compounds. • Nonpolar solvents such as gasoline dissolve nonpolar compounds. • This relationship can be summed up in the expression “like dissolves like. ” Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Which of these compounds should not dissolve in water? A. HCl B. C 4 H 10 C. KI D. NH 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Which of these compounds should not dissolve in water? A. HCl B. C 4 H 10 C. KI D. NH 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Electrolytes and Nonelectrolytes Why are all ionic compounds electrolytes? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Electrolytes and Nonelectrolytes Why are all ionic compounds electrolytes? • An electrolyte is a compound that conducts an electric current when it is in an aqueous solution or in the molten state. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Electrolytes and Nonelectrolytes All ionic compounds are electrolytes because they dissociate into ions. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

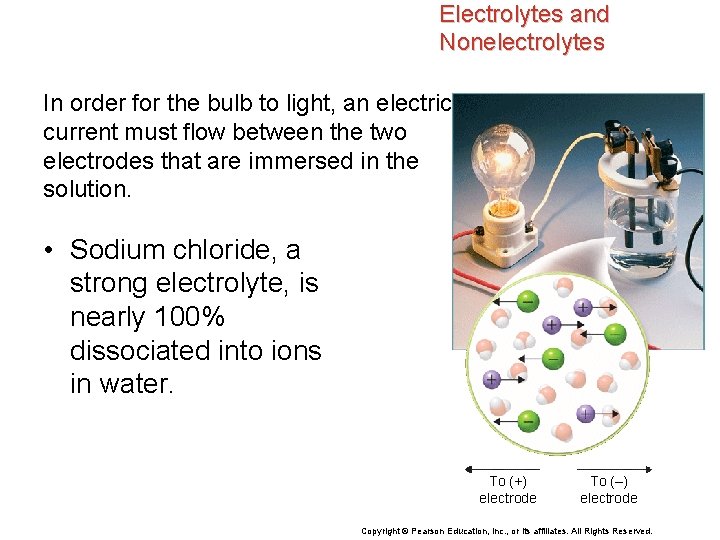
Electrolytes and Nonelectrolytes In order for the bulb to light, an electric current must flow between the two electrodes that are immersed in the solution. • Sodium chloride, a strong electrolyte, is nearly 100% dissociated into ions in water. To (+) electrode To (–) electrode Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

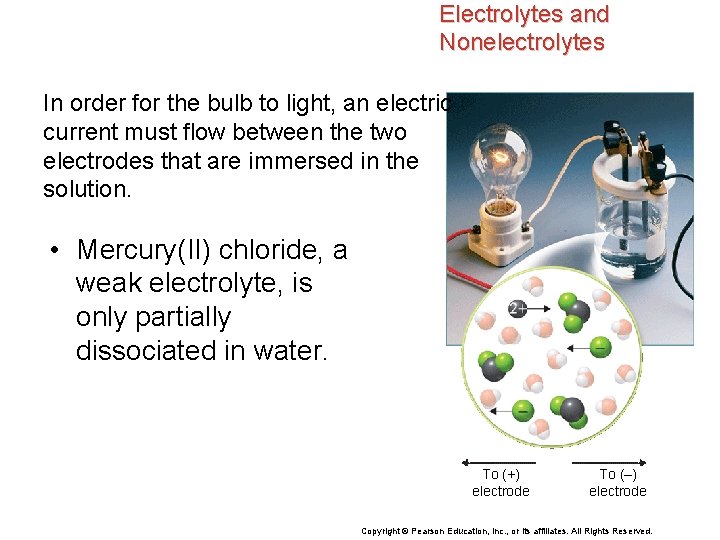
Electrolytes and Nonelectrolytes In order for the bulb to light, an electric current must flow between the two electrodes that are immersed in the solution. • Mercury(II) chloride, a weak electrolyte, is only partially dissociated in water. To (+) electrode To (–) electrode Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

