Chapter 4 Reactions in Aqueous Solutions Some typical






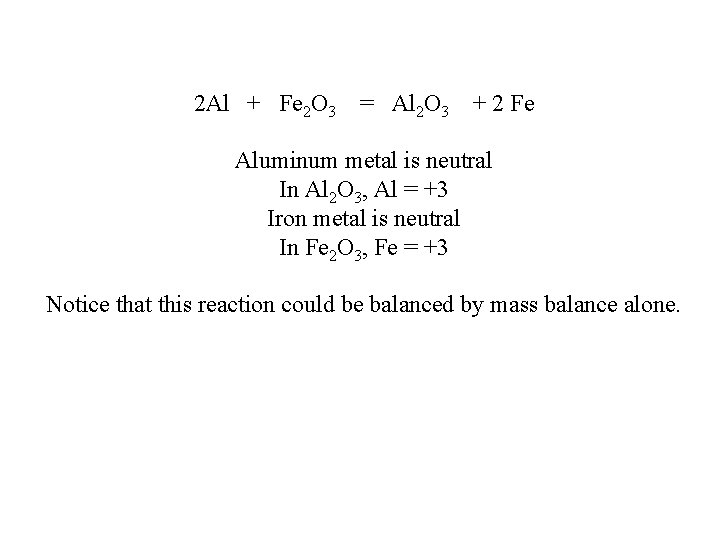






- Slides: 26

Chapter 4 Reactions in Aqueous Solutions

Some typical kinds of chemical reactions: 1. Precipitation reactions: the formation of a salt of lower solubility causes the precipitation to occur. cca 1 precipr 1047 -9 2. Acid Base reactions: the formation of water which is quite stable is a driving force for acid base chemistry. 3. Oxidation Reduction Reactions (reactions where electrons are gained and lost) the driving fore for most reactions including oxidation reduction reactions is the drive to lower the potential energy of the system (that is to convert potential energy to kinetic energy usually in the form of heat) *cca 1 glycerine; thermite Why do these reactions take place?

Electrolyte: the ability of a substance to form ions and conduct electricity Strong electrolytes: a substance when dissolved (usually in water) completely (or nearly so), dissociates into ions : MX +H 2 O = M+ + X- + H 2 O ex. Na. Cl, H 2 SO 4 Weak electrolytes: a substance when dissolved (usually in water) partially dissociates into ions : MX +H 2 O = M+ + X- + MX + H 2 O ex. acetic acid (vinegar, HC 2 H 3 O 2), HF Non-electrolytes: a substance when dissolved (usually in water) does not dissociate at all: MX +H 2 O = MX + H 2 O ex. sugar dvd MF: Media_AssetsChapter 04Electrolytes. Nonelectrolytes Media_AssetsChapter 04Strongand. Weak. Electrolytes

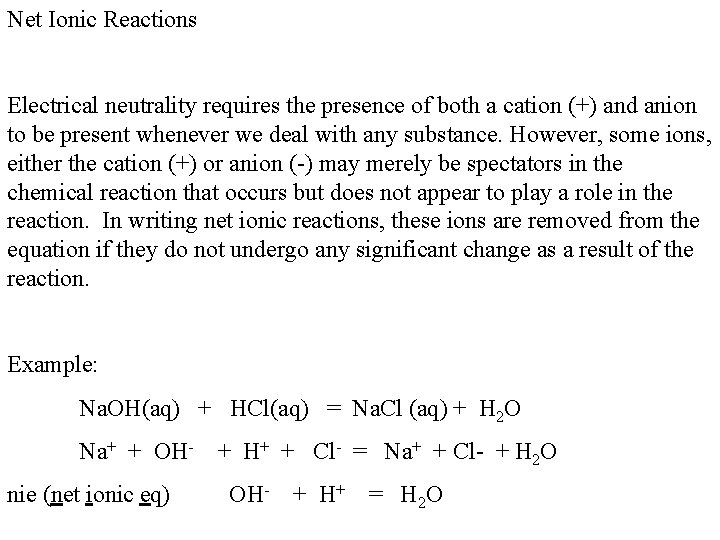
Net Ionic Reactions Electrical neutrality requires the presence of both a cation (+) and anion to be present whenever we deal with any substance. However, some ions, either the cation (+) or anion (-) may merely be spectators in the chemical reaction that occurs but does not appear to play a role in the reaction. In writing net ionic reactions, these ions are removed from the equation if they do not undergo any significant change as a result of the reaction. Example: Na. OH(aq) + HCl(aq) = Na. Cl (aq) + H 2 O Na+ + OH- + H+ + Cl- = Na+ + Cl- + H 2 O nie (net ionic eq) OH- + H+ = H 2 O