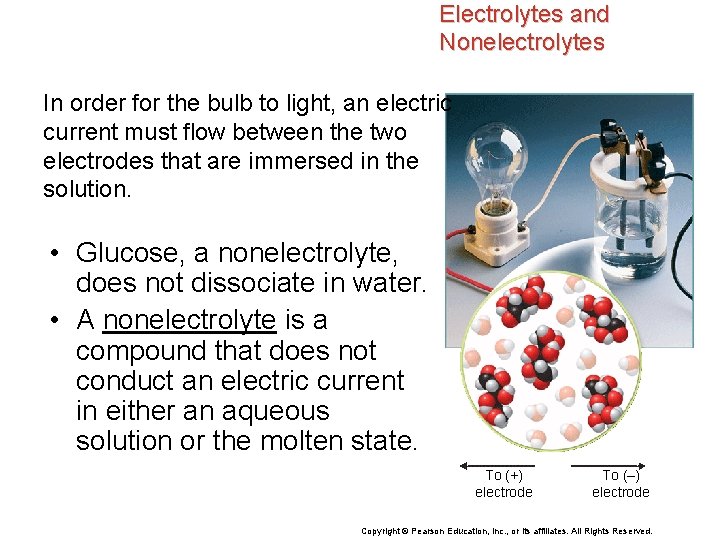
Electrolytes and Nonelectrolytes In order for the bulb to light, an electric current must flow between the two electrodes that are immersed in the solution. • Glucose, a nonelectrolyte, does not dissociate in water. • A nonelectrolyte is a compound that does not conduct an electric current in either an aqueous solution or the molten state. To (+) electrode To (–) electrode Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Electrolytes and Nonelectrolytes Some polar molecular compounds are nonelectrolytes in the pure state but become electrolytes when they dissolve in water. • This change occurs because such compounds ionize in solution. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Electrolytes and Nonelectrolytes Some polar molecular compounds are nonelectrolytes in the pure state but become electrolytes when they dissolve in water. • For example, ammonia (NH 3(g)) is not an electrolyte in the pure state. • Yet an aqueous solution of ammonia conducts an electric current because ammonium ions (NH 4+) and hydroxide ions (OH–) form when ammonia dissolves in water. NH 3(g) + H 2 O(l) NH 4+(aq) + OH–(aq) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Electrolytes and Nonelectrolytes Not all electrolytes conduct electric current to the same degree. • In a solution that contains a strong electrolyte, all or nearly all of the solute exists as ions. • A weak electrolyte conducts an electric current poorly because only a fraction of the solute in the solution exists as ions. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Electrolytes and Nonelectrolytes Your cells use electrolytes, such as sodium and potassium ions, to carry electrical impulses across themselves and to other cells. • An electrolyte imbalance can occur if you become dehydrated. • When you exercise, you can lose water and electrolytes from your body through perspiration. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU Pickles contain table salt. Why can electric current flow through a pickle, causing it to glow? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU Pickles contain table salt. Why can electric current flow through a pickle, causing it to glow? Electrolytes conduct an electric current when they are in an aqueous solution. Table salt, or Na. Cl, is a strong electrolyte. The water and salt in the pickle form a solution that conducts an electric current. The electric current causes the pickle to glow. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Explain why you must be extremely careful when using electricity near a swimming pool. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Explain why you must be extremely careful when using electricity near a swimming pool. The chlorinated water in a swimming pool is a solution that can conduct an electric current. If a current is introduced into the water, any swimmers could be shocked. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates Why do hydrates easily lose and regain water? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates The water contained in a crystal is called the water of hydration or water of crystallization. • A compound that contains water of hydration is called a hydrate. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates The forces holding the water molecules in hydrates are not very strong, so the water is easily lost and regained. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates The forces holding the water molecules in hydrates are not very strong, so the water is easily lost and regained. • A substance that is anhydrous does not contain water. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

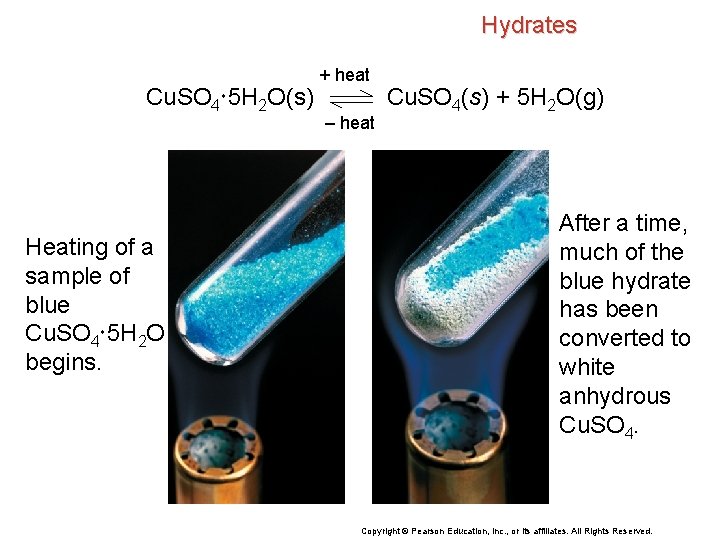
Hydrates Cu. SO 4 5 H 2 O(s) Heating of a sample of blue Cu. SO 4 5 H 2 O begins. + heat – heat Cu. SO 4(s) + 5 H 2 O(g) After a time, much of the blue hydrate has been converted to white anhydrous Cu. SO 4. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates • A piece of filter paper that has been dipped in an aqueous solution of cobalt(II) chloride and then dried is blue in color (anhydrous Co. Cl 2). • When the paper is exposed to moist air, it turns pink because of the formation of the hydrate cobalt(II) chloride hexahydrate (Co. Cl 2 6 H 2 O). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates Each hydrate contains a fixed quantity of water and has a definite composition. Some Common Hydrates Formula Chemical name Common name Mg. SO 4 7 H 2 O Magnesium sulfate heptahydrate Epsom salt Ba(OH)2 8 H 2 O Barium hydroxide octahydrate Ca. Cl 2 2 H 2 O Calcium chloride dihydrate Cu. SO 4 5 H 2 O Copper(II) sulfate pentahydrate Blue vitriol Na 2 SO 4 10 H 2 O Sodium sulfate decahydrate Glauber’s salt KAl(SO 4)2 12 H 2 O Potassium aluminum sulfate dodecahydrate Alum Na 2 B 4 O 7 10 H 2 O Sodium tetraborate decahydrate Borax Fe. SO 4 7 H 2 O Iron(II) sulfate heptahydrate Green vitriol H 2 SO 4 H 2 O Sulfuric acid hydrate (mp 8. 6 C) o Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