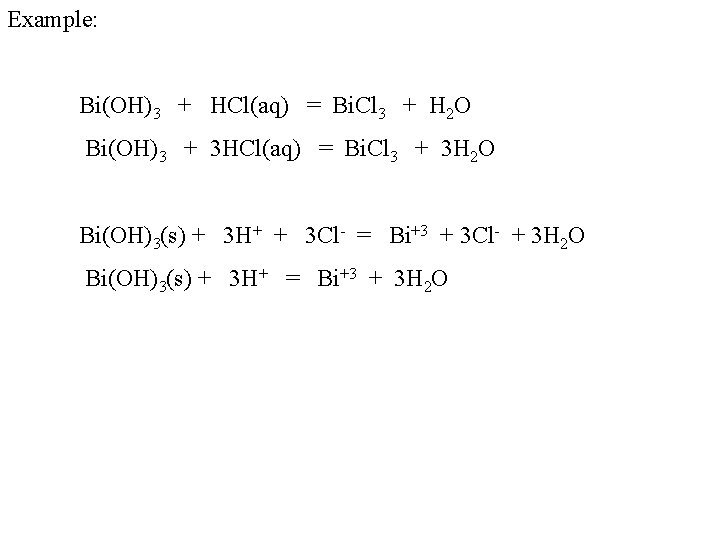
Example: Bi(OH)3 + HCl(aq) = Bi. Cl 3 + H 2 O Bi(OH)3 + 3 HCl(aq) = Bi. Cl 3 + 3 H 2 O Bi(OH)3(s) + 3 H+ + 3 Cl- = Bi+3 + 3 Cl- + 3 H 2 O Bi(OH)3(s) + 3 H+ = Bi+3 + 3 H 2 O

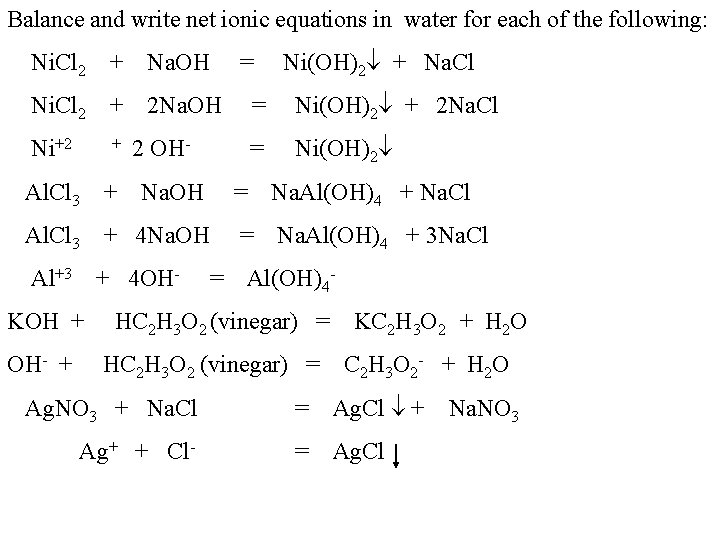
Balance and write net ionic equations in water for each of the following: Ni. Cl 2 + Na. OH = Ni(OH)2 + Na. Cl Ni. Cl 2 + 2 Na. OH = Ni(OH)2 + 2 Na. Cl Ni+2 + 2 OH- = Ni(OH)2 Al. Cl 3 + Na. OH = Na. Al(OH)4 + Na. Cl Al. Cl 3 + 4 Na. OH = Na. Al(OH)4 + 3 Na. Cl Al+3 + 4 OH- = Al(OH)4 - KOH + HC 2 H 3 O 2 (vinegar) = KC 2 H 3 O 2 + H 2 O OH- + HC 2 H 3 O 2 (vinegar) = C 2 H 3 O 2 - + H 2 O Ag. NO 3 + Na. Cl Ag+ + Cl- = Ag. Cl + Na. NO 3 = Ag. Cl

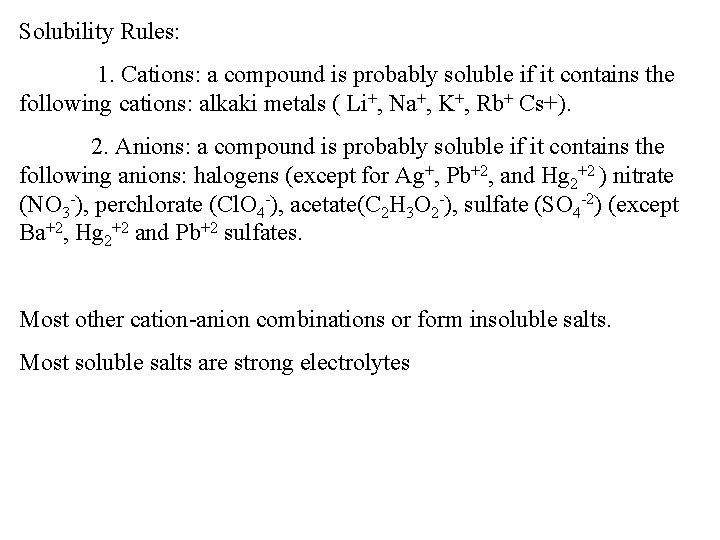
Solubility Rules: 1. Cations: a compound is probably soluble if it contains the following cations: alkaki metals ( Li+, Na+, K+, Rb+ Cs+). 2. Anions: a compound is probably soluble if it contains the following anions: halogens (except for Ag+, Pb+2, and Hg 2+2 ) nitrate (NO 3 -), perchlorate (Cl. O 4 -), acetate(C 2 H 3 O 2 -), sulfate (SO 4 -2) (except Ba+2, Hg 2+2 and Pb+2 sulfates. Most other cation-anion combinations or form insoluble salts. Most soluble salts are strong electrolytes

Solubility Rules: 1. Cations: a compound is probably soluble if it contains the following cation: alkaki metals ( Li+, Na+, K+, Rb+ Cs+). 2. Anions: a compound is probably soluble if it contains the following anions: halogens (except for Ag+, Pb+2, and Hg 2+2 ) nitrate (NO 3 -), perchlorate (Cl. O 4 -), acetate(C 2 H 3 O 2 -), sulfate (SO 4 -2) (except Ba+2, Hg 2+2 and Pb+2 sulfates). Are the following soluble? K 2 Cr. O 4 Zn. Cl 2 Pb(NO 3)2 Ag 2 SO 4 Ca(NO 3)2 Ba. S Hg. S Na 2 S

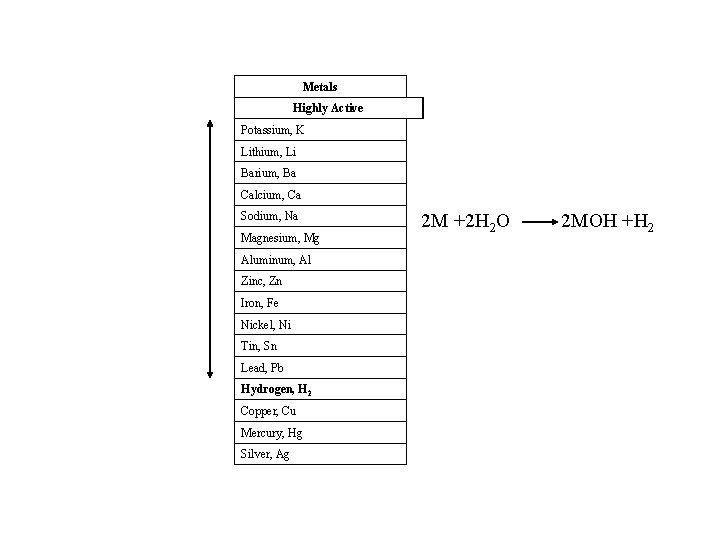
Relative reactivity of metals Na + H 2 O = Na. OH + H 2; this reaction occurs with the elements in first two columns and with Al, Mn, Zn, Co, Ni, Sn. How can we determine which is most and which is least reactive?