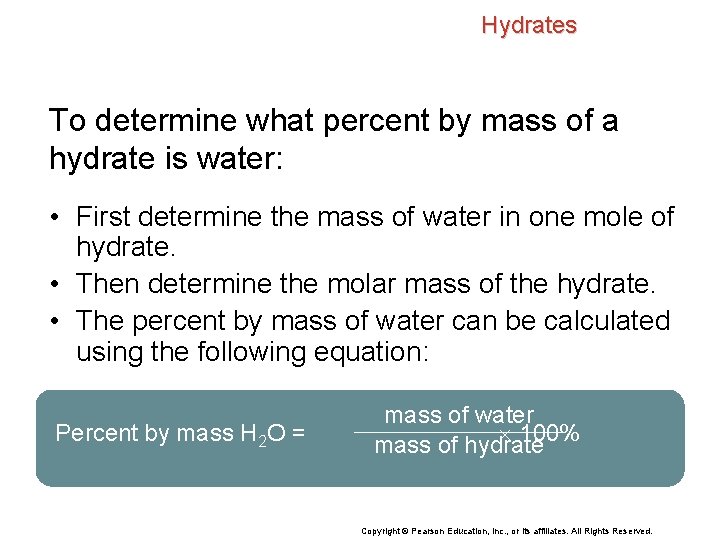
Hydrates To determine what percent by mass of a hydrate is water: • First determine the mass of water in one mole of hydrate. • Then determine the molar mass of the hydrate. • The percent by mass of water can be calculated using the following equation: Percent by mass H 2 O = mass of water 100% mass of hydrate Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates Efflorescent Hydrates The water molecules in hydrates are held by weak forces, so hydrates often have an appreciable vapor pressure. • If a hydrate has a vapor pressure higher than the pressure of water vapor in the air, the hydrate will lose its water of hydration, or effloresce. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates Hygroscopic Hydrates Hydrated ionic compounds that have low vapor pressure remove water from moist air to form higher hydrates. • These hydrates and other compounds that remove moisture from air are called hygroscopic. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates Hygroscopic Hydrates Calcium chloride monohydrate spontaneously absorbs a second molecule of water when exposed to moist air. • Calcium chloride is used as a desiccant in the laboratory. • A desiccant is a substance used to absorb moisture from the air and create a dry atmosphere. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 15. 1 Finding the Percent by Mass of Water in a Hydrate Calculate the percent by mass of water in washing soda, sodium carbonate decahydrate (Na 2 CO 3 10 H 2 O). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Sample Problem 15. 1 1 Analyze List the known and the unknown. To determine the percent by mass, divide the mass of water in one mole of the hydrate by the molar mass of the hydrate and multiply by 100%. KNOWN formula of hydrate = Na 2 CO 3 10 H 2 O UNKNOWN percent H 2 O = ? % Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

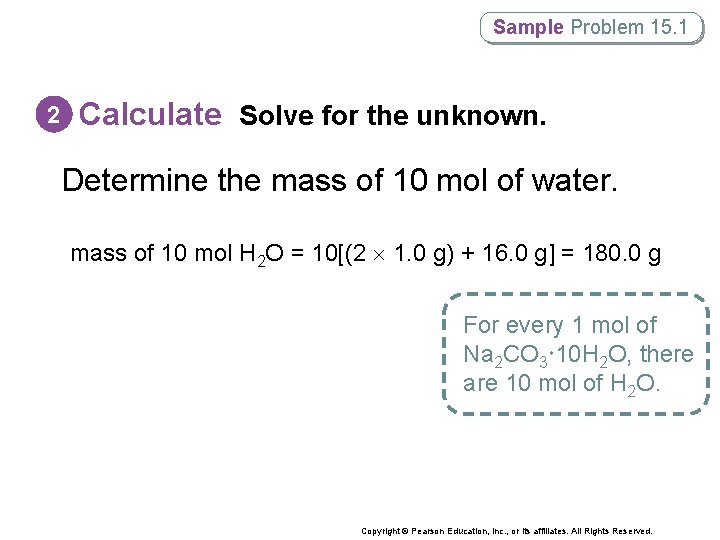
Sample Problem 15. 1 2 Calculate Solve for the unknown. Determine the mass of 10 mol of water. mass of 10 mol H 2 O = 10[(2 1. 0 g) + 16. 0 g] = 180. 0 g For every 1 mol of Na 2 CO 3 10 H 2 O, there are 10 mol of H 2 O. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