Metals Highly Active Potassium, K Lithium, Li Barium, Ba Calcium, Ca Sodium, Na Magnesium, Mg Aluminum, Al Zinc, Zn Iron, Fe Nickel, Ni Tin, Sn Lead, Pb Hydrogen, H 2 Copper, Cu Mercury, Hg Silver, Ag 2 M +2 H 2 O 2 MOH +H 2

Oxidation and Reduction Oxidation: the process by which an element or group of elements loose electrons Reduction: the process by which an element or group of elements gain electrons What is an agent? a facilitator In order to maintain electrical neutrality, for every electron lost by an element, there must be a gain of an electron by some other reactant. The oxidizing agent is the agent responsible for the loss of electrons. In the process the oxidizing agent get reduced The agent that looses electrons causes something else to gain electrons and therefore is the agent responsible for reduction Oxidizing agent is reduced Reducing agent is oxidized

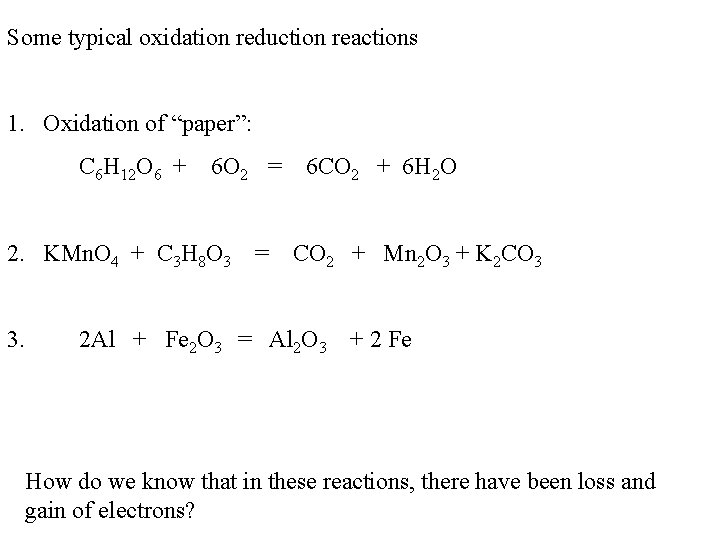
Some typical oxidation reduction reactions 1. Oxidation of “paper”: C 6 H 12 O 6 + 6 O 2 = 6 CO 2 + 6 H 2 O 2. KMn. O 4 + C 3 H 8 O 3 = CO 2 + Mn 2 O 3 + K 2 CO 3 3. 2 Al + Fe 2 O 3 = Al 2 O 3 + 2 Fe How do we know that in these reactions, there have been loss and gain of electrons?
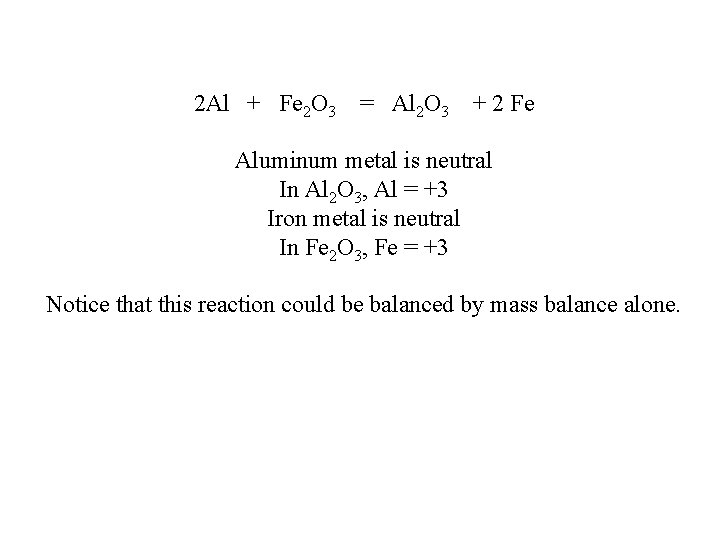
2 Al + Fe 2 O 3 = Al 2 O 3 + 2 Fe Aluminum metal is neutral In Al 2 O 3, Al = +3 Iron metal is neutral In Fe 2 O 3, Fe = +3 Notice that this reaction could be balanced by mass balance alone.

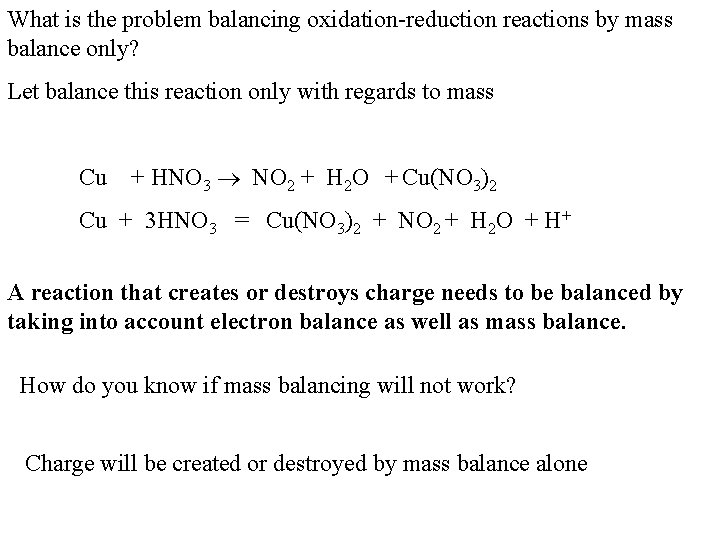
What is the problem balancing oxidation-reduction reactions by mass balance only? Let balance this reaction only with regards to mass Cu + HNO 3 NO 2 + H 2 O + Cu(NO 3)2 Cu + 3 HNO 3 = Cu(NO 3)2 + NO 2 + H 2 O + H+ A reaction that creates or destroys charge needs to be balanced by taking into account electron balance as well as mass balance. How do you know if mass balancing will not work? Charge will be created or destroyed by mass balance alone

Balancing Oxidation and Reduction Reactions Two steps are involved in balancing oxidation-reduction reactions. Step 1: First, it is important to balance the loss and gain in electrons Step 2: Second, it is important to achieve mass balance How do I identify an oxidation reduction reaction that requires both charge and mass balance? If charge is created or destroyed when you mass balance an equation, then you have an oxidation reduction equation that requires balancing both charge and mass

Balancing Oxidation and Reduction Reactions Two steps are involved in balancing oxidation-reduction reactions when the charge on either side of the equation is uneven. Step 1: First, it is important to balance the loss and gain in electrons Step 2: Second, it is important to achieve mass balance What do I do first? 1. Assign oxidation numbers

1. Rules in Assigning oxidation states: All elements are in an oxidation state = 0 Metals usually get oxidized, non-metals usually get reduced Typical oxidation states Alkali metals +1 Halogens -1 Alkaline earths +2 Group 6 A -2 Group 3 A +3 Group 5 A -3 H can be – 1 or +1

Assign oxidation states for each of the element in the following: H 2 SO 4 H 3 PO 4 HCl. O 4 H = +1; O = -2; S = +6 H = +1; O = -2; P = +5 H = +1; O = -2; Cl = +7 Zn. S Zn = +2; S = -2 HNO 3 Cr 2 O 7 -2 Mn. O 4 -1 Mn. O 2 C 6 H 12 O 6 H 2 O 2 H = +1 O = -2; N = +5 Cr = +6; O =-2 Mn = +7; O = -2 Mn = +4; O = -2 C = 0; H = +1; O = -2 H = +1; O = -1

Balancing Oxidation and Reduction Reactions Two steps are involved in balancing oxidation-reduction reactions. Step 1: First, it is important to balance the loss and gain in electrons Step 2: Second, it is important to achieve mass balance What do I do first? 1. Assign oxidation numbers 2. Determine what is oxidized and what is reduced