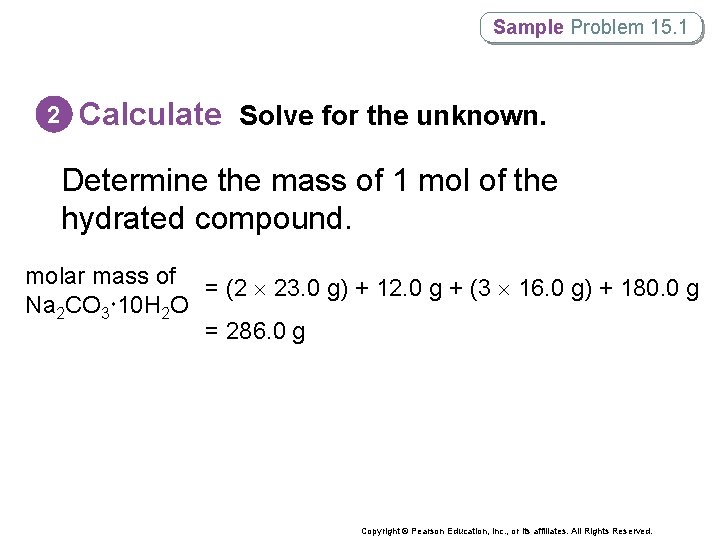
Sample Problem 15. 1 2 Calculate Solve for the unknown. Determine the mass of 1 mol of the hydrated compound. molar mass of = (2 23. 0 g) + 12. 0 g + (3 16. 0 g) + 180. 0 g Na 2 CO 3 10 H 2 O = 286. 0 g Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

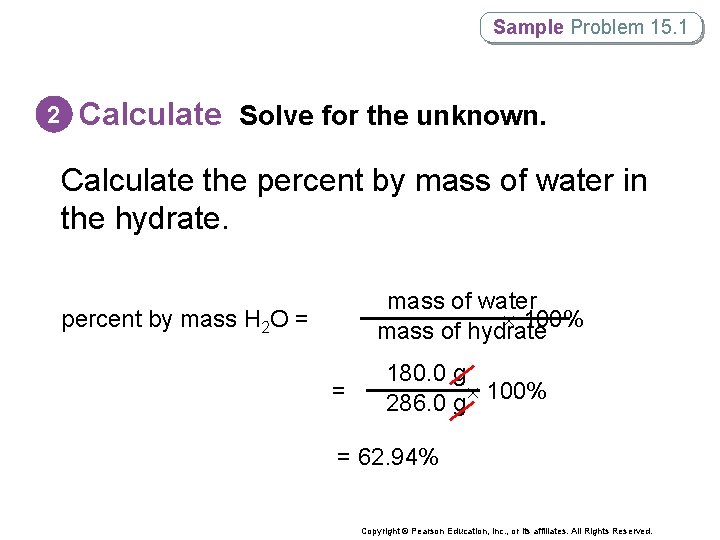
Sample Problem 15. 1 2 Calculate Solve for the unknown. Calculate the percent by mass of water in the hydrate. mass of water 100% mass of hydrate percent by mass H 2 O = = 180. 0 g 286. 0 g 100% = 62. 94% Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

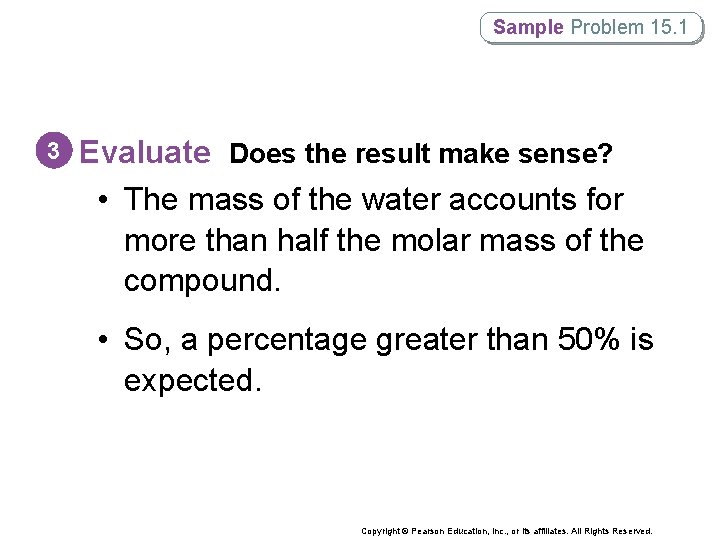
Sample Problem 15. 1 3 Evaluate Does the result make sense? • The mass of the water accounts for more than half the molar mass of the compound. • So, a percentage greater than 50% is expected. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates Deliquescent Compounds Some compounds are so hygroscopic that they become wet when exposed to normally moist air. • These compounds are deliquescent, which means that they remove sufficient water from the air to dissolve completely and form solutions. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Hydrates Pellets of sodium hydroxide are deliquescent. For this reason, containers of Na. OH should always be tightly stoppered. The solution formed by a deliquescent substance has a lower vapor pressure than that of the water in the air. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