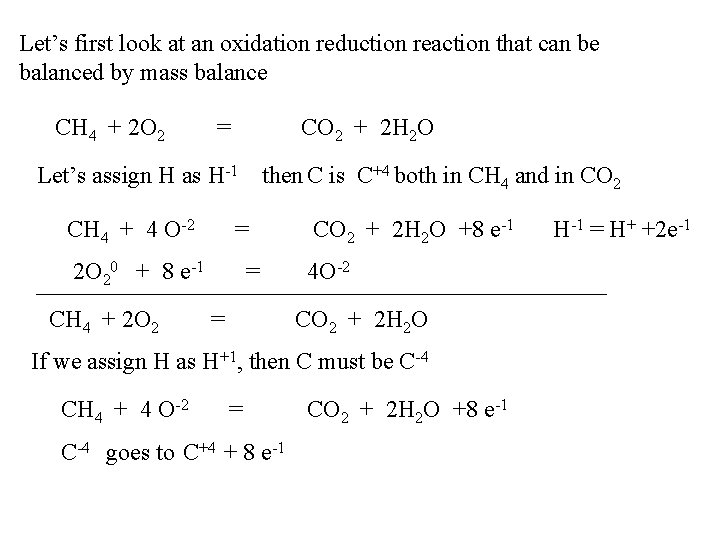
Let’s first look at an oxidation reduction reaction that can be balanced by mass balance CH 4 + 2 O 2 = CO 2 + 2 H 2 O Let’s assign H as H-1 then C is C+4 both in CH 4 and in CO 2 CH 4 + 4 O-2 = CO 2 + 2 H 2 O +8 e-1 2 O 20 + 8 e-1 = 4 O-2 CH 4 + 2 O 2 = CO 2 + 2 H 2 O If we assign H as H+1, then C must be C-4 CH 4 + 4 O-2 = CO 2 + 2 H 2 O +8 e-1 C-4 goes to C+4 + 8 e-1 H-1 = H+ +2 e-1

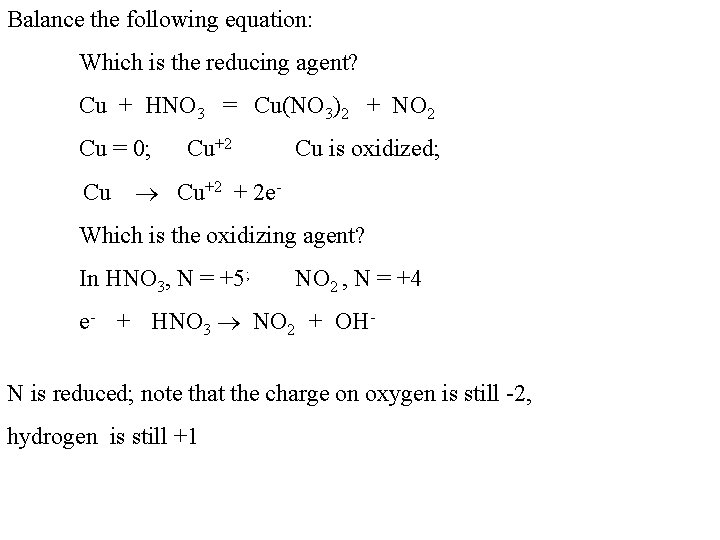
Balance the following equation: Which is the reducing agent? Cu + HNO 3 = Cu(NO 3)2 + NO 2 Cu = 0; Cu+2 Cu Cu is oxidized; +2 + 2 e- Which is the oxidizing agent? In HNO 3, N = +5; NO 2 , N = +4 e- + HNO 3 NO 2 + OHN is reduced; note that the charge on oxygen is still -2, hydrogen is still +1

Balancing Oxidation and Reduction Reactions Two steps are involved in balancing oxidation-reduction reactions. Step 1: First, it is important to balance the loss and gain in electrons Step 2: Second, it is important to achieve mass balance What do I do first? 1. Assign oxidation numbers 2. Determine what is oxidized and what is reduced 3. Mass balance the oxidation half reaction; mass balance the reduction half reaction 4. Combine the two half reactions; if the reaction takes place in H 2 O, it is permissible to break up water to form OH- and H+ as necessary or to form water from OH- and H+.