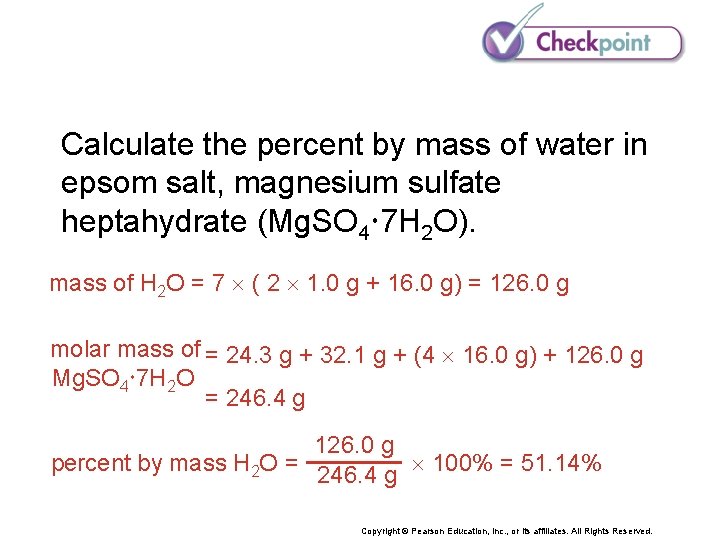
Calculate the percent by mass of water in epsom salt, magnesium sulfate heptahydrate (Mg. SO 4 7 H 2 O). Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Calculate the percent by mass of water in epsom salt, magnesium sulfate heptahydrate (Mg. SO 4 7 H 2 O). mass of H 2 O = 7 ( 2 1. 0 g + 16. 0 g) = 126. 0 g molar mass of = 24. 3 g + 32. 1 g + (4 16. 0 g) + 126. 0 g Mg. SO 4 7 H 2 O = 246. 4 g 126. 0 g percent by mass H 2 O = 246. 4 g 100% = 51. 14% Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Key Concepts and Key Equation Substances that dissolve most readily in water include ionic compounds and polar covalent compounds. All ionic compounds are electrolytes because they dissociate into ions. The forces holding the water molecules in hydrates are not very strong, so the water is easily lost and regained. mass of water percent by mass H 2 O = mass of hydrate 100% Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • aqueous solution: water that contains dissolved substances • solvent: the dissolving medium in a solution • solute: dissolved particles in a solution • solvation: a process that occurs when an ionic solute dissolves; in solution, solvent molecules surround the positive and negative ions Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • electrolyte: a compound that conducts an electric current when it is in an aqueous solution or in the molten state; all ionic compounds are electrolytes, but most covalent compounds are not • nonelectrolyte: a compound that does not conduct an electric current in aqueous solution or in the molten state • strong electrolyte: a solution in which a large portion of the solute exists as ions • weak electrolyte: a solution that conducts electricity poorly because only a fraction of the solute exists as ions Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • water of hydration: water molecules that are an integral part of a crystal structure • hydrate: a compound that has a specific number of water molecules bound to each formula unit • anhydrous: a substance that does not contain water • effloresce: to lose water of hydration; the process occurs when the hydrate has a vapor pressure higher than that of water vapor in the air Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • hygroscopic: a term describing salts and other compounds that remove moisture from the air • desiccant: a hygroscopic substance used as a drying agent • deliquescent: describes a substance that removes sufficient water from the air to form a solution; the solution formed has a lower vapor pressure than that of the water in the air Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

BIG IDEA Bonding and Interactions • Ionic compounds and polar covalent compounds dissolve most readily in water to form aqueous solutions. • Ionic compounds dissolve in water when the polar water molecules attract the ions of the solute, causing the individual solute ions to break away from the ionic crystal. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Chapter 15 Water and Aqueous Systems 15. 1 Water and Its Properties 15. 2 Homogeneous Aqueous Systems 15. 3 Heterogeneous Aqueous Systems Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

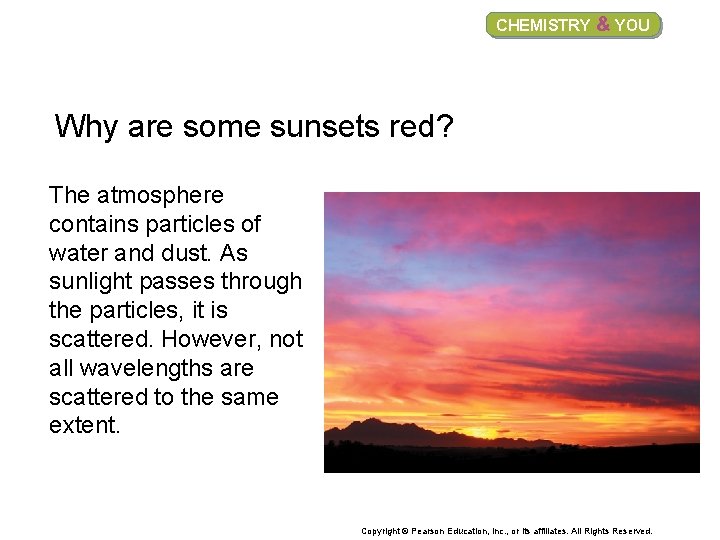
CHEMISTRY & YOU Why are some sunsets red? The atmosphere contains particles of water and dust. As sunlight passes through the particles, it is scattered. However, not all wavelengths are scattered to the same extent. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Suspensions What is the difference between a suspension and a solution? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Suspensions What is the difference between a suspension and a solution? • A suspension is a mixture from which particles settle out upon standing. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Suspensions A suspension differs from a solution because the particles of a suspension are much larger and do not stay suspended indefinitely. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Suspensions A suspension differs from a solution because the particles of a suspension are much larger and do not stay suspended indefinitely. • The particles in a typical suspension have an average diameter greater than 1000 nm. • By contrast, the particle size in a solution is usually about 1 nm. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Suspensions • A solution is a homogeneous mixture. • Suspensions are heterogeneous because at least two substances can be clearly identified. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Suspensions The difference between a solution and suspension is easily seen when the type of mixture is filtered. The small size of the solute particles in a solution allows them to pass through filter paper. The particles of a suspension can be removed by filtration. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Explain why a mixture of sand water can be separated by filtration, but a mixture of salt and water cannot. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Explain why a mixture of sand water can be separated by filtration, but a mixture of salt and water cannot. A mixture of sand water is a suspension, and a mixture of salt and water is a solution. The particles in the sand mixture are much larger than the ions in the salt mixture. The sand particles are too large to pass through filter paper; the ions are not. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids What distinguishes a colloid from a suspension and a solution? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

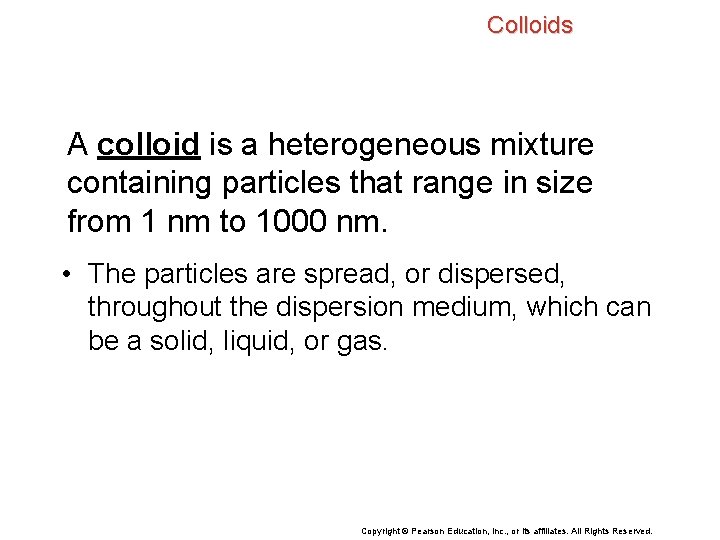
Colloids A colloid is a heterogeneous mixture containing particles that range in size from 1 nm to 1000 nm. • The particles are spread, or dispersed, throughout the dispersion medium, which can be a solid, liquid, or gas. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

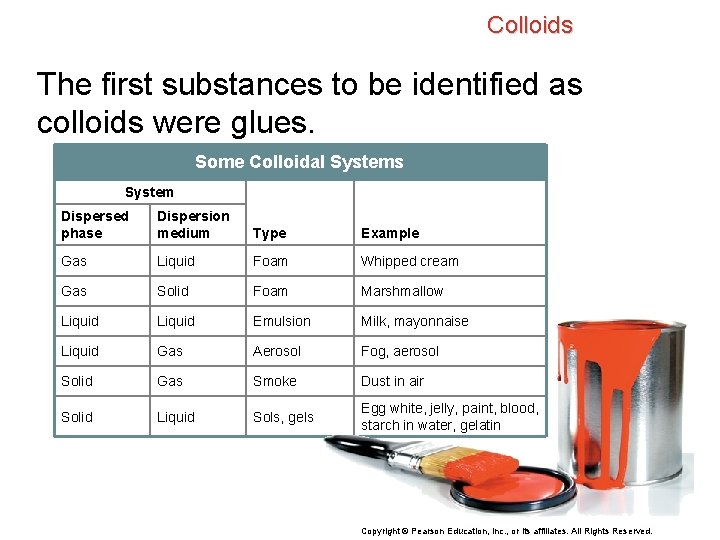
Colloids The first substances to be identified as colloids were glues. Some Colloidal Systems System Dispersed phase Dispersion medium Type Example Gas Liquid Foam Whipped cream Gas Solid Foam Marshmallow Liquid Emulsion Milk, mayonnaise Liquid Gas Aerosol Fog, aerosol Solid Gas Smoke Dust in air Solid Liquid Sols, gels Egg white, jelly, paint, blood, starch in water, gelatin Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids have particles smaller than those in suspensions and larger than those in solutions. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids have particles smaller than those in suspensions and larger than those in solutions. • These intermediate-sized particles cannot be retained by filter paper as are the larger particles of a suspension. • They do not settle out with time. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