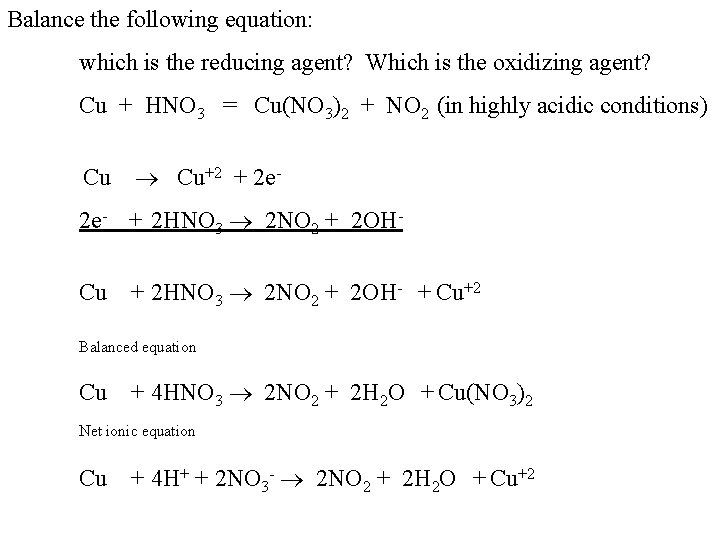
Balance the following equation: which is the reducing agent? Which is the oxidizing agent? Cu + HNO 3 = Cu(NO 3)2 + NO 2 (in highly acidic conditions) Cu +2 + 2 e- + 2 HNO 3 2 NO 2 + 2 OHCu + 2 HNO 3 2 NO 2 + 2 OH- + Cu+2 Balanced equation Cu + 4 HNO 3 2 NO 2 + 2 H 2 O + Cu(NO 3)2 Net ionic equation Cu + 4 H+ + 2 NO 3 - 2 NO 2 + 2 H 2 O + Cu+2

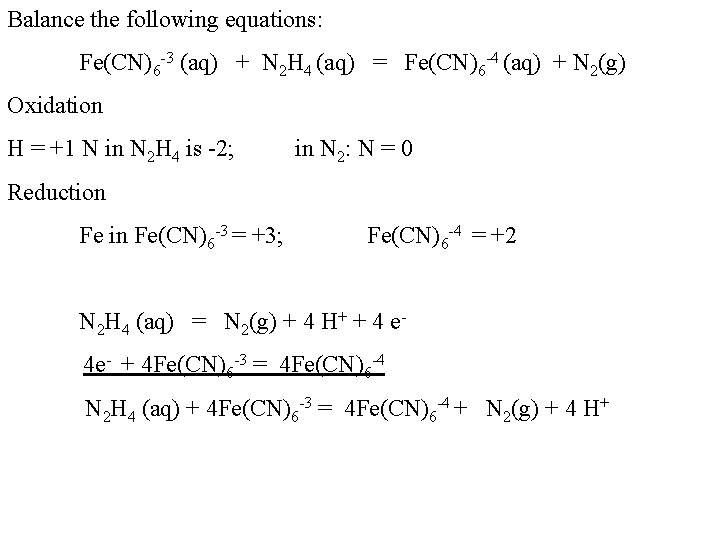
Balance the following equations: Fe(CN)6 -3 (aq) + N 2 H 4 (aq) = Fe(CN)6 -4 (aq) + N 2(g) Oxidation H = +1 N in N 2 H 4 is -2; in N 2: N = 0 Reduction Fe in Fe(CN)6 -3 = +3; Fe(CN)6 -4 = +2 N 2 H 4 (aq) = N 2(g) + 4 H+ + 4 e 4 e- + 4 Fe(CN) -3 = 4 Fe(CN) -4 6 6 N 2 H 4 (aq) + 4 Fe(CN)6 -3 = 4 Fe(CN)6 -4 + N 2(g) + 4 H+

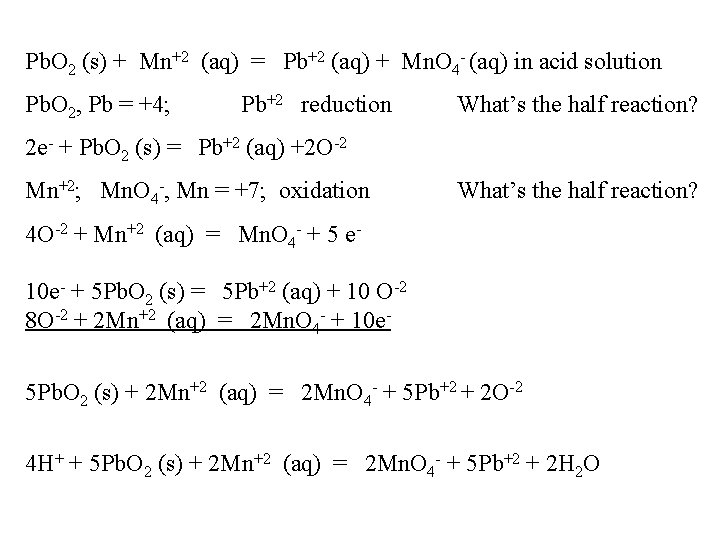
Pb. O 2 (s) + Mn+2 (aq) = Pb+2 (aq) + Mn. O 4 - (aq) in acid solution Pb. O 2, Pb = +4; Pb+2 reduction What’s the half reaction? 2 e- + Pb. O 2 (s) = Pb+2 (aq) +2 O-2 Mn+2; Mn. O 4 -, Mn = +7; oxidation What’s the half reaction? 4 O-2 + Mn+2 (aq) = Mn. O 4 - + 5 e 10 e- + 5 Pb. O 2 (s) = 5 Pb+2 (aq) + 10 O-2 8 O-2 + 2 Mn+2 (aq) = 2 Mn. O 4 - + 10 e 5 Pb. O 2 (s) + 2 Mn+2 (aq) = 2 Mn. O 4 - + 5 Pb+2 + 2 O-2 4 H+ + 5 Pb. O 2 (s) + 2 Mn+2 (aq) = 2 Mn. O 4 - + 5 Pb+2 + 2 H 2 O

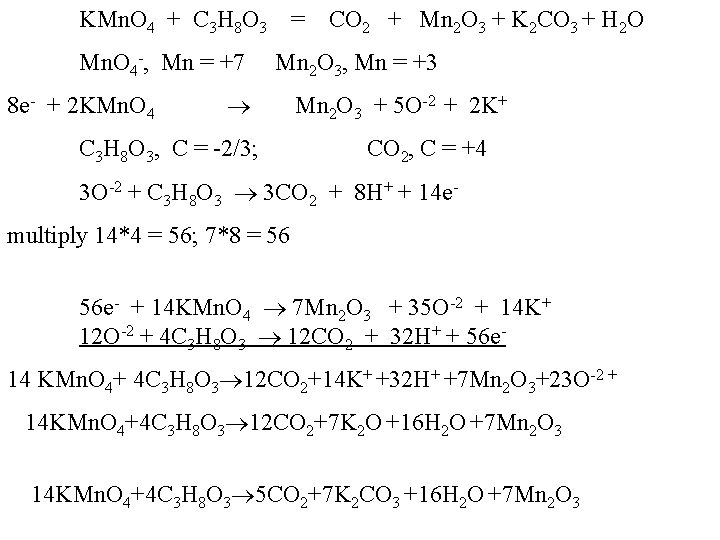
KMn. O 4 + C 3 H 8 O 3 = CO 2 + Mn 2 O 3 + K 2 CO 3 + H 2 O Mn. O 4 -, Mn = +7 Mn 2 O 3, Mn = +3 8 e- + 2 KMn. O 4 C 3 H 8 O 3, C = -2/3; Mn 2 O 3 + 5 O-2 + 2 K+ CO 2, C = +4 3 O-2 + C 3 H 8 O 3 3 CO 2 + 8 H+ + 14 emultiply 14*4 = 56; 7*8 = 56 56 e- + 14 KMn. O 4 7 Mn 2 O 3 + 35 O-2 + 14 K+ 12 O-2 + 4 C 3 H 8 O 3 12 CO 2 + 32 H+ + 56 e 14 KMn. O 4+ 4 C 3 H 8 O 3 12 CO 2+14 K+ +32 H+ +7 Mn 2 O 3+23 O-2 + 14 KMn. O 4+4 C 3 H 8 O 3 12 CO 2+7 K 2 O +16 H 2 O +7 Mn 2 O 3 14 KMn. O 4+4 C 3 H 8 O 3 5 CO 2+7 K 2 CO 3 +16 H 2 O +7 Mn 2 O 3