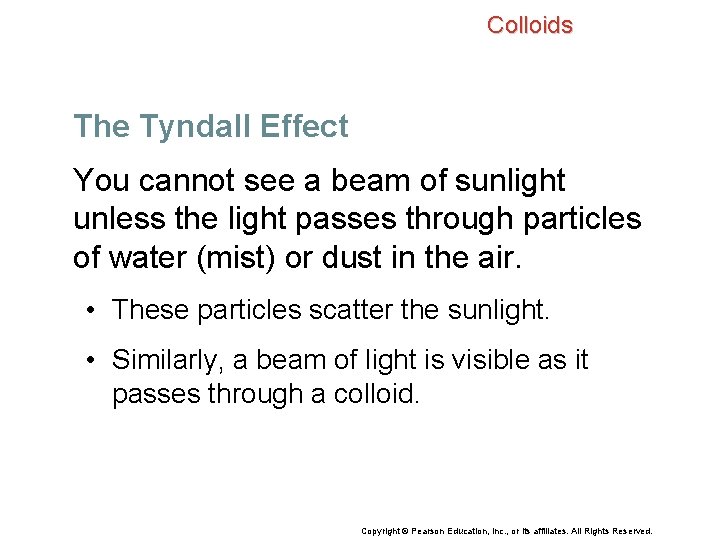
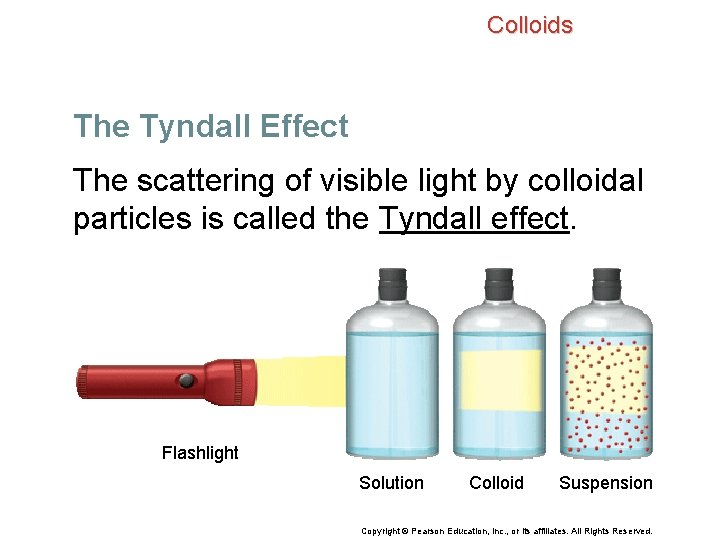
Colloids The Tyndall Effect You cannot see a beam of sunlight unless the light passes through particles of water (mist) or dust in the air. • These particles scatter the sunlight. • Similarly, a beam of light is visible as it passes through a colloid. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids The Tyndall Effect The scattering of visible light by colloidal particles is called the Tyndall effect. Flashlight Solution Colloid Suspension Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

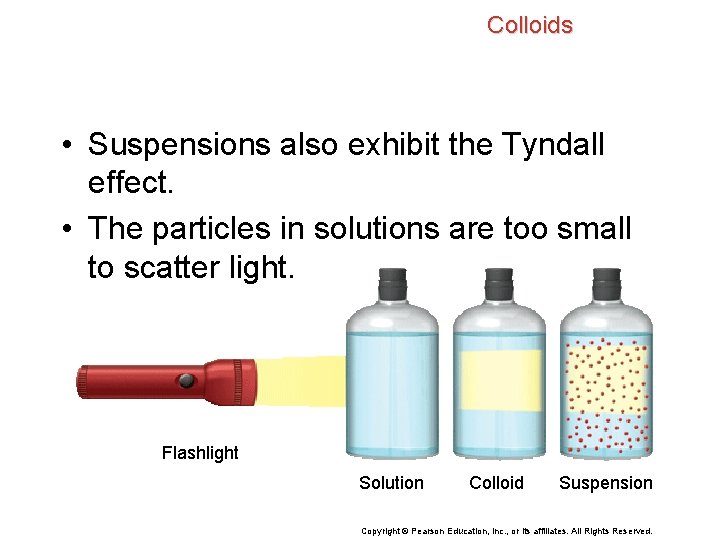
Colloids • Suspensions also exhibit the Tyndall effect. • The particles in solutions are too small to scatter light. Flashlight Solution Colloid Suspension Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU What would be the ideal conditions to see a red sunset? Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

CHEMISTRY & YOU What would be the ideal conditions to see a red sunset? A misty or foggy evening would be ideal for seeing a red sunset. There would be a large number of particles to scatter the sunlight. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Brownian Motion Flashes of light, or scintillations, are seen when colloids are studied under a microscope. • Colloids scintillate because the particles reflecting and scattering the light move erratically. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Brownian Motion The chaotic movement of colloidal particles, which was first observed by the Scottish botanist Robert Brown (1773– 1858), is called Brownian motion. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Brownian Motion Brownian motion is caused by collisions of the molecules of the dispersion medium with the small, dispersed colloidal particles. • These collisions help prevent the colloidal particles from setting. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Coagulation Colloidal particles also tend to stay suspended because they become charged by adsorbing ions from the dispersing medium onto their surface. • Adsorption means to adhere to a surface. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Coagulation All the colloidal particles in a particular colloidal system will have the same charge, although the colloidal system is neutral. • The repulsion between the like-charged particles prevents the particles from forming heavier aggregates that would have a greater tendency to settle out. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Coagulation A colloidal system can be destroyed or coagulated by the addition of electrolytes. • The added ions neutralize the charged colloidal particles. • The particles can clump together to form heavier aggregates and settle out from the dispersion. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Emulsions An emulsion is a colloidal dispersion of a liquid in a liquid. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Emulsions An emulsion is a colloidal dispersion of a liquid in a liquid. • An emulsifying agent is essential for the formation of an emulsion and for maintaining the emulsion’s stability. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Emulsions • Oils and greases are not soluble in water. • However, oils and greases readily form a colloidal dispersion if soap or detergent is added to the water. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Colloids Emulsions • One end of a large soap or detergent molecule is polar and is attracted to water molecules. • The other end of the soap or detergent molecule is nonpolar and is soluble in oil or grease. • Soaps and other emulsifying agents thus allow the formation of colloidal dispersions between liquids that do not ordinarily mix. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

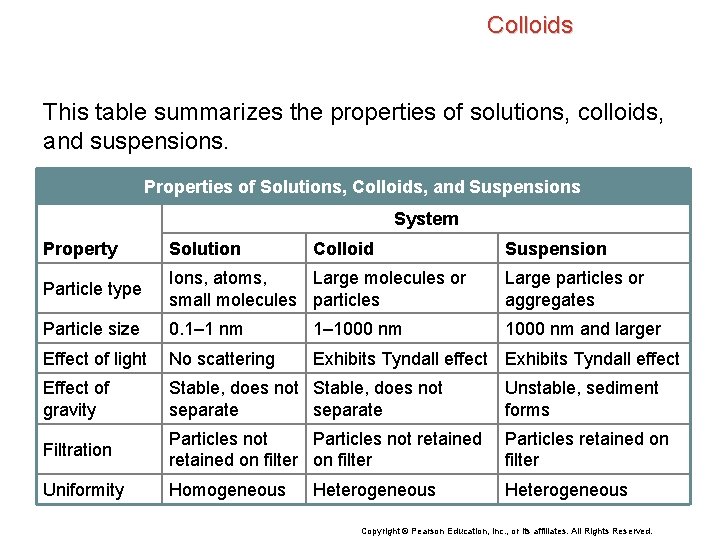
Colloids This table summarizes the properties of solutions, colloids, and suspensions. Properties of Solutions, Colloids, and Suspensions System Property Solution Colloid Particle type Ions, atoms, Large molecules or small molecules particles Large particles or aggregates Particle size 0. 1– 1 nm 1– 1000 nm and larger Effect of light No scattering Exhibits Tyndall effect Effect of gravity Stable, does not separate Unstable, sediment forms Filtration Particles not retained on filter Particles retained on filter Uniformity Homogeneous Heterogeneous Suspension Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Which of the following is a colloidal system? A. mud B. gasoline C. blood D. a mixture of sugar and water Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Which of the following is a colloidal system? A. mud B. gasoline C. blood D. a mixture of sugar and water Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Key Concepts A suspension differs from a solution because the particles of a suspension are much larger and do not stay suspended indefinitely. Colloids have particles smaller than those in suspensions and larger than those in solutions. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • suspension: a mixture from which some of the particles settle out slowly upon standing • colloid: a mixture whose particles are intermediate in size between those of a suspension and a solute solution • Tyndall effect: scattering of light by particles in a colloid or suspension, which causes a beam of light to become visible Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Glossary Terms • Brownian motion: the chaotic movement of colloidal particles, caused by collision with particles of the solvent in which they are dispersed • emulsion: the colloidal dispersion of one liquid in another Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.